Back to Journals » Drug Design, Development and Therapy » Volume 17
Lurasidone for the Treatment of Schizophrenia: Design, Development, and Place in Therapy
Authors Miura I , Horikoshi S, Ichinose M, Suzuki Y, Watanabe K
Received 7 July 2023
Accepted for publication 15 September 2023
Published 28 September 2023 Volume 2023:17 Pages 3023—3031
DOI https://doi.org/10.2147/DDDT.S366769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Itaru Miura,1 Sho Horikoshi,1,2 Mizue Ichinose,1,3 Yuhei Suzuki,1 Kenya Watanabe4
1Department of Neuropsychiatry, Fukushima Medical University School of Medicine, Fukushima, Japan; 2Department of Neuropsychiatry, Horikoshi Psychosomatic Clinic, Fukushima, Japan; 3Department of Neuropsychiatry, Hoshigaoka Hospital, Koriyama, Japan; 4Department of Pharmacy, Fukushima Medical University Hospital, Fukushima, Japan
Correspondence: Itaru Miura, Department of Neuropsychiatry, Fukushima Medical University School of Medicine, Hikarigaoka 1, Fukushima, 960-1295, Tel +81-24-547-1331, Fax +81-24-548-6735, Email [email protected]
Abstract: This review aims to provide a comprehensive overview of the current literature on the drug design, development, and therapy of lurasidone for the treatment of schizophrenia. Lurasidone has antagonistic effects on the dopamine D2, 5-hydroxytryptamine (5-HT)2A, and 5-HT7 receptors and a partial agonistic effect on the 5-HT1A receptor with low affinities for muscarinic M1, histamine H1, and a1 adrenergic receptors. The receptor-binding profile of lurasidone is thought to be associated with fewer side effects such as anticholinergic effects, lipid abnormalities, hyperglycemia, and weight gain. Behavioral pharmacological studies have demonstrated that lurasidone exerts anxiolytic and antidepressive effects and improves cognitive function, which are associated with the modulation of 5-HT7 and 5-HT1A receptors. Literature search using PubMed was performed to find published studies of randomized controlled trials and recent meta-analyses regarding efficacy and safety, particularly metabolic side effects of lurasidone in schizophrenia. In short-term studies, the results of randomized placebo-controlled trials and meta-analyses have suggested that lurasidone was superior to placebo in improving total psychopathology, positive symptoms, negative symptoms, and general psychopathology in patients with acute schizophrenia. Regarding safety, lurasidone had minimal metabolic side effects, and was identified as one of the drugs with the most benign profiles for metabolic side effects. Long-term trials revealed that lurasidone had the preventive effects on relapse, with minimal effects on weight gain and other metabolic side effects. Furthermore, lurasidone improves cognitive and functional performance of patients with schizophrenia, especially in long-term treatment. Patients with schizophrenia require long-term treatment with antipsychotics for relapse prevention; thus, minimizing weight gain and other side effects is crucial. Lurasidone is suitable as one of the first-line antipsychotic drugs in the acute phase, and a switching strategy should be considered during the maintenance phase, to balance efficacy and adverse effects and achieve favorable outcomes in the long-term course of schizophrenia.
Keywords: schizophrenia, lurasidone, serotonin-dopamine antagonist, antipsychotics
Introduction
Schizophrenia is a complex and severe psychiatric disease that causes various burdens, including cognitive impairment, depression, social isolation, and poor physical health.1 Schizophrenia is associated not only with poor occupational functioning and economic burdens but also with a higher mortality rate and a 10- to 25-year shorter life expectancy.2 This mortality gap is only partially due to a higher risk of suicide,3,4 and cardiovascular disease is a strong contributor to the high mortality rate in patients with schizophrenia.2
After the onset and first episode of acute psychosis, patients with schizophrenia need long-term treatment with antipsychotics for relapse prevention; however, many patients (up to 50%) are not adherent to antipsychotic medication.5 Non-adherence to antipsychotics is the strongest predictor for relapse,5 and various adverse effects of antipsychotics are important factors for adherence to the treatment of schizophrenia. In particular, weight gain and metabolic side effects, such as hyperlipidemia and diabetes, are common adverse effects of second-generation antipsychotics (SGAs),6 which can lead to cardiovascular diseases. Patients with schizophrenia require long-term treatment with antipsychotics for relapse prevention; thus, minimizing weight gain and other side effects is crucial.7 Although long-acting injectable antipsychotics are theoretically strong treatment tools for relapse prevention, balancing the efficacy and adverse effects of antipsychotic medications also contributes to improved adherence, leading to favorable outcomes in schizophrenia treatment.
Lurasidone, a serotonin–dopamine antagonist, has been widely used to treat schizophrenia and bipolar depression in many countries for >10 years.8 SDAs improve positive symptoms of schizophrenia through the inhibition of dopamine D2 receptors in mesolimbic dopaminergic neural pathway, and the 5-HT2A antagonism contributes to decrease extrapyramidal symptoms (EPS). Although SDAs have common side effects such as weight gain, hyperlipidemia and diabetes, akathisia, and hyperprolactinemia, lurasidone has a relatively favorable tolerability profile, with minimal effects on metabolic parameters and prolactin levels.8 In this review, we provide an overview of drug development and summarize the results of clinical trials of lurasidone in patients with schizophrenia. Literature search using PubMed was performed to find published studies of randomized controlled trials and recent meta-analyses regarding efficacy and safety, particularly metabolic side effects of lurasidone in schizophrenia. Furthermore, we discussed the current clinical position of lurasidone considering mid- to long-term treatment.
Design and Development
Despite advancements in research and treatment, antipsychotic treatment for schizophrenia still has unmet needs, including limited efficacy, residual symptoms such as negative, cognitive, and mood symptoms, and side effects.9 First-generation antipsychotics (FGAs) and SGAs have some differences in efficacy.10 SGAs outperform FGAs regarding treatment discontinuation and improvements in negative, cognitive, and depressive symptoms, and they have fewer extrapyramidal symptoms (EPS).11,12 However, patients treated with SGAs often experience side effects such as weight gain, metabolic disturbance, and sedation. These side effects can be particularly problematic in patients who require long-term antipsychotic treatment. The dopamine D2 receptor remains the primary target for antipsychotic drugs; 5-hydroxytryptamine (serotonin) 5-HT2A and 5-HT1A, and off-target receptors such as 5-HT2c, histamine H1, and muscarinic M1 receptors should also be considered in the design process of novel multitarget antipsychotic drugs.13
Lurasidone was synthesized in the early 1990s by Dainippon Sumitomo Pharma Co., Ltd. and approved by the United States Food and Drug Administration for the treatment of schizophrenia in 2010 and bipolar depression in 2013. Prior to the creation of lurasidone, tandospirone, a 5-HT1A partial agonist, was synthesized from buspirone, an anxiolytic azapirone derivative. Lurasidone was synthesized using tandospirone as the lead compound, and the imide moiety was common to tandospirone. The aryl moiety is benzisothiazole, common to perospirone, and a cyclic structure was newly introduced in the linker region (Figure 1).14 These successfully enhanced D2 and 5-HT2A receptor-binding affinities, as well as reduced other receptors associated with side effects, such as H1 receptor, M1 receptor, and adrenaline a1 receptor, while maintaining 5-HT1A receptor binding affinity.
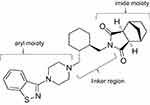 |
Figure 1 Chemical structure of lurasidone. |
Pharmacodynamics of Lurasidone
The receptor-binding affinities of lurasidone and other antipsychotics are presented in Table 1.15 Lurasidone has antagonistic effects on the D2, 5-HT2A, and 5-HT7 receptors and a partial agonistic effect on the 5-HT1A receptor. Lurasidone has a predominant affinity for 5-HT7 receptors and a high affinity for 5-HT1A receptors. Although the role of 5-HT7 receptor antagonism in clinical efficacy remains unclear, behavioral pharmacological studies in animals have demonstrated that lurasidone exerts anxiolytic and antidepressant effects and improves cognitive function, which are associated with the modulation of 5-HT7 and 5-HT1A receptors. Lurasidone dose-dependently and significantly increased the number of shocks received, anxiolytic-like activities, in the Vogel’s conflict test in rats.15 In the tail suspension and forced swim tests, lurasidone decreased immobility, an antidepressant-like response, and these effects required functional 5-HT7 receptors.16,17 In cognitive function tests in rats, lurasidone improved the N-methyl-D-aspartate receptor antagonist phencyclidine (PCP)- and MK-801-induced learning and memory impairment, and these effects were reduced by the 5-HT1A receptor antagonist WAY-100635 and 5-HT7 receptor agonist AS19.18–21 Moreover, lurasidone ameliorated the cognitive deficits produced by PCP, altering the efflux of neurotransmitters and metabolites in the cortex and nucleus accumbens, and increased the cortical efflux of dopamine, acetylcholine, and glutamate.22,23 Overall, 5-HT1A receptor partial agonistic and 5-HT7 receptor antagonistic properties of lurasidone may be involved in these effects, and increased neurotransmitters efflux in the frontal cortex may contribute to the improvement of cognitive disorder and antidepressant effects.
 |
Table 1 Comparison of Receptor Binding Profiles Between Lurasidone and Other Psychotic agents15 |
Moreover, other mechanisms may be involved in the cognitive improvement and antidepressant effects of lurasidone. Calabrese et al24 investigated the ability of lurasidone to modulate the involvement of genomic and non-genomic glucocorticoid receptor signaling in behavioral alterations in rats following chronic stress exposure, and lurasidone normalized anhedonia, cognitive impairment induced by chronic stress, and abnormalities in glucocorticoid receptor signaling. Fumagalli et al25 investigated the expression of brain-derived neurotrophic factor (BDNF), a neurotrophin that plays a crucial role in brain plasticity, following chronic lurasidone treatment in response to acute stress in rats. Chronic lurasidone treatment increased total BDNF mRNA levels in the prefrontal cortex, and the modulation of BDNF mRNA levels in response to acute swim stress in lurasidone-treated rats was markedly potentiated in the hippocampus. Moreover, the same research group investigated the ability of lurasidone to modulate behavioral and neuroplastic alterations in a chronic mild stress model of depression and revealed that chronic lurasidone treatment improved anhedonia in rats with chronic mild stress and restored BDNF mRNA levels in the prefrontal cortex.26 These results suggested that lurasidone regulates BDNF expression through modulation of its transcripts, thereby contributing to the amelioration of cognitive impairment and depression associated with neuronal plasticity.
Lurasidone has low affinities for M1, H1, and a1 receptors, which is associated with fewer side effects such as anticholinergic effects, lipid abnormalities, hyperglycemia, orthostatic hypotension, sedation, and weight gain.
Pharmacokinetics and Drug Interaction of Lurasidone
Lurasidone is rapidly absorbed after oral administration, with peak plasma concentrations typically occurring within 1–3 h.27 It has a bioavailability of approximately 9%–19% owing to its poor absorption after oral ingestion and first-pass metabolism, and its pharmacokinetics are linear and dose-proportional over a dose range of 20–160 mg/day.28 The absorption of lurasidone is increased when taken with food, resulting in higher peak plasma concentrations and greater bioavailability. Therefore, lurasidone should be administered as food. Lurasidone is extensively metabolized in the liver via cytochrome P450 (CYP) 3A4-mediated oxidation and subsequent glucuronidation. Its primary active metabolites, ID-14283 and ID-14326, also exhibit activity against D2 and 5-HT2A receptors.28,29 Lurasidone is metabolized by CYP3A4, and the concomitant use of strong CYP3A4 inhibitors such as itraconazole, ritonavir, and clarithromycin can increase lurasidone exposure and should be avoided. Similarly, strong inducers of CYP3A4, such as rifampicin or phenytoin, can decrease lurasidone exposure.28,29
Results of Clinical Trials
Short-Term Studies
Double-blind randomized controlled trials (RCTs) have demonstrated the efficacy and safety of lurasidone in short-term studies of patients with acute schizophrenia. Meltzer et al30 performed a 6-week RCT comparing lurasidone 40 mg, lurasidone 120 mg, olanzapine 15 mg, and placebo in patients with schizophrenia and acute exacerbation of psychotic symptoms, demonstrating that lurasidone 40 mg and 120 mg and olanzapine 15 mg were superior to placebo in terms of efficacy (Positive and Negative Syndrome Scale [PANSS] total, positive and negative scores, and Clinical Global Impression – Severity [CGI-S] score). No significant differences in the improvement of the mean PANSS total or CGI-S scores were observed between the lurasidone and olanzapine groups. The incidence of akathisia was higher in the lurasidone 120 mg group (22.9%) than that in the lurasidone 40 mg (11.8%), olanzapine 15 mg (7.4%), or placebo (0.9%) groups, whereas the incidence of ≥7% weight gain was 5.9% in the lurasidone group, 34.4% in the olanzapine 34.4%, and 7.0% in the placebo group. A meta-analysis31 including eight RCTs with total of 2373 patients compared lurasidone (n=1570, 20–160 mg) and placebo (n=803) in terms of efficacy and safety, which revealed that lurasidone was superior to placebo in terms of the improvement of total psychopathology [standardized mean difference (SMD): −0.34, (95% confidence interval [CI]: −0.48, −0.20)], positive symptoms [SMD: −0.47, (95% CI: −0.57, −0.36)], negative symptoms [SMD: −0.34, (95% CI: −0.45, −0.22)], and general psychopathology symptoms [SMD: −0.36, (95% CI: −0.48, −0.24)]. In the subgroup analysis by dosages, although lurasidone 20 mg/day (n=96) [SMD: 0.27, (95% CI: −0.19, 0.72)] did not indicate a significant difference from placebo, lurasidone 40 mg (n=667) [SMD: −0.26, (95% CI: −0.51, −0.01)], 80 mg (n=777) [SMD: −0.42, (95% CI: −0.57, −0.26)], 120 mg (n=410) [SMD: −0.32, (95% CI: −0.53, −0.11)], and 160 mg/day (n=181) [SMD: −0.82, (95% CI: −1.14, −0.49)] were significantly superior to placebo in improving total psychopathology. Additionally, a recent 6-week double-blind RCT32 has demonstrated that lurasidone was non-inferior to risperidone in terms of the change in the PANSS total score from baseline to week 6, with minimal effects on weight, metabolic parameters, and prolactin levels. Importantly, a dose–response meta-analysis33 revealed that the efficacy of lurasidone in acute schizophrenia is dose dependent, suggesting that higher doses are more efficacious. In addition to the positive, negative, and total symptoms, lurasidone is efficacious for depressive symptoms,31 and a recent meta-analysis12 has revealed that lurasidone significantly decreased depressive symptoms compared to placebo (SMD: −0.19, (95% CI: −0.38, −0.04)). Regarding safety, a meta-analysis by Zheng et al31 demonstrated that lurasidone was well-tolerated in the treatment of acute schizophrenia. Compared with placebo, although vomiting (number needed to harm [NNH] = 50, 95% CI: 33-∞), akathisia (NNH = 11, 95% CI:8–17), dystonia (NNH = 33, 95% CI: 20–100), parkinsonism (NNH = 14, 95% CI: 11–20), somnolence (NNH = 20, 95% CI: 17–33), dizziness (NNH = 33, 95% CI:17–100), sedation (NNH = 25, 95% CI: 17–50), nausea (NNH = 17, 95% CI: 17–33), and ≥7% weight gain (NNH = 50, 95% CI: 25-∞) were more frequent in the lurasidone group, lurasidone had minimal metabolic side effects including increase in weight and glucose and lipid levels. Another study also showed similar significant differences between lurasidone and placebo in terms of akathisia (NNH = 11) and sedation or somnolence (NNH = 20), suggesting that lurasidone is categorized as a predominantly activating antipsychotic.34 Higher rates of akathisia are also observed in a recent meta-analysis,35 with odds ratio = 3.74 (95% CI: 2.32–6.02) compared with placebo. A network meta-analysis (NMA)6 which focused on metabolic side effects identified lurasidone as one of the drugs with the most benign profiles for metabolic side effects. In that study, the effect of lurasidone on changes in body weight [mean difference [MD] 0.48, (95% CI: −0.01, 0.97)], total cholesterol [MD −0.03, (95% CI: −0.15, 0.09)], low-density lipoprotein cholesterol [MD −0.05, (95% CI: −0.14, 0.04)], and triglycerides [MD 0.00, (95% CI: −0.14, 0.13)] was not significantly different from placebo. Moreover, lurasidone had a significantly lower effect than placebo on blood glucose (MD: −0.29, [95% CI: −0.55, −0.03]).
Long-Term Studies
A double-blind, placebo-controlled withdrawal RCT of lurasidone for maintenance therapy for schizophrenia revealed that lurasidone (40 mg or 80 mg) significantly delayed the time to relapse compared to placebo, with a 33.7% reduction in relapse risk (Cox hazard: 0.663 (95% CI: 0.447–0.983); p=0.041).36 Effects of lurasidone on weight gain and levels of glucose, lipid, and prolactin were minimal during the study period. A double-blind RCT37 evaluating the long-term safety and tolerability of lurasidone (40–120 mg) for 12 months, with risperidone (2–6 mg) as an active control, demonstrated that the three most frequent adverse events of lurasidone were nausea (16.7%), insomnia (15.8%), and sedation (14.6%) (10.9%, 13.4%, and 13.9% in the risperidone group, respectively). The frequency of ≥7% weight gain was higher in the risperidone group than in the lurasidone group (14% and 7%, respectively). Changes in prolactin levels were significantly higher in the risperidone group than in the lurasidone group, and minimal changes were observed in the lurasidone group. In terms of efficacy measures, both groups demonstrated comparable improvement and similar relapse rates.
Cognitive impairment affects social function and quality of life in patients with schizophrenia, thus making it one of the most important treatment targets. However, although SGAs are superior to FGAs in improving cognitive function, the effect size is small.11 Harvey et al have reported that lurasidone improves cognitive and functional performance of patients, and the effects can be expected in long-term treatment rather than short-term.38–40 High doses (≧120 mg) of lurasidone are effective for overall cognitive function, whereas even low doses (≦80 mg) may be effective in improving specific cognitive functions.38–40 Moreover, a long-term improvement of cognitive function was observed in a trial of lurasidone in patients with treatment-resistant schizophrenia,41 which demonstrated that 24-week treatment with lurasidone improved the PANSS total score and 2 of 7 cognitive domains (speed of processing and executive function).
Lurasidone for Adolescents with Schizophrenia
Arango et al performed a systematic review and NMA to compare lurasidone and other SGAs in adolescent patients with schizophrenia.42 The NMA revealed that lurasidone outperformed the placebo in terms of the PANSS total score ([MD −7.95, (95% CI: −11.76, −4.16)]) and did not significantly differ from other SGAs (ziprasidone, asenapine, paliperidone, aripiprazole, quetiapine, risperidone, and olanzapine) in terms of efficacy. Regarding safety, lurasidone did not differ from the placebo, ziprasidone, or aripiprazole in increasing weight gain and was associated with significantly less weight gain than olanzapine, quetiapine, risperidone, asenapine, and paliperidone. In contrast, lurasidone was associated with significantly higher rates of EPS and akathisia, similar to other SGAs.
Furthermore, a recent two-year open-label study has demonstrated the long-term safety and efficacy of lurasidone for treatment-naive youth with schizophrenia.43
Place in Therapy of Lurasidone
Overall, lurasidone is efficacious and well-tolerated in the acute and long-term treatment of schizophrenia, as described above. The favorable profile of lurasidone for metabolic side effects, including weight gain and changes in lipid and glucose levels, is advantageous in patients with schizophrenia. These are consistent with a recent review, which suggests that lurasidone is among the best-tolerated antipsychotics.44 In the acute phase of schizophrenia, although the improvement of positive and/or excitement/agitation symptoms is important, minimizing side effects is needed to improve adherence to treatment, which leads to relapse prevention. Furthermore, the efficacy of lurasidone is dose-dependent, and a higher dose should be considered if a sufficient response is not obtained from a lower dose of lurasidone, with caution regarding the increase in EPS and/or akathisia. According to a recent Italian expert opinion,44 akathisia is the most reported adverse event of lurasidone, but it may be controlled by dose reduction. Based on its efficacy and safety, lurasidone is suitable as a first-line antipsychotic drug together with a dopamine D2 partial agonist in the acute phase, particularly during the early course of schizophrenia. However, because the effect of lurasidone on sedation is not strong, a reasonable combination with benzodiazepines, other antipsychotics, or mood stabilizers may be needed for the management of acute symptoms of schizophrenia, such as agitation, excitement, or other aggressive behaviors during the acute phase.
In the maintenance phase of schizophrenia, the treatment goals are to prevent relapse, reduce mortality risk, improve cognitive function and dysfunction, and achieve personal recovery. In addition to the preventive effects on relapse, the minimal effects of lurasidone on weight gain and other metabolic side effects advance the clinical position of lurasidone as a preferential and significant treatment choice during the maintenance phase. A recent study investigating the comparative safety signal assessment of hospitalization associated with the use of atypical antipsychotics revealed that no hospitalization-related safety signals were observed with the use of lurasidone, along with other atypical antipsychotics.45 Moreover, lurasidone was associated with the lowest lifetime costs when initiated as acute or maintenance treatments, which is primarily due to the avoidance of diabetes and cardiovascular adverse events.46 When switching to antipsychotics is necessary because of side effects, lurasidone is recommended as the first-line antipsychotic for the management of dyslipidemia, impaired glucose tolerance, hyperprolactinemia, QT prolongation, sexual dysfunction, and weight gain.47 A recent NMA revealed that excessive dose reduction of antipsychotics is associated with a higher risk of relapse,48 and switching antipsychotics to other drugs is a useful and important treatment strategy when patients experience various side effects. In fact, switching from risperidone or olanzapine to lurasidone results in a decrease in weight and other improvements in side effects,49,50 although caution is needed for an increase in the incidence of akathisia when switching from olanzapine.50 Moreover, lurasidone can improve cognitive function via 5-HT1A receptor partial agonistic and 5-HT7 receptor antagonistic properties, which have beneficial effects on social and occupational function and quality of life in the long-term course of schizophrenia. Altogether, lurasidone can prevent relapse with minimal effects on weight and metabolic parameters and contribute to patient recovery during the long-term maintenance phase of schizophrenia.
In addition to the beneficial effects on cognitive function in patients with treatment-resistant schizophrenia,41 recent studies have reported the effectiveness of lurasidone augmentation of clozapine in treatment-resistant schizophrenia.51,52 In these studies, therapeutic effects of lurasidone were observed in a broad range of symptom dimensions, although there were some patients who discontinued treatment due to side effects.52 These suggest the possibility of lurasidone augmentation in patients with treatment-resistant schizophrenia, although RCTs with larger sample size are needed to confirm these results.
Although efficacy and safety of lurasidone are observed in many RCTs and meta-analyses, further studies should report more findings for its effects on cognitive function, particularly in patients with treatment-resistant schizophrenia. Moreover, further research is needed to investigate the role of 5-HT7 receptor antagonistic properties of lurasidone in the treatment of schizophrenia.
Conclusion
Lurasidone is an antipsychotic drug with a benign profile of metabolic side effects and non-inferior efficacy to olanzapine or risperidone during the acute phase of schizophrenia. Given these attributes, lurasidone can potentially shape clinical practices by providing a treatment option that not only addresses the acute symptoms of schizophrenia but also mitigates side effects, thereby enhancing patient adherence. These findings open avenues for future research into developing similar therapeutics with minimal side effects. Patients with schizophrenia require long-term treatment with antipsychotics for relapse prevention; thus, minimizing weight gain and metabolic side effects is crucial to prevent the development of cardiovascular disease, which is a strong contributor to the high mortality rate among patients with schizophrenia. Furthermore, lurasidone also exhibits beneficial impacts on cognitive function, anxiolytic, and antidepressive effects, adding to its advantages as a treatment option for schizophrenia. Lurasidone is suitable as a first-line antipsychotic drug in the acute phase, and a switching strategy should be considered during the maintenance phase, to balance efficacy and adverse effects and achieve favorable outcomes in the long-term course of schizophrenia.
Disclosure
Dr. Miura has received speaker’s honoraria from Sumitomo, Eisai, Janssen, Meiji Seika Pharma, Otsuka, Takeda, Towa, Tanabe-Mitsubishi, and Yoshitomi. Dr. Horikoshi has received honoraria for lectures from Sumitomo, Janssen, Meiji Seika Pharma, Otsuka, Takeda, Lundbeck, and Yoshitomi. Drs. Ichinose, Suzuki, and Watanabe declare no conflicts of interest in this work.
References
1. Rössler W, Salize HJ, van Os J, Riecher-Rössler A. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol. 2005;15(4):399–409. doi:10.1016/j.euroneuro.2005.04.009
2. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–341. doi:10.1001/jamapsychiatry.2014.2502
3. Lu L, Dong M, Zhang L, et al. Prevalence of suicide attempts in individuals with schizophrenia: a meta-analysis of observational studies. Epidemiol Psychiatr Sci. 2019;29:e39. doi:10.1017/S2045796019000313
4. Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4(4):295–301. doi:10.1016/S2215-0366(17)30078-0
5. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. doi:10.1002/wps.20060
6. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. doi:10.1016/S2215-0366(19)30416-X
7. Fagiolini A, Olivola M, Lavatelli L, et al. Treatment persistence in patients with schizophrenia treated with lurasidone in Italian clinical practice. Ann Gen Psychiatry. 2022;21(1):49. doi:10.1186/s12991-022-00425-y
8. Fiorillo A, Cuomo A, Sampogna G, et al. Lurasidone in adolescents and adults with schizophrenia: from clinical trials to real-world clinical practice. Expert Opin Pharmacother. 2022;23(16):1801–1818. doi:10.1080/14656566.2022.2141568
9. Spaulding WD, Sullivan M, Weiler M, Reed D, Richardson C, Storzbach D. Changing cognitive functioning in rehabilitation of schizophrenia. Acta Psychiatr Scand Suppl. 1994;384(s384):116–124. doi:10.1111/j.1600-0447.1994.tb05900.x
10. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. doi:10.1016/S0140-6736(19)31135-3
11. Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2013;16(6):1205–1218. doi:10.1017/S1461145712001277
12. Miura I, Nosaka T, Yabe H, Hagi K. Antidepressive effect of antipsychotics in the treatment of schizophrenia: meta-regression analysis of randomized placebo-controlled trials. Int J Neuropsychopharmacol. 2021;24(3):200–215. doi:10.1093/ijnp/pyaa082
13. Kondej M, Stępnicki P, Kaczor AA. Multi-target approach for drug discovery against schizophrenia. Int J Mol Sci. 2018;19(10):3105. doi:10.3390/ijms19103105
14. Maruyama M. SAR study, synthesis, and biological activity of lurasidone hydrochloride (LATUDA (R)): new treatment drug for schizophrenia. Admin. Pap Am Chem Soc. 2012;244.
15. Ishibashi T, Horisawa T, Tokuda K, et al. Pharmacological Profile of Lurasidone, a Novel Antipsychotic Agent with Potent 5-Hydroxytryptamine 7 (5-HT 7) and 5-HT 1A Receptor Activity. J Pharmacol Exp Ther. 2010;334(1):171–181. doi:10.1124/jpet.110.167346
16. Cates LN, Roberts AJ, Huitron-Resendiz S, Hedlund PB. Effects of lurasidone in behavioral models of depression. Role of the 5-HT7 receptor subtype. Neuropharmacology. 2013;70:211–217. doi:10.1016/j.neuropharm.2013.01.023
17. Sarkisyan G, Roberts AJ, Hedlund PB. The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behav Brain Res. 2010;209(1):99–108. doi:10.1016/j.bbr.2010.01.022
18. Horiguchi M, Hannaway KE, Adelekun AE, Jayathilake K, Meltzer HY. Prevention of the phencyclidine-induced impairment in novel object recognition in female rats by co-administration of lurasidone or tandospirone, a 5-HT(1A) partial agonist. Neuropsychopharmacology. 2012;37(10):2175–2183. doi:10.1038/npp.2012.64
19. Horisawa T, Nishikawa H, Toma S, et al. The role of 5-HT7 receptor antagonism in the amelioration of MK-801-induced learning and memory deficits by the novel atypical antipsychotic drug lurasidone. Behav Brain Res. 2013;244:66–69. doi:10.1016/j.bbr.2013.01.026
20. Horiguchi M, Huang M, Meltzer HY. The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J Pharmacol Exp Ther. 2011;338(2):605–614. doi:10.1124/jpet.111.180638
21. Horisawa T, Ishibashi T, Nishikawa H, et al. The effects of selective antagonists of serotonin 5-HT7 and 5-HT1A receptors on MK-801-induced impairment of learning and memory in the passive avoidance and Morris water maze tests in rats: mechanistic implications for the beneficial effects of the novel atypical antipsychotic lurasidone. Behav Brain Res. 2011;220(1):83–90. doi:10.1016/j.bbr.2011.01.034
22. Huang M, Panos JJ, Kwon S, Oyamada Y, Rajagopal L, Meltzer HY. Comparative effect of lurasidone and blonanserin on cortical glutamate, dopamine, and acetylcholine efflux: role of relative serotonin (5-HT) 2A and DA D 2 antagonism and 5-HT 1A partial agonism. J Neurochem. 2014;128(6):938–949. doi:10.1111/jnc.12512
23. Huang M, Horiguchi M, Felix AR, Meltzer HY. 5-HT1A and 5-HT7 receptors contribute to lurasidone-induced dopamine efflux. NeuroReport. 2012;23(7):436–440. doi:10.1097/WNR.0b013e328352de40
24. Calabrese F, Brivio P, Sbrini G, et al. Effect of lurasidone treatment on chronic mild stress-induced behavioural deficits in male rats: the potential role for glucocorticoid receptor signalling. J Psychopharmacol. 2020;34(4):420–428. doi:10.1177/0269881119895547
25. Fumagalli F, Calabrese F, Luoni A, Bolis F, Racagni G, Riva MA. Modulation of BDNF expression by repeated treatment with the novel antipsychotic lurasidone under basal condition and in response to acute stress. Int J Neuropsychopharmacol. 2012;15(02):235–246. doi:10.1017/S1461145711000150
26. Luoni A, Macchi F, Papp M, Molteni R, Riva MA. Lurasidone exerts antidepressant properties in the chronic mild stress model through the regulation of synaptic and neuroplastic mechanisms in the rat prefrontal cortex. Int J Neuropsychopharmacol. 2014;18:yu061. doi:10.1093/ijnp/pyu061
27. Food and Drug Administration. Drug approval package. Latuda (lurasidone hydrochloride) tablets. Sunovion Pharmaceuticals, Inc. clinical review; pharmacology review(s); clinical pharmacology biopharmaceutics review(s). Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/200603Orig1s000PharmR.pdf.
28. Greenberg WM, Citrome L. Pharmacokinetics and pharmacodynamics of lurasidone hydrochloride, a second-generation antipsychotic: a systematic review of the published literature. Clin Pharmacokinet. 2017;56(5):493–503. doi:10.1007/s40262-016-0465-5
29. Meyer JM, Loebel AD, Schweizer E. Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs. 2009;18(11):1715–1726. doi:10.1517/13543780903286388
30. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957–967. doi:10.1176/appi.ajp.2011.10060907
31. Zheng W, Cai DB, Yang XH, et al. Short-term efficacy and tolerability of lurasidone in the treatment of acute schizophrenia: a meta-analysis of randomized controlled trials. J Psychiatr Res. 2018;103:244–251. doi:10.1016/j.jpsychires.2018.06.005
32. Feng Y, Shi J, Wang L, et al. Randomized, double-blind, 6-week non-inferiority study of lurasidone and risperidone for the treatment of schizophrenia. Psychiatry Clin Neurosci. 2020;74(6):336–343. doi:10.1111/pcn.12965
33. Leucht S, Crippa A, Siafis S, Patel MX, Orsini N, Davis JM. Dose–response meta-analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry. 2020;177(4):342–353. doi:10.1176/appi.ajp.2019.19010034
34. Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138–147. doi:10.1097/JCP.0000000000000665
35. Demyttenaere K, Detraux J, Racagni G, Vansteelandt K. Medication-induced akathisia with newly approved antipsychotics in patients with a severe mental illness: a systematic review and meta-analysis. CNS Drugs. 2019;33(6):549–566. doi:10.1007/s40263-019-00625-3
36. Tandon R, Cucchiaro J, Phillips D, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. J Psychopharmacol. 2016;30(1):69–77. doi:10.1177/0269881115620460
37. Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27(3):165–176. doi:10.1097/YIC.0b013e32835281ef
38. Harvey PD, Siu CO, Hsu J, Cucchiaro J, Maruff P, Loebel A. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: a short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur Neuropsychopharmacol. 2013;23(11):1373–1382. doi:10.1016/j.euroneuro.2013.08.003
39. Harvey PD, Siu CO, Ogasa M, Loebel A. Effect of lurasidone dose on cognition in patients with schizophrenia: post-hoc analysis of a long-term, double-blind continuation study. Schizophr Res. 2015;166(1–3):334–338. doi:10.1016/j.schres.2015.06.008
40. Harvey PD, Siu CO, Loebel AD. Change in daytime sleepiness and cognitive function in a 6-month, double-blind study of lurasidone and quetiapine XR in patients with schizophrenia. Schizophr Res Cogn. 2016;5:7–12. doi:10.1016/j.scog.2016.05.002
41. Meltzer HY, Share DB, Jayathilake K, Salomon RM, Lee MA. Lurasidone improves psychopathology and cognition in treatment-resistant schizophrenia. J Clin Psychopharmacol. 2020;40(3):240–249. doi:10.1097/JCP.0000000000001205
42. Arango C, Ng-Mak D, Finn E, Byrne A, Loebel A. Lurasidone compared to other atypical antipsychotic monotherapies for adolescent schizophrenia: a systematic literature review and network meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1195–1205. doi:10.1007/s00787-019-01425-2
43. Correll CU, Tocco M, Pikalov A, Hsu J, Goldman R. Long-term safety and effectiveness of open-label lurasidone in antipsychotic-naïve versus previously treated adolescents with schizophrenia: a post-hoc analysis. Schizophr Res. 2022;240:205–213. doi:10.1016/j.schres.2021.12.046
44. Riva MA, Albert U, de Filippis S, Vita A, De Berardis D. Identification of clinical phenotypes in schizophrenia: the role of lurasidone. Ther Adv Psychopharmacol. 2021;11:20451253211012250. doi:10.1177/20451253211012250
45. Yunusa I, Teng C, Karaye IM, Crounse E, Alsahali S, Maleki N. Comparative safety signal assessment of hospitalization associated with the use of atypical antipsychotics. Front Psychiatry. 2022;13:917351. doi:10.3389/fpsyt.2022.917351
46. Kearns B, Cooper K, Cantrell A, Thomas C. Schizophrenia Treatment with Second-Generation Antipsychotics: a Multi-Country Comparison of the Costs of Cardiovascular and Metabolic Adverse Events and Weight Gain. Neuropsychiatr Dis Treat. 2021;17:125–137. doi:10.2147/NDT.S282856
47. Taylor D, Paton C, Kerwin R. The Maudsley Prescribing Guideline in Psychiatry.
48. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614–624. doi:10.1016/S2215-0366(22)00158-4
49. Mattingly GW, Haddad PM, Tocco M, et al. Switching to lurasidone following 12 months of treatment with risperidone: results of a 6-month, open-label study. BMC Psychiatry. 2020;20(1):199. doi:10.1186/s12888-020-02523-1
50. Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74(05):507–515. doi:10.4088/JCP.12m08084
51. Olivola M, Arienti V, Bassetti N, Giovanna G, Brondino N. Lurasidone augmentation of clozapine in refractory schizophrenia: a case series. J Clin Psychopharmacol. 2023;43(2):157–160. doi:10.1097/JCP.0000000000001662
52. Siwek M, Chrobak AA, Gorostowicz A, Król P, Dudek D. Lurasidone augmentation of clozapine in schizophrenia-retrospective chart review. Brain Sci. 2023;13(3):445. doi:10.3390/brainsci13030445
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
