Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Lymphocyte to Monocyte Ratio is Independently Associated with Futile Recanalization in Acute Ischemic Stroke After Endovascular Therapy
Authors Guan J , Wang Q, Zhao Q
Received 9 September 2023
Accepted for publication 16 November 2023
Published 28 November 2023 Volume 2023:19 Pages 2585—2596
DOI https://doi.org/10.2147/NDT.S434225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Jincheng Guan,1 Qiong Wang,2 Qingshi Zhao1
1Department of Neurology, People’s Hospital of Longhua, Shenzhen, People’s Republic of China; 2Department of Neurology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China
Correspondence: Qingshi Zhao, Department of Neurology, People’s Hospital of Longhua, 38 Jinglong Construction Road, Shenzhen, 518109, People’s Republic of China, Email [email protected]
Background and Purpose: Acute ischemic stroke (AIS) caused by large artery occlusion (LAO) poses considerable risks in terms of mortality and disability. Endovascular treatment (EVT) has emerged as a primary intervention for this condition. However, the occurrence of futile recanalization (FR) following EVT remains common, necessitating the identification of predictive markers for treatment outcomes. Although the lymphocyte to monocyte ratio (LMR) has been linked to various diseases, its association with FR after EVT in AIS patients has not been investigated.
Methods: An analysis was conducted on patients with AIS who underwent EVT within 24 hours of symptom onset. The success of reperfusion was evaluated using the modified Thrombolysis in Cerebral Infarction (mTICI) scale, with patients achieving an mTICI score of ≥ 2b being included in the study. Various clinical, radiological, and laboratory variables, including lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR), were collected. Logistic regression analysis was used to determine factors associated with FR, and receiver operating characteristic (ROC) analysis was performed to assess the predictive value of LMR.
Results: Among the cohort of 101 patients, it was observed that 52.4% experienced FR. Upon admission, lower levels of lymphocyte-to-monocyte ratio (LMR) were found to be associated with older age, higher baseline NIHSS scores, lower ASPECTS, and poorer mRS scores at 90 days. Both univariate and multivariate logistic regression analyses indicated that low LMR independently predicted FR, with an adjusted odds ratio of 0.64 (95% CI = 0.412– 0.984, p = 0.042). ROC analysis further demonstrated that LMR had an area under the curve (AUC) of 0.789 for predicting FR.
Conclusion: This study establishes the potential value of the lymphocyte-to-monocyte ratio (LMR) as a prognostic marker for predicting FR in patients with AIS undergoing EVT. Decreased LMR levels are associated with unfavorable clinical outcomes.
Keywords: acute ischemic stroke, large artery occlusion, endovascular therapy, lymphocyte-to-monocyte ratio, futile recanalization, prognostic marker, inflammation, treatment outcomes
Introduction
Large artery occlusion (LAO) leading to acute ischemic stroke (AIS) has emerged as a significant burden on public health, given its heightened rates of mortality and disability.1 According to the latest statistics from 2020, the standardized prevalence, morbidity, and mortality estimates of stroke among adults aged 40 years and above in southern China were 1.8% (95% CI, 1.7–2.0%), 522.9 (95% CI, 461.7–584.4) per 100,000 person-years, and 368.8 (95% CI, 317.4–420.2) per 100,000 person-years, respectively. These figures indicate that the incidence and mortality rates were higher than the national average.2,3 The clinical evidence has shown that expeditious reperfusion in patients with acute ischemic stroke (AIS) and large vessel occlusion can lead to a reduction in mortality rates. Several randomized controlled trials (RCTs) have provided evidence of the superior effectiveness of endovascular treatment (EVT) compared to conventional treatment for AIS caused by occlusion of the proximal anterior circulation large arteries.4–6 EVT has become a widely utilized frontline treatment for AIS patients with large artery occlusion.7 However, futile recanalization (FR) is a common occurrence among patients with acute ischemic stroke (AIS) who undergo endovascular therapy (EVT).8–11 Several studies have indicated that nearly half of these patients experience FR, with rates ranging from 40.5% to 54.5%.12–15 In AIS patients, even when successful vessel recanalization is achieved (mTICI grade 2b or 3), the clinical outcomes are considered unfavorable if the modified Rankin Scale (mRS) score exceeds 2 at 90 days, thus defining it as futile recanalization.
Lymphocytes and monocytes, which are integral constituents of the immune system, exert substantial influence on inflammation and immune regulation.16–18 Considerable research has been dedicated to exploring the relationship between the lymphocyte to monocyte ratio and disease prognosis, spanning various disciplines such as cardiovascular diseases, malignant tumors, and others, both within domestic and international contexts.19–22 However, the association between the lymphocyte to monocyte ratio and futile recanalization subsequent to endovascular treatment in the realm of acute ischemic stroke has received limited attention in the literature.
In recent times, there has been a growing interest among research institutions globally in investigating the correlation between the ratio of lymphocytes to monocytes and the prognosis of individuals suffering from stroke. Professor Gao Yanjun16 conducted a study on innovative prognostic factors for acute ischemic stroke (AIS), wherein he established the substantial predictive capability of the lymphocyte to monocyte ratio (LMR) upon admission for determining the prognosis of AIS. This study emphasized the robust connection between a low LMR and the severity of stroke as well as an unfavorable prognosis. Dr. Gong Pengyu’s research team discovered23 that the lymphocyte to monocyte ratio prior to thrombolysis functions as an autonomous safeguarding element against early neurological deterioration. In a pioneering investigation by Kim et al20 it was initially demonstrated that a connection exists between the lymphocyte to monocyte ratio and unfavorable prognosis in individuals suffering from acute ischemic stroke. Moreover, numerous studies have explored the correlation between the systemic inflammatory response index and futile recanalization subsequent to endovascular treatment in patients with acute ischemic stroke. A study conducted by an Italian research group24 unveiled a noteworthy correlation between elevated systemic immune-inflammation index (SIRI) upon admission and futile recanalization subsequent to endovascular treatment. Additionally, multiple international studies25 have demonstrated the independent predictive capability of systemic immune-inflammation index (SII) in determining futile recanalization post endovascular treatment. These studies have shed light on the association between the lymphocyte to monocyte ratio and the inflammatory response as well as immune function in stroke patients, thereby providing valuable insights into the mechanisms underlying stroke pathogenesis and the potential for novel treatment strategies. However, there is presently a scarcity of research examining the correlation between the lymphocyte to monocyte ratio and futile recanalization subsequent to endovascular treatment. Consequently, it is crucial to undertake studies to elucidate the prognostic importance of the lymphocyte to monocyte ratio in individuals afflicted with acute ischemic stroke.
Materials and Methods
Patient Selection
In this retrospective investigation, we collected data from patients with acute ischemic stroke who underwent EVT within 24 hours of symptom onset at the Third Affiliated Hospital of Guangzhou Medical University, China, between April 2020 and July 2022. We utilized the modified Thrombolysis in Cerebral Infarction (mTICI) scale to evaluate EVT reperfusion. Eligible patients underwent EVT and achieved an mTICI score of ≥2b, indicating recanalization after the procedure. Patients were included in the analysis if they had complete 3-month modified Rankin Scale (mRS) data, age ≥18 years, pre-stroke mRS score ≤2, and onset to arrival (OTA) duration ≤24 hours. The rationale for including patients with a pre-stroke mRS score ≤2 in the analysis should be clarified. On the one hand, patients with a pre-stroke mRS score ≤2 had no pre-existing disability, which may reduce additional risk factors for cerebrovascular diseases.16 However, in most clinical investigations centered on EVT for acute ischemic stroke in the anterior circulation, patients with a pre-stroke mRS score ≤2 are considered for inclusion in the trials.23,26–28 This inclusion is grounded on the observation that patients with a pre-stroke mRS score ≤2 can benefit from EVT and may experience reduced mortality rates.29,30 Consequently, we incorporated this patient group to ensure impartiality in the outcomes. The Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University reviewed and sanctioned this study. Every participant included in this study furnished informed consent by signing. This study was reviewed and approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University, and all patients involved signed the informed consent form.
Patient Clinical and Radiological Variables
We examined a variety of clinical and radiological characteristics of the individuals, including age, gender, risk factors, baseline National Institutes of Health Stroke Scale (NIHSS) score (ranging from 0 to 42; higher scores indicate greater neurological impairment), baseline Alberta Stroke Program Early CT Score (ASPECTS), etiology of ischemic stroke, location of arterial occlusion, intravenous thrombolysis, and modified Thrombolysis in Cerebral Infarction (mTICI) grade. Additionally, we documented the medical and lifestyle histories of the patients, including hypertension, diabetes, dyslipidemia, coronary artery disease, smoking habits, and alcohol consumption. The Alberta Stroke Program Early CT Score (ASPECTS) was employed to evaluate the extent of early infarction. The ASPECTS score ranges from 0 to 10, with higher scores indicating lesser early ischemic changes. We categorized the etiology of ischemic stroke using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria.31 In summary, we assessed the durations of two variables: onset to puncture (OTP) and puncture to recanalization (PTR). Furthermore, we evaluated complications, including symptomatic intracerebral hemorrhage (sICH), defined as any intracranial hemorrhage resulting in a four-point increment in the total NIHSS score.
Laboratory Measurements
The collected laboratory data included baseline systolic (SBP) and diastolic blood pressure (DBP), glucose levels upon admission, differential white blood cell counts, platelet counts, levels of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), creatinine (Cr), and homocysteine (HCY). The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the neutrophil count by the lymphocyte count. Lymphocyte-to-monocyte ratio (LMR) was calculated by dividing the lymphocyte count by the monocyte count. Platelet-to-lymphocyte ratio (PLR) was calculated by dividing the platelet count by the lymphocyte count.32
Patient Outcome
Participants with mTICI ≥2b were subsequently grouped based on their 90-day modified Rankin Scale (mRS) score, forming the futile recanalization group (90-day mRS score of 3–6) and the meaningful recanalization group (90-day mRS score of 0–2). The evaluation of the 90-day modified Rankin Scale (mRS) was conducted through telephonic interviews or outpatient appointments at the 3-month follow-up following the onset of stroke.
Statistical Analysis
Data analyses were conducted utilizing SPSS 26.0 (IBM Corporation, Armonk, New York, USA) and R software (version 4.2.1). A clinical comparison was performed between the futile recanalization and meaningful recanalization patient cohorts, and the normality of continuous variables was examined using the Shapiro–Wilk test. Normally distributed data were analyzed using the t-test and presented as mean and standard deviation (SD). Data with non-normal distributions were assessed using the Mann–Whitney U-test and presented as the median and interquartile range (IQR). Categorical information was analyzed using either Pearson’s chi-squared test or Fisher’s exact test. In this study, only creatinine for this variable had missing data, including 6 missing data. Missing data were imputed using the missForest algorithm. Variables with P < 0.05 in the univariate analysis were selected for univariate logistic regression analysis. Subsequently, variables with P < 0.05 in the univariate logistic regression analysis were included in the multivariate logistic regression analysis. Multivariate logistic regression was employed to verify independent predictive factors associated with futile recanalization following endovascular treatment. The odds ratio (OR) with a 95% confidence interval (CI) was computed as the estimate for each endpoint. Receiver operating characteristic (ROC) analysis was conducted to determine the optimal cutoff value of LMR for discriminating clinical outcomes after mechanical thrombectomy (MT). Based on the LMR cutoff value, all patients were divided into two groups, and the clinical characteristics of the two groups were compared. Statistical significance was defined as a two-tailed P-value of 0.05.
Result
Baseline Characteristics of Patients
A total of 134 patients were recruited between April 2020 and July 2022. After excluding patients with failed recanalization (n=16), a pre-stroke mRS score >2 (n=6), lost to follow-up (n=5), OTA duration > 24 hours (n=2), and age <18 years (n=1), a final sample of 101 patients remained (Figure 1). These patients were then allocated to two groups: the meaningful recanalization group and the futile recanalization group. The group of patients who underwent futile recanalization demonstrated an incidence rate of 52.4%. These patients exhibited advanced age, with a median age of 78 compared to 66.5 years in the meaningful recanalization group (p<0.001), and a higher prevalence of hypertension (71.7% vs 47.9%, p=0.025). In comparison to the meaningful recanalization group, the futile recanalization group displayed elevated mean NIHSS scores of 17.02±7.22 compared to 11.42±5.88 (p<0.001) and significantly lower median ASPECTS scores upon admission (9 vs 10 points, p=0.039). Furthermore, the group classified as having futile recanalization exhibited a significantly higher median baseline systolic blood pressure (155.00 vs 141.00mmHg, p=0.003), and a larger proportion of individuals experienced symptomatic intracranial hemorrhage after undergoing endovascular treatment (18.9% vs 2.1%, p=0.017). Our laboratory examination and evaluation revealed higher neutrophil (median 8.97 vs 6.61, p=0.010) and monocyte counts (median 0.61 vs 0.46, p<0.001) in patients belonging to the futile recanalization group, while lymphocyte counts were lower (median 0.97 vs 1.33, p=0.019). Additionally, the futile recanalization group exhibited elevated NLR (median 9.23 vs 5.22, p=0.001) and PLR (median 224.11 vs 148.48, p=0.004), along with lower LMR values (median 1.56 vs 2.86, p<0.001) when compared to the meaningful recanalization group. (Table 1)
 |
Table 1 Comparison of Characteristics Between the Futile Recanalization Group and the Favorable Recanalization Group |
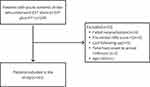 |
Figure 1 Flow chart presenting the process of patient inclusion and exclusion in this study. |
Univariable and Multivariable Logistic Regression Analyses for Futile Recanalization
Table 2 presents the results of univariable and multivariable logistic regression analyses aimed at identifying independent factors associated with futile recanalization. The findings indicate that lower ASPECTS on admission (adjusted odds ratio [aOR] = 0.53, 95% confidence interval [CI] = 0.321–0.868, p = 0.012), higher baseline NIHSS (aOR = 1.12, 95% CI = 1.020–1.240, p < 0.018), higher baseline SBP (aOR = 1.03, 95% CI = 1.001–1.058, p < 0.039), and sICH (aOR = 45.91, 95% CI = 2.607–808.259, p < 0.009) were independently associated with futile recanalization. After controlling for potential confounding variables such as age, ASPECTS on admission, baseline NIHSS, baseline SBP, hypertension, NLR, PLR, and sICH, the lymphocyte-to-monocyte ratio was determined to be an independent negative predictor for futile recanalization (adjusted odds ratio [aOR] = 0.64, 95% confidence interval [CI] = 0.412–0.984, p = 0.042, see Table 2). The results of the multivariate analysis revealed a sensitivity of 0.792, specificity of 0.791, positive predictive value of 0.808, negative predictive value of 0.776, and diagnostic accuracy of 0.792. The multivariate logistic regression model did not exhibit collinearity issues, as indicated by variance inflation factors ranging from 1.090 to 3.973.(Table 3)
 |
Table 2 Univariate and Multivariate Logistic Regression Analysis for Futile Recanalization After Endovascular Therapy |
 |
Table 3 Multicollinearity Assessment |
Association of LMR with Futile Recanalization
The study findings indicate that LMR exhibited a robust predictive capacity for futile recanalization subsequent to endovascular therapy, as evidenced by an AUC of 0.789 (95% CI: 0.709–0.886) and a cut-off value of 1.9 (sensitivity: 83.3%; specificity: 69.8%) (Table 4). In comparison, the AUC values for baseline NIHSS, baseline SBP, ASPECTS on admission, and sICH were 0.732, 0.669, 0.611, and 0.584, respectively. Notably, LMR demonstrated a superior predictive ability for futile recanalization when compared to baseline NIHSS, baseline SBP, ASPECTS on admission, and sICH. Furthermore, LMR exhibited a higher predictive capacity for futile recanalization in comparison to baseline NIHSS, baseline SBP, ASPECTS on admission, and sICH (Figure 2).
 |
Table 4 ROC Curve Analysis of LMR |
To investigate the correlation between the lymphocyte-to-monocyte ratio (LMR) and the clinical status of patients with acute ischemic stroke (AIS), a total of 101 patients were categorized into two groups based on the receiver operating characteristic (ROC) cut-off values: LMR <1.92 (n = 45) and LMR ≥ 1.92 (n = 56). A lower LMR level (<1.92) exhibited significant associations with older age, higher initial National Institutes of Health Stroke Scale (NIHSS) score, more unfavorable modified Rankin Scale (mRS) score at 90 days, and elevated homocysteine levels (P = 0.031, P = 0.009, P < 0.001, and P = 0.018, respectively) (Table 5). Figure 3 displays the ordinal analysis of the distribution of modified Rankin Scale (mRS) scores between the high lymphocyte-to-monocyte ratio (LMR) group and the low LMR group. The univariate analysis revealed a statistically significant association between a lower LMR, dichotomized at 1.92, and a poorer functional outcome (odds ratio [OR] = 0.607, 95% confidence interval [CI] = 0.485–0.760, p < 0.001).
 |
Table 5 Association of Lymphocyte-to-Monocyte Ratio with Baseline Characteristics Characteristics |
 |
Figure 3 Distribution of mRS score at 90 days between different level of LMR. |
Discussion
The primary novel discovery of this study is the correlation between lymphocyte-to-monocyte ratio (LMR) and futile recanalization in patients with acute ischemic stroke (AIS). Among AIS patients who undergo endovascular treatment (EVT) and achieve meaningful recanalization, those with lower LMR upon admission are at a higher risk of experiencing unfavorable functional outcomes at 3 months. LMR can serve as a readily accessible independent predictive factor for futile recanalization. Furthermore, when the systemic immune-inflammation index (SIRI) exceeds the established threshold of 1.92× 109/L, the likelihood of adverse outcomes at 3 months nearly triples for patients. These findings are consistent with an expanding body of scholarly research that emphasizes the pivotal role of inflammation in the pathobiology of stroke and its influence on prognosis.
Following the onset of stroke, an inflammatory reaction occurs as a result of the release of inflammatory mediators in the damaged tissue of the brain, leading to the activation of microglia (brain macrophages) and the recruitment of circulating immune cells to the site of injury.16 The brain undergoes a substantial influx of polymorphonuclear and mononuclear cells, which subsequently form macrophages, representing one of the initial events in the inflammatory cascade. During the acute phase, white blood cells that have infiltrated release inflammatory cytotoxic mediators, which result in cellular damage, heightened microvascular permeability, and activation of pathways that facilitate the formation of blood clots. Consequently, this exacerbates ischemic injury, tissue edema, and secondary damage.20 While the ischemic core cannot be rescued, prompt restoration of blood flow can salvage the surrounding penumbra. Activation of endothelial cells leads to the aggregation of leukocytes and obstruction of microvessels, thereby causing microvascular injury and impacting tissue reperfusion at the microvascular level. Therefore, despite the effective restoration of larger blood vessels, there is a potential negative impact on the viability of the penumbral region. Empirical findings suggest that specific blood flows within the brain, which are randomly distributed, do not fully recover following the reestablishment of cerebral circulation. This phenomenon, referred to as “no-reflow”, is facilitated by platelets and white blood cells. The recanalization and reperfusion processes in the penumbral region enhance the pro-inflammatory role of platelets, consequently triggering intricate thrombo-inflammatory pathways that contribute to ischemia-reperfusion injury.17 Importantly, the interaction between T cells and platelets may contribute to an increased infarct area. Consequently, microvascular events and secondary thrombus formation involve reciprocal interactions among endothelial cells, white blood cells, and platelets.
White blood cells, such as neutrophils, lymphocytes, and monocytes, have unique effects on the inflammatory response. Neutrophils play a role in initiating inflammation and causing brain damage through the release of inflammatory mediators. Following an ischemic stroke, neutrophils rapidly and extensively infiltrate the central nervous system, particularly accumulating in brain microvessels.23 During the second phase of blood-brain barrier disruption, these neutrophils locally release proteolytic enzymes, resulting in damage to the blood-brain barrier and infiltration of brain tissue. This process contributes to endothelial injury and adjacent vascular damage, and hemorrhage transformation. Contrarily, lymphocytes possess a regulatory impact on inflammation, functioning as a specific category of leukocytes and assuming a contentious role in post-ischemic inflammation. Certain experiments conducted on rodent stroke models have demonstrated that heightened lymphocyte levels upregulate the anti-inflammatory cytokine IL-10, thereby suppressing inflammatory cytokines such as IL-6 and tumor necrosis factor-alpha, and consequently exerting neuroprotective effects. Conversely, monocytes contribute to inflammation and thrombus formation by engaging in interactions with platelets and endothelial cells.33
The findings of our study indicate that a decrease in lymphocyte-to-monocyte ratio (LMR) can serve as a predictive factor for futile recanalization after endovascular treatment in individuals with stroke. Consequently, incorporating lymphocytes and monocytes into a consolidated index could offer a fresh perspective on forecasting futile recanalization in patients with acute ischemic stroke (AIS). It is imperative to emphasize that evaluating a solitary cell lineage may not fully encompass the intricacies of immune status and response. Additionally, the analysis of individual blood cells may be susceptible to external influences and biases, such as variations in hydration, dehydration, and handling of blood specimens. Hence, combining multiple cell measurements as indices and ratios may offer greater reliability in clinical practice.
The precise mechanisms by which LMR affects futile recanalization are not yet fully understood. Nevertheless, a review of existing literature demonstrates that the association between neutrophils within white blood cells and futile recanalization has been confirmed through animal experimentation. El Amki et al conducted a study using a rat model of middle cerebral artery occlusion (MCAO) and observed the occurrence of the no-reflow phenomenon following rt-PA thrombolysis. Their findings indicated that white blood cells, particularly neutrophils, adhered to and obstructed distal capillaries, resulting in blockage.34 In their study, Erdener et al employed high spatiotemporal resolution imaging techniques in a mouse model of transient middle cerebral artery occlusion (MCAO) to observe the occurrence of dynamic capillary stasis within the ischemic penumbral region. Notably, this stasis was found to be reversible upon administration of a neutrophil antibody (Ly6G antibody).35 These findings have significant implications, suggesting that the adhesion and blockade of white blood cells may play a critical role in impeding the effectiveness of recanalization following reperfusion therapy in cases of ischemic stroke. Although animal experiments have demonstrated the potential of neutrophil antibody (Ly6G antibody) in alleviating no-reflow, the absence of the Ly6G antigen in human white blood cells poses challenges for further research in this area. While initial research has provided some understanding of the connection between neutrophils and futile recanalization, additional inquiry is necessary to fully comprehend the underlying mechanisms through which the lymphocyte-to-monocyte ratio (LMR) influences futile recanalization. This further investigation will offer novel avenues for translational research and potential targets for intervention to improve the effectiveness of endovascular therapy (EVT). In patients with varying stroke types, particularly in the context of lymphocyte to monocyte ratio as a potential prognostic marker for predicting recanalization after endovascular treatment, the prognostic impact of LMR may differ. This distinction is particularly relevant when comparing lacunar infarcts to nonlacunar infarcts. Given the distinct pathophysiology, prognostic factors, and clinical characteristics of lacunar stroke compared to other acute cerebrovascular diseases, investigating the prognostic significance of LMR in this context could be a valuable topic for future research.36
This study is characterized by several limitations. Firstly, it is a retrospective study conducted at a single center, featuring a relatively small sample size that solely includes cases from our hospital. As a result, there is a possibility of patient selection bias and statistical analysis bias. Secondly, due to the nature of being a hospital-based study, there may have been inaccuracies and incompleteness in the documentation of pre-admission medication history, which hindered the inclusion of past anti-thrombotic medication history for analysis. Furthermore, our study solely focused on the initial calculation of the Lymphocyte-to-Monocyte Ratio (LMR) upon admission, neglecting the exploration of dynamic fluctuations in inflammatory markers through repeated assessments or the monitoring of LMR throughout the remainder of the hospital stay or at the time of discharge. This constraint may potentially influence the correlation between LMR and ineffective reperfusion in patients with Acute Ischemic Stroke (AIS), underscoring the necessity for future investigations to scrutinize the influence of dynamic changes in LMR on the likelihood of ineffective reperfusion. Additionally, the study did not assess various other inflammatory markers or potential confounding factors, such as plasma coagulation factors, TNF-α, IL-1, and IL-6. Therefore, it is imperative to conduct further extensive and prospective studies with larger sample sizes to corroborate these findings and comprehensively evaluate other variables, including collateral circulation status, cerebral reserve, and infarct volume. These variables have the potential to impact the relationship between LMR and ineffective reperfusion. Notwithstanding these constraints, this study serves as the initial manifestation of the prognostic significance of LMR in individuals afflicted with acute ischemic stroke induced by large vessel occlusion subsequent to intravascular intervention.
Conclusion
The results of our study provide evidence of a significant association between the lymphocyte to monocyte ratio (LMR) and futile recanalization in individuals who have undergone endovascular treatment. Lower LMR values are associated with positive clinical outcomes following this treatment. The LMR, which serves as an affordable and noninvasive serological marker of inflammation, shows potential as a prognostic indicator for acute ischemic stroke patients undergoing endovascular treatment for large artery occlusion (LAO). Further research is necessary to substantiate these findings and provide a deeper understanding of their underlying mechanisms. A comprehensive understanding of the factors and mechanisms that contribute to the lack of successful restoration of blood flow could aid in identifying new therapeutic targets and devising strategies to enhance the prognosis of individuals suffering from acute ischemic stroke.
Ethics Approval and Informed Consent
This study protocol was reviewed and approved by the Institutional Research Ethic Committee of The Third Affiliated Hospital of Guangzhou Medical, approval number [IIT20220240B-R1]. All participants wrote informed consent. We followed the guidelines outlined in the Declaration of Helsinki.
Acknowledgments
This study was supported by High Level Project of Medicine in Longhua, ShenZhen (HLPM201907020102), construction funds of key medical disciplines in Longhua District, Shenzhen (MKD202007090208), the Scientific Research Projects of Medical and Health Institutions of Longhua District, Shenzhen (2021067).
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–1935. doi:10.1016/S0140-6736(14)60584-5
2. W-J T, Zhao Z, Yin P, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. 2023;6(3):e231455. doi:10.1001/jamanetworkopen.2023.1455
3. Tu WJ, Wang LD; Special Writing Group of China Stroke Surveillance R. China stroke surveillance report 2021. Mil Med Res. 2023;10(1):33. doi:10.1186/s40779-023-00463-x
4. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi:10.1056/NEJMoa1411587
5. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi:10.1056/NEJMoa1414905
6. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. doi:10.1056/NEJMoa1503780
7. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
8. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi:10.1016/S0140-6736(16)00163-X
9. Hussein HM, Georgiadis AL, Vazquez G, et al. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol. 2010;31(3):454–458. doi:10.3174/ajnr.A2006
10. Yang J, Jin Z, Song J, et al. Futile recanalization after endovascular treatment in patients with acute basilar artery occlusion. Neurosurgery. 2023;92(5):1006–1012. doi:10.1227/neu.0000000000002313
11. Lin S, Lin X, Zhang J, et al. A visualized nomogram to online predict futile recanalization after endovascular thrombectomy in basilar artery occlusion stroke. Front Neurol. 2022;13:968037. doi:10.3389/fneur.2022.968037
12. Hassan AE, Shariff U, Saver JL, et al. Impact of procedural time on clinical and angiographic outcomes in patients with acute ischemic stroke receiving endovascular treatment. J Neurointerv Surg. 2019;11(10):984–988. doi:10.1136/neurintsurg-2018-014576
13. Hussein HM, Saleem MA, Qureshi AI. Rates and predictors of futile recanalization in patients undergoing endovascular treatment in a multicenter clinical trial. Neuroradiology. 2018;60(5):557–563. doi:10.1007/s00234-018-2016-2
14. Tateishi Y, Wisco D, Aoki J, et al. Large deep white matter lesions may predict futile recanalization in endovascular therapy for acute ischemic stroke. Interv Neurol. 2015;3(1):48–55. doi:10.1159/000369835
15. van Horn N, Kniep H, Leischner H, et al. Predictors of poor clinical outcome despite complete reperfusion in acute ischemic stroke patients. J Neurointerv Surg. 2021;13(1):14–18. doi:10.1136/neurintsurg-2020-015889
16. Ren H, Liu X, Wang L, et al. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(11):2595–2602. doi:10.1016/j.jstrokecerebrovasdis.2017.06.019
17. Song Q, Pan R, Jin Y, et al. Lymphocyte-to-monocyte ratio and risk of hemorrhagic transformation in patients with acute ischemic stroke. Neurol Sci. 2020;41(9):2511–2520. doi:10.1007/s10072-020-04355-z
18. Herz J, Sabellek P, Lane TE, et al. Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke. 2015;46(10):2916–2925. doi:10.1161/STROKEAHA.115.010620
19. Switonska M, Slomka A, Korbal P, et al. Association of neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio with treatment modalities of acute ischaemic stroke: a Pilot Study. Medicine. 2019;55(7):342. doi:10.3390/medicina55070342
20. Park MG, Kim MK, Chae SH, et al. Lymphocyte-to-monocyte ratio on day 7 is associated with outcomes in acute ischemic stroke. Neurol Sci. 2018;39(2):243–249. doi:10.1007/s10072-017-3163-7
21. Wang L, Song Q, Wang C, et al. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: a cohort study and systematic review. J Neurol Sci. 2019;406:116445. doi:10.1016/j.jns.2019.116445
22. Guan J, Wang Q, Hu J, et al. Nomogram-based prediction of the futile recanalization risk among acute ischemic stroke patients before and after endovascular therapy: a retrospective study. Neuropsychiatr Dis Treat. 2023;19:879–894.
23. Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18(1):51. doi:10.1186/s12974-021-02090-6
24. Lattanzi S, Norata D, Divani AA, et al. Systemic inflammatory response index and futile recanalization in patients with ischemic stroke undergoing endovascular treatment. Brain Sci. 2021;11(9):1164.
25. Yi HJ, Sung JH, Lee DH. Systemic inflammation response index and systemic immune-inflammation index are associated with clinical outcomes in patients treated with mechanical thrombectomy for large artery occlusion. World Neurosurg. 2021;153:e282–e289. doi:10.1016/j.wneu.2021.06.113
26. Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the devt randomized clinical trial. JAMA. 2021;325(3):234–243.
27. Goyal N, Tsivgoulis G, Alexandrov AV, et al. Author response: comparative safety and efficacy of combined IVT and MT with direct MT in large vessel occlusion. Neurology. 2018;91(24):1116. doi:10.1212/WNL.0000000000006654
28. LeCouffe NE, Kappelhof M, Treurniet KM, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385(20):1833–1844. doi:10.1056/NEJMoa2107727
29. Bala F, Beland B, Mistry E, et al. Endovascular treatment of acute ischemic stroke in patients with pre-morbid disability: a meta-analysis. J Neurointerv Surg. 2023;15(4):343–349.
30. Zhao H, Bai X, Li W, et al. Influence of pre-stroke dependency on safety and efficacy of endovascular therapy: a systematic review and meta-analysis. Front Neurol. 2022;13:956958. doi:10.3389/fneur.2022.956958
31. Adams HP
32. Oh SW, Yi HJ, H LD, et al. Prognostic significance of various inflammation-based scores in patients with mechanical thrombectomy for acute ischemic stroke. World Neurosurg. 2020;141:e710–e717. doi:10.1016/j.wneu.2020.05.272
33. Zhou YX, Li WC, Xia SH, et al. Predictive value of the systemic immune inflammation index for adverse outcomes in patients with acute ischemic stroke. Front Neurol. 2022;13:836595. doi:10.3389/fneur.2022.836595
34. El Amki M, Gluck C, Binder N, et al. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep. 2020;33(2):108260. doi:10.1016/j.celrep.2020.108260
35. Erdener SE, Tang J, Kilic K, et al. Dynamic capillary stalls in reperfused ischemic penumbra contribute to injury: a hyperacute role for neutrophils in persistent traffic jams. J Cereb Blood Flow Metab. 2021;41(2):236–252. doi:10.1177/0271678X20914179
36. Rudilosso S, Rodríguez-Vázquez A, Urra X, et al. The potential impact of neuroimaging and translational research on the clinical management of lacunar stroke. Int J Mol Sci. 2022;23(3):1497. doi:10.3390/ijms23031497
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

