Back to Journals » Journal of Inflammation Research » Volume 16
Lymphocyte to C-Reactive Protein Ratio as an Early Biomarker to Distinguish Sepsis from Pneumonia in Neonates
Received 7 June 2023
Accepted for publication 11 August 2023
Published 17 August 2023 Volume 2023:16 Pages 3509—3517
DOI https://doi.org/10.2147/JIR.S424897
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Xinrui Liu,1 Yuan Mu2
1Zhengzhou Key Laboratory of Children’s Infection and Immunity, Children’s Hospital Affiliated to Zhengzhou University, Henan Children’s Hospital, Zhengzhou Children’s Hospital, Zhengzhou, People’s Republic of China; 2Institute of Thermology, Henan Institute of Metrology and Testing Sciences, Zhengzhou, People’s Republic of China
Correspondence: Xinrui Liu, Email [email protected]
Background: Neonatal sepsis is an acute and severe disease that seriously threatens the life and health of newborns. Neonates with pneumonia may also have unrecognized neonatal sepsis. Early diagnosis of neonatal sepsis is beneficial for early treatment. This study aimed to evaluate the clinical significance of the lymphocyte-to-C-reactive protein ratio (LCR) as an early biomarker to distinguish sepsis from pneumonia.
Methods: This retrospective study enrolled 1635 neonates with pneumonia from February 2016 to March 2022. Among them, 182 cases were diagnosed with sepsis based on the positive blood culture results. Clinical and laboratory data were extracted from the electronic medical records. LCR was calculated as the ratio of the total lymphocyte count (× 109 cells/L) to the C-reactive protein level (mg/L). Binary logistic regression analysis was conducted to evaluate the clinical significance of LCR as an early biomarker in distinguishing sepsis from pneumonia. Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic value of LPCR in sepsis cases. All statistical analyses were conducted using Statistical Product and Service Solutions, version 24.0.
Results: The neonates with pneumonia combined with sepsis had a lower LCR than that of the neonates with pneumonia. Further analysis showed that the prevalence of neonatal pneumonia combined with sepsis was significantly higher in the low-LCR group than in the high-LCR group (20.7% vs 5.5%, P < 001). Binary logistic regression revealed that LCR was an independent risk factor for identifying pneumonia combined with sepsis. The ROC curve analysis revealed that LCR had better power than the lymphocyte count and CRP level individually in diagnosing neonatal pneumonia combined with sepsis (0.72 vs 0.65 vs 0.66, P < 0.001), with 62% sensitivity and 72% specificity.
Conclusion: LCR can be a potential early biomarker in distinguishing neonates with sepsis from those with pneumonia.
Keywords: lymphocyte to C-reactive protein ratio, neonates, pneumonia, sepsis
Introduction
Neonates are susceptible to pathogenic microorganisms and may develop into neonatal pneumonia or sepsis owing to their immature immune systems.1 Neonatal sepsis is a severe threat to the life and health of newborns, affecting 2/1000 live births, with a high mortality rate ranging from 11% to 19%.2 Furthermore, neonates with pneumonia may also have unrecognized neonatal sepsis. In some cases, neonates have both pneumonia and sepsis, and they are not treated according to the intervention guidelines for sepsis. Hence, these neonates cannot benefit from the early treatment of sepsis, as encouraged by the Surviving Sepsis Campaign Physician’s management guidelines.3 However, there are many difficulties and uncertainties in the early diagnosis of neonatal sepsis, such as non-specific clinical symptoms and lower rates of positive blood culture results. Therefore, exploring more convenient and accurate indicators for the early detection of neonatal sepsis is critical.
Neonatal sepsis is characterized by systemic inflammatory response syndrome,4 indicating that inflammatory markers may be clinically relevant in identifying neonatal sepsis. Lymphocytes are the primary white blood cells that help the body fight infection and disease.5,6 In sepsis, various cytokines can induce apoptosis of the lymphocytes.7,8 Several studies have reported that lymphocytopenia is frequently observed in patients with sepsis, and persistent lymphocytopenia is associated with high mortality rates.9–13 C-reactive protein (CRP) is a protein produced by the liver and increases significantly when there is inflammation in the body.14 CRP is one of the most investigated and widely used laboratory parameters to evaluate the body’s inflammation status.15 Multiple studies have confirmed that CRP has high clinical significance in the early diagnosis of sepsis and is an important predictor and risk factor for poor outcomes in neonates with sepsis.16,17 The lymphocyte-to-CRP ratio (LCR) is an index calculated as the ratio of the total lymphocyte count (×109 cells/L) to the CRP level (mg/L). Studies have reported that LCR decreases in patients with infectious diseases and is a helpful biomarker in the early screening and prediction of infectious diseases, such as coronavirus disease 2019 (COVID-19) and sepsis.18–20 However, no studies have evaluated the clinical significance of LCR in distinguishing neonatal sepsis from neonatal pneumonia. Thus, we aimed to examine whether LCR can serve as an early biomarker in distinguishing sepsis from pneumonia in neonates.
Materials and Methods
Study Design and Population
This single-center retrospective observational study was conducted at Henan Children’s Hospital Zhengzhou, China, between February 2016 and March 2022. The inclusion criteria for the study were aged ≤ 28 days and a diagnosis of pneumonia. The exclusion criteria were the presence of hematological system diseases, malignancies, or major congenital malformations and incomplete data during the admission, such as body temperature, CRP level, and lymphocyte count. The study followed the principles of the Declaration of Helsinki, and it was approved by the Hospital Ethics Review Board of Henan Children’s Hospital. The data used in this study were retrospectively collected, and the datasets were entirely anonymized before analysis. Considering the retrospective nature of this investigation, the need for informed consent was waived by the Hospital Ethics Review Board.
Clinical Definition
The diagnosis of neonatal pneumonia is mainly based on the medical history, clinical symptoms, and laboratory test results. Medical history refers to the current presence of any high-risk factors or previous contact with infected patients. Clinical symptoms include abnormal body temperature, respiratory distress, snoring, and coughing. Laboratory findings refer to abnormal levels of inflammatory cells and markers, such as CRP and procalcitonin (PCT). Additionally, chest radiography usually shows new pulmonary infiltration. Neonatal sepsis is defined as systemic inflammatory response syndrome in the presence of a positive blood culture according to the published International Pediatric Sepsis Consensus.21 The neonatal sequential organ failure assessment (nSOFA) score is used to assess the severity of neonatal sepsis.22 Both neonatal pneumonia and sepsis were diagnosed by two independent doctors for these patients.
Data Collection
Clinical and laboratory data during hospital admission were extracted from the electronic medical records, including age, sex, weight, body temperature, respiratory rate, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), the length of hospital stay, PCT, CRP, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (ALB), creatinine (CREA) and urea nitrogen (UREA). The PCT levels were detected using Cobas® 8000 modular analyzer (Roche Diagnostics, Switzerland). The CRP levels were detected using a latex-enhanced immunoturbidimetric assay on an UPPER analyzer (Ultrasensitive CRP kit, Upper Bio-Tech, China). The ALT, AST, ALB, CREA, and UREA levels were recorded by the conventional clinical analytical approach using an automatic Beckman biochemical analyzer (Beckman Coulter, Brea, California, USA). The total white blood cells, neutrophils, lymphocytes, and platelets were recorded using an automated blood cell counter (Sysmex Corporation, Japan). In our data analysis, PCT levels>100 ng/mL or <0.02 ng/mL were defined as 101 ng/mL and 0.01 ng/mL, respectively, and CRP levels <0.8 mg/L were defined as 0.7 mg/L. The LCR was calculated as the total lymphocyte count (×109 cells/L)/CRP (mg/L).
Statistical Analysis
Normally distributed data are presented as means ± standard deviations. They were analyzed using independent t-tests or one-way analysis of variance. Non-normally distributed data are presented as medians and interquartile ranges. They were analyzed using the Mann–Whitney U-test. Categorical data are expressed as numbers and percentages. They were evaluated using chi-square tests. The association between the LCR and other continuous variables was assessed using Spearman correlation test. Binary logistic regression analysis was conducted to evaluate the clinical significance of LCR as an early biomarker in distinguishing sepsis from pneumonia. Receiver operating characteristic (ROC) curve was used to assess the diagnostic value of LCR in sepsis. Delong’s test was used to compare the area under the ROC curves (AUC) among different variables. The optimal cut-off point was calculated using Youden’s index (sensitivity + specificity − 1).23 All statistical analyses were performed using IBM Statistical Product and Service Solutions version 24.0 (SPSS Inc., Chicago, Illinois, USA) and MedCalc version 15.2.2 (MedCalc Software, Belgium).
Results
Study Population Characteristics
This study enrolled 1635 neonates with pneumonia, of which 182 (11.1%) had sepsis and were allotted to pneumonia with sepsis group. The remaining 1453 (88.9%) neonates were included in the pneumonia group. The baseline characteristics of the two groups are presented in Table 1. Compared with the neonates in the pneumonia group, neonates in pneumonia with sepsis group were older and had lower body weight, SBP, and DBP. Moreover, the body temperature, respiratory rate, and heart rate were higher, and the hospital stay was lengthier in pneumonia with sepsis group than in the pneumonia group. Laboratory tests showed that neonates in the pneumonia with sepsis group had higher PCT, CRP, CREA, and UREA levels, neutrophil count, and nSOFA scores, and lower ALB level, lymphocyte count, and LCR than those of the pneumonia group.
 |
Table 1 Basic Characteristics of the Study Participants by Groups |
Association Between LCR and the Presence of Neonatal Sepsis
To further explore the association of the LCR with the presence of neonatal pneumonia combined with sepsis, the neonates were classified into the following three groups according to the LCR tertiles: low-LCR group (LCR <3.56), intermediate-LCR group (LCR, 3.56–6.29), and high-LCR group (LCR >6.29). The neonates in the low-LCR group were younger and had a higher proportion of males, higher PCT levels and nSOFA scores, and lengthier hospital stay when compared with the neonates in the other two groups (Table 2). Further analysis revealed that the prevalence of neonatal pneumonia combined with sepsis was significantly higher in the low-LCR group than in the high-LCR group (20.7% vs 5.5%, P < 0.001), while that of neonatal pneumonia was significantly lower in the low-LCR group than in the high-LCR group (79.3% vs 94.5%, P < 0.001).
 |
Table 2 Clinical and Demographic Characteristics According to the Lymphocyte-to-C-Reactive Protein Ratio Tertiles |
Correlation Between LCR and Clinical Parameters
Spearman correlation analysis was performed to investigate the relationship between the LCR and various clinical parameters. LCR was positively correlated with age (r = 0.320, P < 0.001), body weight (r = 0.161, P < 0.001), SBP (r = 0.148, P < 0.001), DBP (r = 0.097, P < 0.001), ALT (r = 0.058, P = 0.020), and ALB (r = 0.367, P < 0.001) (Table 3). However, LCR was negatively correlated with the respiratory rate (r = –0.084, P = 0.001), PCT (r = –0.481, P < 0.001), CREA (r = –0.290, P < 0.001), UREA (r = –0.174, P < 0.001), neutrophil count (r = –0.217, P < 0.001), nSOFA score (r = –0.333, P < 0.001), and length of hospital stay (r = –0.225, P < 0.001). No significant association was found between the LCR and body weight, heart rate, and AST.
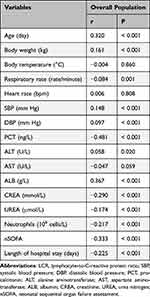 |
Table 3 Correlations Between LCR and Clinical Parameters |
LCR as an Independent Factor in Identifying Neonatal Sepsis
Univariate and multivariable binary logistic analyses were conducted to evaluate the clinical significance of LCR as an early biomarker in distinguishing neonatal pneumonia combined with sepsis from pneumonia. Variables with a P-value <0.05 in the univariate analysis were used in the multivariable binary logistic analysis, including body weight, body temperature, heart rate, SBP, DBP, PCT, ALB, ALT, AST, CREA, UREA, and neutrophil count. After adjusting for the above variables, LCR remained an independent biomarker for identifying neonatal sepsis in neonates with pneumonia (odds ratio [OR] = 0.902, 95% confidence interval [CI] = 0.847–0.962, P = 0.002) (Table 4). Further analysis showed that the LCR tertiles were independently associated with an increased prevalence of neonatal pneumonia combined with sepsis.
 |
Table 4 Predictive Value of the LCR for Sepsis in Neonates with Pneumonia |
Diagnostic Value of LCR in Neonatal Sepsis
ROC curve analysis was conducted to assess the efficacy of LCR in distinguishing neonatal sepsis from pneumonia in neonates. The AUC of LCR revealed that it had good power in diagnosing neonatal pneumonia combined with sepsis (AUC = 0.72, 95% CI = 0.67–0.76, P < 0.001), which was higher than that of the lymphocyte count (AUC = 0.65, 95% CI, 0.60–0.70, P < 0.001) and CRP (AUC = 0.66, 95% CI = 0.62–0.71, P < 0.001) (P < 0.05) (Figure 1). The optimal cut-off value of LCR in predicting neonatal pneumonia combined with sepsis was 3.35, with 62% sensitivity and 72% specificity.
 |
Figure 1 ROC curve of the lymphocyte count, CRP, and LCR in predicting neonatal sepsis. |
Discussion
Compared with adults, neonates have limited immunological memory and an immature immune system, which makes them vulnerable to pathogenic microorganisms and the development of pneumonia and sepsis.1 Pneumonia is a significant neonatal infection that affects the lung and has a high incidence. Compared to neonatal pneumonia, neonatal sepsis is an acute and severe disease that seriously threatens the life and health of newborns.24 Neonatal sepsis is an infection of the bloodstream in newborn infants under 28 days old. It is the leading cause of morbidity and mortality, especially in low- and middle-income countries.25 Oza et al26 analyzed the neonatal mortality data from 193 countries between 2000 and 2013 and found that neonatal sepsis was the third leading cause of neonatal deaths after preterm birth and postpartum complications, accounting for 15.6% of neonatal deaths. In the late neonatal period (7–27 days), the sepsis-related mortality rate increased to 37.2%.26 Accurate early diagnosis of neonatal sepsis can help to provide treatment according to the Surviving Sepsis Campaign Physician’s management guidelines.3 However, the clinical symptoms of neonatal pneumonia and sepsis are similar, making it difficult to distinguish them based on the clinical signs. Moreover, blood culture, the gold standard method for diagnosing neonatal sepsis, usually has a long waiting time and a low positive detection rate of pathogenic microorganisms.27 Therefore, circulating biomarkers may help to distinguish neonatal sepsis from pneumonia.
Lymphocytes are the type of white blood cells with the highest peripheral blood count in healthy newborns.28 They comprise an essential arm of the innate immune response during sepsis and can recognize microbial antigens and present them to the T cells.29 Moreover, lymphocytes can also release chemokines and regulatory cytokines.30 However, during sepsis, the number of lymphocytes undergoes apoptosis, which significantly reduces their number.7,31 Clinical studies have confirmed that patients with sepsis have a lower lymphocyte count, and lymphocytopenia is commonly observed in them.10,32–35 The decreased lymphocyte count contributes to the immunosuppressed state, making patients susceptible to new infections.9 CRP is an acute-phase protein produced by the liver in response to inflammation. The CRP level is the most studied and commonly used laboratory parameter in sepsis cases. Studies have confirmed that CRP significantly increases in patients with sepsis and is an independent sepsis predictor for sepsis.
Recently, the lymphocyte count and CRP level were combined to improve the potential predictive value of the individual markers. LCR is an index calculated by dividing the lymphocyte count by the CRP level. LCR has garnered increasing attention as a newly emerging inflammatory marker. It has been proven to be a helpful predictor for postoperative complications in various types of tumor diseases, such as colorectal cancer, gastric cancer, and hepatocellular carcinoma.36–40 Furthermore, it is closely associated with infectious diseases. A meta-analysis study showed that LCR values were decreased significantly in patients with COVID‐19.41 Demirkol et al42 further reported that the CRP-to-lymphocyte ratio (CLR) level was elevated in deceased COVID-19 patients indicating that the elevated levels were associated with poor outcomes in COVID-19 patients. Yang et al43 reported that LCR proved more beneficial than the CRP level or lymphocyte count alone in evaluating severe COVID-19. However, published studies have not paid sufficient attention to the relationship between LCR and sepsis in adults and newborns.
In this study, we first evaluated the ability of LCR to identify sepsis in neonates with pneumonia. Our data showed that the levels of LCR were lower in neonates with pneumonia combined with sepsis than in those with pneumonia. The prevalence of neonatal pneumonia combined with sepsis was significantly higher in the low-LCR group than in the high-LCR group. Moreover, LCR correlated negatively with PCT, nSOFA score, and the length of hospital stay. However, the correlation between LCR and other indicators is not very strong (r < 0.5), which may be related to the physiological increase/decrease of some indicators in newborns. Multivariate analysis revealed that LCR is an independent risk factor for neonatal pneumonia combined with sepsis. ROC curve showed that LCR had better power than the lymphocyte count and CRP level alone in distinguishing sepsis from neonatal pneumonia.
This study has several limitations. First, since this was a single-center retrospective observational study, the results require further validation via future multicenter studies. Second, we did not differentiate between early-onset sepsis and late-onset sepsis due to the limited number of children diagnosed with sepsis in this study. Finally, LCR was only calculated at admission, and serial LCR measurements may prove helpful in evaluating the clinical significance of LCR in distinguishing neonatal sepsis from neonatal pneumonia.
Conclusions
This study elicited the relationship between the LCR and the presence of sepsis in neonates with pneumonia. LCR is a potentially helpful biomarker in distinguishing neonates with sepsis from those with pneumonia.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval
The study was conducted according to the principles of the Declaration of Helsinki and was approved by the Hospital Ethics Review Board of Henan Children’s Hospital, Zhengzhou, China. The study guarantees that the identity of the participants and other related data has been kept anonymous and confidential. The requirement for informed consent was waived considering the retrospective nature of the study.
Acknowledgments
We thank Bullet Edits Limited for the language editing and proofreading of the manuscript.
Funding
This work was funded by the Medical Science and Technology Project of Henan Province (LHGJ20210637 and LHGJ20210661).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am. 2013;60:367–389. doi:10.1016/j.pcl.2012.12.003
2. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6:223–230. doi:10.1016/s2213-2600(18)30063-8
3. Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46:10–67. doi:10.1007/s00134-019-05878-6
4. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390:1770–1780. doi:10.1016/s0140-6736(17)31002-4
5. Grossman Z, Paul WE. Dynamic tuning of lymphocytes: physiological basis, mechanisms, and function. Annu Rev Immunol. 2015;33:677–713. doi:10.1146/annurev-immunol-032712-100027
6. Mastrogiovanni M, Di Bartolo V, Alcover A. Cell polarity regulators, multifunctional organizers of lymphocyte activation and function. Biomed J. 2022;45:299–309. doi:10.1016/j.bj.2021.10.002
7. Le Tulzo Y, Pangault C, Gacouin A, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–494. doi:10.1097/00024382-200212000-00001
8. Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi:10.4049/jimmunol.174.8.5110
9. Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care. 2012;16:R112. doi:10.1186/cc11404
10. Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383–391. doi:10.1097/SHK.0000000000000234
11. Chung KP, Chang HT, Lo SC, et al. Severe lymphopenia is associated with elevated plasma interleukin-15 levels and increased mortality during severe sepsis. Shock. 2015;43(6):569–575. doi:10.1097/SHK.0000000000000347
12. Sheikh Motahar Vahedi H, Bagheri A, Jahanshir A, Seyedhosseini J, Vahidi E. Association of lymphopenia with short term outcomes of sepsis patients; a brief report. Arch Acad Emerg Med. 2019;7(1):e14.
13. Jiang J, Du H, Su Y, et al. Nonviral infection-related lymphocytopenia for the prediction of adult sepsis and its persistence indicates a higher mortality. Medicine. 2019;98(29):e16535. doi:10.1097/MD.0000000000016535
14. Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279(47):48487–48490. doi:10.1074/jbc.R400025200
15. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi:10.3389/fimmu.2018.00754
16. Stocker M, van Herk W, El Helou S, et al. C-reactive protein, procalcitonin, and white blood count to rule out neonatal early-onset sepsis within 36 hours: a secondary analysis of the neonatal procalcitonin intervention study. Clin Infect Dis. 2021;73(2):e383–e390. doi:10.1093/cid/ciaa876
17. Wang HE, Shapiro NI, Safford MM, et al. High-sensitivity C-reactive protein and risk of sepsis. PLoS One. 2013;8(7):e69232. doi:10.1371/journal.pone.0069232
18. Tonduangu N, Le Borgne P, Lefebvre F, et al. Prognostic value of C-Reactive Protein to Lymphocyte Ratio (CLR) in emergency department patients with SARS-CoV-2 infection. J Pers Med. 2021;12(1):11. doi:10.3390/jpm11121274
19. Ullah W, Basyal B, Tariq S, et al. Lymphocyte-to-C-reactive protein ratio: a novel predictor of adverse outcomes in COVID-19. J Clin Med Res. 2020;12(7):415–422. doi:10.14740/jocmr4227
20. Li X, Wei Y, Xu Z, et al. Lymphocyte-to-C-reactive protein ratio as an early sepsis biomarker for neonates with suspected sepsis. Mediators Inflamm. 2023;2023:9077787. doi:10.1155/2023/9077787
21. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi:10.1097/01.Pcc.0000149131.72248.E6
22. Wynn JL, Polin RA. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr Res. 2020;88:85–90. doi:10.1038/s41390-019-0517-2
23. Hughes G. Youden’s index and the weight of evidence. Methods Inf Med. 2015;54:198–199. doi:10.3414/ME14-04-0003
24. Molloy EJ, Bearer CF. Paediatric and neonatal sepsis and inflammation. Pediatr Res. 2022;91:267–269. doi:10.1038/s41390-021-01918-4
25. Celik IH, Hanna M, Canpolat FE, Mohan P. Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res. 2022;91:337–350. doi:10.1038/s41390-021-01696-z
26. Oza S, Lawn JE, Hogan DR, Mathers C, Cousens SN. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000–2013. Bull World Health Organ. 2015;93:19–28. doi:10.2471/BLT.14.139790
27. Iroh Tam PY, Bendel CM. Diagnostics for neonatal sepsis: current approaches and future directions. Pediatr Res. 2017;82:574–583. doi:10.1038/pr.2017.134
28. Fleisher TA, Rosenzweig SD. Lymphocyte reference intervals in the era of newborn screening. J Allergy Clin Immunol. 2019;144:1516–1517. doi:10.1016/j.jaci.2019.09.022
29. Ochoa JB, Makarenkova V. T lymphocytes. Crit Care Med. 2005;33:S510–S513. doi:10.1097/01.ccm.0000186788.71460.53
30. Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--An updated view. Mediators Inflamm. 2013;2013:165974. doi:10.1155/2013/165974
31. Monneret G, Venet F. A rapidly progressing lymphocyte exhaustion after severe sepsis. Crit Care. 2012;16:140. doi:10.1186/cc11416
32. Li T, Qi M, Dong G, et al. Clinical value of prognostic nutritional index in prediction of the presence and severity of neonatal sepsis. J Inflamm Res. 2021;14:7181–7190. doi:10.2147/JIR.S343992
33. Cilloniz C, Peroni HJ, Gabarrus A, et al. Lymphopenia is associated with poor outcomes of patients with community-acquired pneumonia and sepsis. Open Forum Infect Dis. 2021;8:ofab169. doi:10.1093/ofid/ofab169
34. Muszynski JA, Nofziger R, Greathouse K, et al. Early adaptive immune suppression in children with septic shock: a prospective observational study. Crit Care. 2014;18:R145. doi:10.1186/cc13980
35. Finfer S, Venkatesh B, Hotchkiss RS, Sasson SC. Lymphopenia in sepsis-an acquired immunodeficiency? Immunol Cell Biol. 2022;101(6):535–544. doi:10.1111/imcb.12611
36. Angin YS, Yildirim M, Dasiran F, Okan I. Could lymphocyte to C-reactive protein ratio predict the prognosis in patients with gastric cancer? ANZ J Surg. 2021;91:1521–1527. doi:10.1111/ans.16913
37. Iseda N, Itoh S, Yoshizumi T, et al. Lymphocyte-to-C-reactive protein ratio as a prognostic factor for hepatocellular carcinoma. Int J Clin Oncol. 2021;26:1890–1900. doi:10.1007/s10147-021-01985-x
38. Yildirim M, Koca B. Lymphocyte C-reactive protein ratio: a new biomarker to predict early complications after gastrointestinal oncologic surgery. Cancer Biomark. 2021;31:409–417. doi:10.3233/CBM-210251
39. Aoyama T, Nakazano M, Nagasawa S, et al. The association of the Lymphocyte-to-C-Reactive-Protein Ratio with gastric cancer patients who receive curative treatment. In Vivo. 2022;36:482–489. doi:10.21873/invivo.12728
40. Utsumi M, Inagaki M, Kitada K, et al. Preoperative lymphocyte-to-C-reactive protein ratio predicts hepatocellular carcinoma recurrence after surgery. Ann Surg Treat Res. 2022;103:72–80. doi:10.4174/astr.2022.103.2.72
41. Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92:1733–1734. doi:10.1002/jmv.25819
42. Demirkol ME, Bilgin S, Kahveci G, et al. C-reactive protein-to-lymphocyte ratio is a reliable marker in patients with COVID-19 infection: the CLEAR COVID study. Cir Cir. 2022;90:596–601. doi:10.24875/CIRU.22000124
43. Yang M, Chen X, Xu Y. A retrospective study of the C-reactive protein to lymphocyte ratio and disease severity in 108 patients with early COVID-19 pneumonia from January to March 2020 in Wuhan, China. Med Sci Monit. 2020;26:e926393. doi:10.12659/MSM.926393
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
