Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Lung Deposition and Inspiratory Flow Rate in Patients with Chronic Obstructive Pulmonary Disease Using Different Inhalation Devices: A Systematic Literature Review and Expert Opinion
Authors Baloira A , Abad A, Fuster A, García Rivero JL, García-Sidro P, Márquez-Martín E, Palop M, Soler N, Velasco JL, González-Torralba F
Received 15 January 2021
Accepted for publication 21 March 2021
Published 19 April 2021 Volume 2021:16 Pages 1021—1033
DOI https://doi.org/10.2147/COPD.S297980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Adolfo Baloira, 1 Araceli Abad, 2 Antonia Fuster, 3 Juan Luis García Rivero, 4 Patricia García-Sidro, 5 Eduardo Márquez-Martín, 6, 7 Marta Palop, 8 Néstor Soler, 9 JL Velasco, 10 Fernando González-Torralba 11
1Complejo Hospitalario Universitario de Pontevedra, Pontevedra, Spain; 2Hospital Universitario de Getafe, Madrid, Spain; 3Hospital Unvidersitario Son Llàtzer, Palma de Mallorca, Spain; 4Hospital Comarcal de Laredo, Cantabria, Spain; 5Hospital Universitario de la Plana, Castellón, Spain; 6Hospital Virgen del Rocío, Sevilla, Spain; 7CIBERES, Instituto de Salud Carlos III, Madrid, Spain; 8Hospital de Sagunto, Valencia, Spain; 9Hospital Universitario Clínic, Barcelona, Spain; 10Hospital Universitario Virgen de la Victoria, Málaga, Spain; 11Pulmonology Section, Hospital Universitario del Tajo, Aranjuez, Spain
Correspondence: Fernando González-Torralba
Pulmonology Section, Hospital Universitario del Tajo, Av. Amazonas Central, s/n, Aranjuez, 28300, Madrid, Spain
Tel +0034 918 01 41 00
Email [email protected]
Background: Our aim was to describe: 1) lung deposition and inspiratory flow rate; 2) main characteristics of inhaler devices in chronic obstructive pulmonary disease (COPD).
Methods: A systematic literature review (SLR) was conducted to analyze the features and results of inhaler devices in COPD patients. These devices included pressurized metered-dose inhalers (pMDIs), dry powder inhalers (DPIs), and a soft mist inhaler (SMI). Inclusion and exclusion criteria were established, as well as search strategies (Medline, Embase, and the Cochrane Library up to April 2019). In vitro and in vivo studies were included. Two reviewers selected articles, collected and analyzed data independently. Narrative searches complemented the SLR. We discussed the results of the reviews in a nominal group meeting and agreed on various general principles and recommendations.
Results: The SLR included 71 articles, some were of low–moderate quality, and there was great variability regarding populations and outcomes. Lung deposition rates varied across devices: 8%– 53% for pMDIs, 7%-69% for DPIs, and 39%– 67% for the SMI. The aerosol exit velocity was high with pMDIs (more than 3 m/s), while it is much slower (0.84– 0.72 m/s) with the SMI. In general, pMDIs produce large-sized particles (1.22– 8 μm), DPIs produce medium-sized particles (1.8– 4.8 μm), and 60% of the particles reach an aerodynamic diameter < 5 μm with the SMI. All inhalation devices reach central and peripheral lung regions, but the SMI distribution pattern might be better compared with pMDIs. DPIs’ intrinsic resistance is higher than that of pMDIs and SMI, which are relatively similar and low. Depending on the DPI, the minimum flow inspiratory rate required was 30 L/min. pMDIs and SMI did not require a high inspiratory flow rate.
Conclusion: Lung deposition and inspiratory flow rate are key factors when selecting an inhalation device in COPD patients.
Keywords: COPD, lung deposition, inspiratory flow, inhalation devices, systematic literature review
Corrigendum for this paper has been published
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by a persistent airflow limitation that is usually progressive, according to guidelines from the Global Initiative for Chronic Obstructive Lung Disease (GOLD).1 In recent years, the prevalence of COPD has dramatically increased, growing by 44.2% from 1990 to 2015.2 The impact on patients, society, and health systems is correspondingly huge. More than 3 million people die of COPD worldwide each year, accounting for 6% of all deaths worldwide.3 In 2010, the cost of COPD in the USA was projected to be approximately US $50 billion.4
One of the primary treatment modalities for COPD is medications that are delivered via inhalation devices. Currently, in clinical practice, a variety of devices are available for the treatment of these patients, including pressurized metered-dose inhalers (pMDIs), which are used with or without a valved holding chamber or spacer, as well as dry powder inhalers (DPIs) and the soft mist inhaler (SMI). Inhaler devices vary in several ways, including how the inhaler dispenses the drug, whether the treatment is passively or actively generated (using propellant, mechanical, or compressed air), and the drug’s formulation (solution, dry powder, or mist).
The selection of an inhalation device is a key point in COPD because it impacts patient adherence, the drug’s effectiveness, and long–term outcomes.5 A range of studies have assessed which factors/characteristics should be considered when selecting the most appropriate device.6–8 Interestingly, according to many expert opinions, the most important factors involved in achieving optimal disease outcomes are the generation of high lung deposition and correct dispensation with low inspiratory flow rates.9 Other relevant factors include inhalation technique, potential difficulties with the device, and patient preferences.
On the other hand, data regarding lung deposition and inspiratory flow rates across inhalation devices in COPD patients are usually described and evaluated as absolute, static numbers. However, a theoretical framework and pathophysiological and clinical evidence all suggest that both are influenced by several factors that relate to the patients and their COPD, all of which can change over time.6,10–17 Therefore, analyzing lung deposition and inspiratory flow rates in COPD patients who use inhalation devices requires a more careful, holistic, and dynamic approach.
Considering all the aspects described above, we performed a systematic literature review (SLR) and a narrative review to assess lung deposition and inspiratory flow rates, as well as data related to these inhalation devices in COPD patients. Using this information, we propose related conclusions and recommendations that can contribute to the selection of inhalation devices. We are confident that this information will be very useful for health professionals who are involved in the care of patients with COPD.
Methods
This project consisted of an SLR, a narrative review, and an expert opinion based on a nominal group meeting. A nominal group meeting is a structured method for brainstorming that encourages contributions from everyone and facilitates quick agreement on the relative importance of issues, problems, or solutions.
Experts’ Selection
We first established a group of 10 pneumologists (two of us were project coordinators). We are all specialized in COPD with demonstrated clinical experience (a minimum of 8 years and ≥5 publications and members of the Sociedad Española de Neumología y Cirugía Torácica (SEPAR). Besides, we are located in different parts of Spain. Then, we defined the project’s objectives, established the protocol of the SLR, and decided that this would be complemented by a narrative review.
Systematic Literature Review
The SLR was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The objective of the SLR was to analyze lung deposition, inspiratory flow, and other characteristics of different inhaler devices (pMDIs, DPIs, and SMI) in both COPD patients and healthy subjects. Studies were identified using sensitive search strategies in the main medical databases. For this purpose, an expert librarian checked the search strategies (Tables 1–7 of the Supplementary material). Disease- and inhaler device-related terms were used as search keywords, which employed a controlled vocabulary, specific MeSH headings, and additional keywords. The following bibliographic databases were screened up to April 2019: Medline (PubMed) and Embase from 1961 to April 2019, and the Cochrane Library up to April 2019. Retrieved references were managed in Endnote X5 (Thomson Reuters). Finally, a manual search was performed by reviewing the references of the included studies and all the publications, as well as other information provided by the authors. Retrieved studies were included if they met the following pre-established criteria: Patients had to be diagnosed with COPD, aged 18 or older, and treated with an inhaler device, and studies had to include outcomes related to lung deposition and inspiratory flow, including the rate of lung deposition, the particles’ mass median aerodynamic diameter (MMAD) expressed as µm (micrometer), the aerosol exit velocity (AEV) in meter per second (m/s), the lung distribution pattern, the inspiratory flow rate expressed as liter per minute (L/min), or the device’s intrinsic resistance. Other variables, such as safety, were also considered. Only SLRs, meta-analyses, randomized controlled trials (RCTs), observational studies, and in vitro studies in English, French, or Spanish were included. Animal studies were excluded. The screening of studies, data collection (including the evidence tables), and analysis were independently performed by two reviewers. In the case of a discrepancy between the reviewers, a consensus was reached by including a third reviewer. The 2011 levels of evidence from the Oxford Center for Evidence-Based Medicine (OCEBM)18 were used to grade the quality of the studies.
Narrative Review
To supplement the SLR, additional searches were performed specifically to explore the basis of lung deposition and inspiratory flow, including their determinants and the effect of COPD on these aspects. For this purpose, apart from the results of the SLR, we performed different searches in Medline using PubMed’s Clinical Queries tool and small search strategies using MeSH and text–word terms (Table 8 of the Supplementary material).
Nominal Group Meeting
The results of the SLR and narrative searches were presented and discussed in a guided nominal group meeting. In this meeting, we agreed on a series of general conclusions and clinical recommendations.
Results
The SLR retrieved 3064 articles, of which 979 were duplicates. A total of 120 articles were reviewed in detail, as well as a further 20 articles that were retrieved using the manual search. Eventually, 75 articles were excluded (Table 9 of the Supplementary material), most of them due to lack of relevant data, and 71 were included, 24 were in vitro studies. Some of the included articles were of low–moderate quality (due to the study design, and poor description of the methodology, especially for the articles published before the 1990s). We found great variability regarding study designs, populations, outcomes, and measures. There were 24 in vitro studies,16,17,19–40 and the rest of the articles comprised one SLR41 and several RCTs and cross-sectional studies. The studies analyzed more than 1600 COPD patients, most of whom were men, with age ranges from 27 to 89 years, and with forced expiratory volume in 1 second from 25% to 80%. Many of these studies assessed one type of inhalation device, but others compared pMDIs and DPIs,19,20,30,33,37,40–46 pMDIs and SMI,47–49 or DPIs and SMI.17,27,28 One study also evaluated the three inhalation devices.17 The narrative searches found almost 1000 articles.
Here, we summarize the main results of the SLR and narrative review, according to the project’s objectives (lung deposition, inspiratory flow rate, and data regarding these aspects for different inhaler devices). We also present the general conclusions and recommendations. Tables 1–3 show the main characteristics of the inhalation devices.
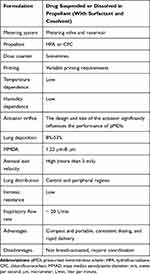 |
Table 1 Main Characteristics of Pressurized Metered-Dose Inhalers |
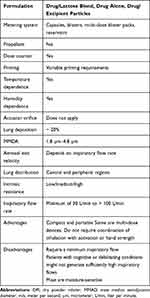 |
Table 2 Main Characteristics of Dry Powder Inhalers |
 |
Table 3 Main Characteristics of the Soft Mist Inhaler |
Lung Deposition
Different factors have been associated with lung deposition, some of which relate to the patient’s features (eg, airway geometry, inspiratory capacity, inhalation technique, breath–hold time, etc.) and to COPD (eg, exacerbations or hyperinflation).10–12,50–52 In fact, it has been shown during COPD exacerbations patients present decreased lung function and respiratory muscle strength that eventually influence on lung deposition.53 However, other factors are connected to the inhaler device (eg, the aerosol-generating system, speed of the aerosol plume, intrinsic resistance, inhaled carrier gas, oral/nasal inhalation, etc.), formulation (eg, the particle charge, lipophilicity, hygroscopicity, etc.), inhaled particle (eg, MMDA, its effect on lung distribution, etc.), and inhalation pattern (eg, the inspiration flow rate, volume, breath–hold time, etc.).52,54
With regards to the lung deposition (in relation to the emitted dose) across inhaler devices, data from in vitro and in vivo studies have estimated that 10%–20% of the delivered dose reaches the airways.54–56
Lung deposition rates (from individual studies) ranging from 8% to 53% have been reported for pMDIs.49,54,56–59 However, this rate increased to 11%–68% with the addition of a valved holding chamber or spacer31,35,46,59–63 and to 50%–60% with press-and-breathe actuators.64 More specifically, when Modulite® was used, lung deposition could reach up to 31%–34%.65,66 The K–haler® has a reported lung deposit of 39%.67 As exposed before, different factors might be contributing to these rate variability.
The studies included that analyzed DPIs have shown that the lung deposition rate is low, at around 20%,68 which is negatively influenced by a suboptimal inspiratory flow rate, humidity, and changes in temperature.69 Furthermore, clear differences in lung deposition were not observed when patients performed inhalation correctly.68 For the main DPI devices, the published lung deposition rates from individual studies (without direct comparisons) are as follows: Accuhaler® 7.6%,70 Aerolizer® 13%–20%,34,71 Breezhaler® 26.8%–39%,24,29 Easyhaler® 18.5%–31%,71,72 Genuair® 30.1%–51.1%,27,73,74 Handihaler® 9.8%–46.7%,19,24,71 Ingelheim inhaler® 16%–59%,75 NEXThaler® 39.4%–56%,11,76 Spinhaler® 11.5%,75 Turbuhaler® 14.2%–69.3%,21,42,77–79 and Twisthaler® 36%–37%.80 Similarly to all inhaler devices, other factors are probably influencing the lung deposition rate.81 Although some studies have compared lung deposition in pMDIs and DPIs, their results are contradictory.19,33
Respimat® (SMI) has largely exhibited high lung deposition rates that range from 39.2% to 67%,27,38,48,49,74,82–84 with different inspiratory flow rates (high and low) and irrespective of humidity.85 Compared with other devices, SMI showed higher lung deposition than pMDIs (including those with a chamber or spacer) or DPIs.27,48,74,83,86
We also evaluated the AEV. Inhalation devices with a high AEV might have a short spray duration and vice versa. With pMDIs, the aerosol exits through a nozzle at a very high rate of more than 3 m/s.87 However, the AEV of the SMI is much slower, at 0.84–0.72 m/s, and the aerosol cloud lasts longer.88–90
It has also been observed that the distribution of the deposition sites of inhaled particles is strongly dependent on their aerodynamic diameters.69 This SLR found that pMDIs generally produce at least medium-sized particles, with a significant rate of extrafine particles. The observed MMAD of conventional pMDIs varies from 1.22 to 8 μm,35,91,92 from 1.19 to 3.57 μm when a valved holding chamber or spacer is used,31,35,93 and from 0.72 to 2.0 μm with Modulite®.65,66 Regarding particle size data for DPIs, depending on the device and drug, MMDAs vary from 1.40 to 4.8 µm.11,19,21,24,27–29,36,37,74,76 Conversely, SMI generates a cloud that contains an aerosol with a fine particle fraction of around 3.7 μm.74 It is estimated that 60% of the particles reach a MMAD <5 μm with SMI.85 The reported rate with pMDIs and DPIs (indirect comparison) is not that high.27,28,74,94
Another relevant outcome when using inhalation devices is the lung distribution pattern (through the central and peripheral regions). All inhalation devices have been shown to reach both central and peripheral areas. SMI data suggest that lung distribution pattern might be better than pMDIs, with a higher distribution in bronchial trees and peripheral regions.11,28,49,60,65,66,73,74,82,95,96 More specifically, a comparative study found mean peripheral, intermediate and central lung deposition, and peripheral zone/central zone ratio of 5.0%–9.4%, 4.8%–11.3%, 4.5%–10.4%, 1.01–1.16 with Respimat® vs 3.8%, 4.9%, 5.6%, 1.36 with pMDIs, respectively.49 Comparative data between pMDIs and DPIs are conflicting.33,46
Inspiratory Flow Rate
The other main focus of this project was the inspiratory flow rate. First, it is important to consider the factors associated with inspiratory flow rate (Table 4). Similar to lung deposition, some of these factors relate to the patient’s and COPD’s characteristics, while other factors relate to the inhaler device, such as the intrinsic resistance.6,10,13–17,43,45,97,98
 |
Table 4 Main Factors Associated to Inspiratory Flow Rate |
Overall, two main driving forces can affect the performance of DPIs: the inspiratory flow generated by the patient and the turbulence produced inside the device, the latter of which solely depends on the original technical characteristics of the device, including the intrinsic resistance. These two parameters affect the disaggregation of the drug dose, the diameter of the particles to inhale, the lung distribution of the dose, and eventually, the efficacy of the delivered drug. Essentially, a higher intrinsic resistance results in the patient needing to generate a higher inspiratory flow.
In general, although variable, DPIs’ intrinsic resistance is higher than that of pMDIs or SMI, which are relatively similar and low. Therefore, pMDIs and SMI do not require the patient to generate a high inspiratory flow (and inspiratory effort).
According to the results of the SLR, pMDIs require low inspiratory flow rates of around 20 L/min (59, 70, 132) to achieve an adequate lung deposition.10,17,43,45,57,82,99–101 There were no major differences between the use of one propellant and another.57 In order to generate the correct inspiratory airflow and lung deposition with this type of inhalation device, it is recommended the patients start breathing from their functional residual capacity, then they should activate the inhalation device and start inhalation using an inspiratory flow rate that is below 60 L/min. Then, at the end of inspiration, patients should hold their breath for around 10 seconds.100 Consequently, patients need a correct inhalation technique and coordination. The K-haler® is triggered by an inspiratory flow rate of approximately 30 L/min.67
Inhaler devices are many times classified as low- (30 L/min or below), medium– (~30–60 L/min), and high-resistance (>60 L/min) devices.10,17 DPIs with low intrinsic resistance include Aerolizer®, Spinhaler®, and Breezhaler®; DPIs with medium resistance include Accuhaler®/Diskhaler®, Genuair®/Novolizer®, and NEXThaler®; DPIs with medium/high resistance include Turbuhaler®; and DPIs with high resistance include Easyhaler®, Handihaler®, and Twisthaler®. The estimated inspiratory flow rates required thus vary across devices, from a minimum of 30 L/min to more than 100 L/min.6,26,32,43,45,101–108
Based on the information presented above, when using a high-resistance DPI, the disaggregation and micro-dispersion of the powdered drug are relatively independent of the patient’s inspiratory effort because the driving force depends on the intrinsic resistance of the DPI itself, which is able to produce the turbulence required for effective drug micro-dispersion. However, when a low-resistance device is used, the only force that can generate turbulence is the patient’s inspiratory airflow, which should be high.
Finally, the studies showed that the SMI inhalation device uses mechanical energy (from a spring) to generate a fine, slow–moving mist from an aqueous solution, which is independent of the patient’s inspiratory effort. Therefore, the required inspiratory flow rate and/or effort are less relevant than with DPIs.83,88,89,109 Moreover, the inhalation maneuver with SMI is more similar to physiological inhalation. One study observed that drug delivery to the lungs with SMI was more efficient than with pMDIs, even with poor inhalation technique.82
General Conclusions and Recommendations
The experts discussed the results of the reviews, and, based on the evidence, they formulated a series of general conclusions and recommendations that are outlined in Tables 5 and 6. In summary, health professionals involved in the management of COPD patients should be aware of all factors involved in adequate drug distribution when using inhalation devices. Two main objective factors emerged at this point: lung deposition and the required inspiratory flow rate. Both of these factors are highly influenced by patient, COPD, and inhaler device characteristics. Moreover, COPD is a heterogeneous and dynamic chronic disease, in which lung deposition and inspiratory flow rates vary across patients and also within the same patient.
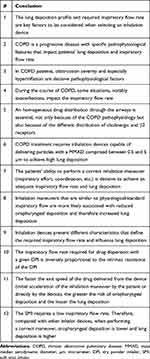 |
Table 5 General Conclusions Regarding Lung Deposition and Inspiratory Flow Rate in Chronic Obstructive Pulmonary Disease |
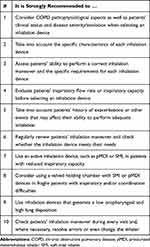 |
Table 6 Experts’ Recommendations for the Selection of the Appropriate Inhalation Device in Chronic Obstructive Pulmonary Disease |
Thus, it is strongly recommended that, in addition to the standard variables for COPD, inspiratory flow rate and the patient’s inspiratory capacity are evaluated (on a regular basis), and the selection of an inhaler device should be based on the COPD patient’s features, needs, and clinical situation. This selection should consider the different characteristics of the devices to ensure physicians choose the device that best matches that patient’s needs.
Finally, we considered it important to systematically review the patient’s inhalation maneuver,110 see Tables 3 and 6. This should be checked during every visit, so that errors can be resolved, and inhalers can be checked and even changed, where necessary. The same way, before considering a change in the patient’s treatment, possible errors with the inhalation maneuver should be evaluated.
Discussion
We have presented a critical and detailed review of data related to lung deposition and inspiratory flow rates in COPD patients across different inhalation devices, while also taking into account all the factors and bases that determine the effectiveness of the inhaled route of administration.
The delivery of drugs by inhalation is an integral component in the treatment of COPD. A growing number of inhalation devices, whose designs and characteristics vary, have been engineered in recent years to treat COPD and other respiratory diseases.111 Therefore, selecting the most appropriate device that meets each individual patient’s needs is vital in clinical practice. Several factors have been proposed that should be considered when choosing an inhalation device. These include the patient’s lung function, device handling, inhalation technique, and preferences.8 However, according to a published expert opinion, the two most important characteristics for an inhaler used by patients with COPD are that the device permits a high pulmonary deposition of the drug and allows its delivery at low inspiratory flows.9
With regards to lung deposition, the selected inhalation device should guarantee the maximum lung deposition and distribution of the drug in the context of a given patient. At this point, we would like to highlight that it is extremely relevant to consider that COPD presents specific pathophysiological features that (negatively) impact a patient’s lung deposition, especially hyperinflation.10–12,50,51 Moreover, COPD is considered a progressive disease that carries the risk of clinical exacerbation, suggesting that the impact on lung deposition might also change during the disease course. Similarly, a wealth of evidence indicates that patient-related factors, such as the inhalation technique and the presence of debilitating conditions, influence lung deposition.112 Finally, lung distribution is also important because β2 and cholinergic receptors are present in both central and peripheral areas.113,114 It is important to bear in mind that the receptors are present in different amounts in the central and peripheral areas, so ideally the active ingredients should also be delivered to the appropriate area in correspondence with the receptor concentrations.
Data from the SLR show that the lung deposition rate (of the emitted dose) of pMDIs is generally low, although it can be increased with the addition of a valved holding chamber or spacer or with the use of Modulite®.49,54,56–59 Data for DPIs vary depending on the device, but lung deposition rates are quite low and negatively influenced by different factors.69 Individual studies and comparisons with pMDIs and DPIs indicate that SMI generates higher lung deposition rates, irrespective of other factors, such as inspiratory flow or humidity.27,38,48,49,74,82–86 We also found that AEV is high with pMDIs,87 compared to SMI, the latter of which is distinctly slower and produces a longer-lasting aerosol cloud.88–90 Further research is necessary to corroborate individual (and some comparative results) that suggest that SMI also generates a higher rate of fine particle fraction.27,28,74,85,94 It is well established that the generation of particles with smaller diameters is essential for passing the mouth–throat region.69 Finally, all inhalation devices can reach both central and peripheral airways.
Inspiratory flow rate analysis also generated interesting data. We have identified several factors that are associated with the inspiratory flow rate, of which physicians should be aware. Some of these factors relate to the patient and COPD, such as inspiratory capacity and hyperinflation.6,10,13–17,43,45,97,98 However, other factors relate to the inhalation device’s characteristics, including its intrinsic resistance.
Most DPIs require a high inspiratory flow to overcome the device’s resistance and to achieve effective drug delivery. Therefore, the inspiratory airflow generated by the patient represents the only active force that can produce the disaggregation of the powdered drug for inhalation. This point is critical because many patients with COPD, especially those with severe COPD (but also many patients with less severe disease), might not achieve the required inspiratory flow. It has been described that up to 20% of patients with severe COPD are not able to generate the required inspiratory flows with some DPIs (126). Similarly, it is estimated that 30% of elderly patients with COPD and 40% of patients hospitalized for COPD exacerbations do not achieve required inspiratory flows with Turbuhaler®.44,102 However, we also found the opposite situation, in which an excessive inspiratory flow rate that overcomes the resistance might lead to an increased oropharyngeal deposition.6 Thus, DPIs might not be the best option when the required inspiratory rate cannot be assured.
Conversely, pMDIs and SMI require low inspiratory effort.10,17,43,45,57,82,99–101 However, the pMDI’s inhalation technique is quite complex, compared to the SMI inhalation maneuver, which is similar to physiological inhalation. Moreover, SMI generates a fine, slow-moving mist that might reduce oropharyngeal deposition, when compared with pMDIs.82 This finding has implications for clinical practice. For example, patients with difficulties between breathing and actuation of the device may be unable to effectively use a pMDI. In these cases, SMI or DPIs might be more appropriate.
We would also like to note some limitations of the SLR, the first of which is the great heterogeneity regarding the studies’ designs, populations, and outcomes. This could have limited comparability across inhalation devices. Furthermore, the quality of many of the studies was low or moderate. As previously mentioned, some studies were published more than 20 years ago. Consequently, it was quite difficult to draw robust conclusions. The recommendations were also not formally evaluated using a Delphi process. However, we agree that the recommendations reflect general but objective facts.
Conclusions
The choice of inhalation devices for COPD patients depends on a combination of factors, but lung deposition and inspiratory flow rate are key aspects of this selection process. When selecting an inhalation device, all health professionals who are involved in the care of patients with COPD must consider the basis of lung deposition and inspiratory flow rate, among other aspects. The clinician can then select the most adequate inhalation device, depending on the patient, their COPD, and the inhalation device’s characteristics, which will ultimately achieve the maximum lung deposition and distribution.
Abbreviations
COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; GOLD, Global Initiative for Chronic Obstructive Lung Disease; L/min, liter per minute; MMAD, mass median aerodynamic diameter; m/s, meter per second; OCEBM, Oxford Centre for Evidence-Based Medicine; pMDI, pressurized metered-dose inhaler; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; SLR, systematic literature review; SMI, soft mist inhaler; µm, micrometer; USA, United States of America.
Data Sharing Statement
The tables and datasets analysed during the current systematic review are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Estibaliz Loza (InMusc) for her contribution in the systematic literature review and Carmen Gonzalez (Gotit) for her help in the nominal group meeting.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The project was funded by Boehringer-Ingelheim. Boehringer-Ingelheim had no role in the design of the study, the collection, analysis, and interpretation of the data, and the writing of the manuscript. The authors received no direct compensation related to the development of the manuscript. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Disclosure
The authors report no conflicts of interest.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
2. Collaborators GBDCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi:10.1016/S2213-2600(17)30293-X
3. Kostikas K, Clemens A, Patalano F. Prediction and prevention of exacerbations and mortality in patients with COPD. Expert Rev Respir Med. 2016;10(7):739–753. doi:10.1080/17476348.2016.1185371
4. Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi:10.2147/CEOR.S34321
5. Lopez-Campos JL, Quintana Gallego E, Carrasco Hernandez L. Status of and strategies for improving adherence to COPD treatment. Int J Chron Obstruct Pulmon Dis. 2019;14:1503–1515. doi:10.2147/COPD.S170848
6. Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13. doi:10.1186/s40248-015-0012-5
7. Area de asma de S, Area de enfermeria de S, Departamento de asma A. [SEPAR-ALAT consensus for inhaled therapies]. Arch Bronconeumol. 2013;49(Suppl 1):2–14. doi:10.1016/S0300-2896(13)70068-1
8. Usmani OS. <p>Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manag. 2019;15:461–472. doi:10.2147/TCRM.S160365
9. Garcia-Rio F, Soler-Cataluna JJ, Alcazar B, Viejo JL, Miravitlles M. Requirements, Strengths and Weaknesses of Inhaler Devices for COPD Patients from the Expert Prescribers’ Point of View: results of the EPOCA Delphi Consensus. COPD. 2017;14(6):573–580.
10. Ghosh S, Ohar JA, Drummond MB. Peak Inspiratory Flow Rate in Chronic Obstructive Pulmonary Disease: implications for Dry Powder Inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381–387. doi:10.1089/jamp.2017.1416
11. Virchow JC, Poli G, Herpich C, et al. Lung Deposition of the Dry Powder Fixed Combination Beclometasone Dipropionate Plus Formoterol Fumarate Using NEXThaler((R)) Device in Healthy Subjects, Asthmatic Patients, and COPD Patients. J Aerosol Med Pulm Drug Deliv. 2018;31(5):269–280. doi:10.1089/jamp.2016.1359
12. Lippmann M, Yeates DB, Albert RE. Deposition, retention, and clearance of inhaled particles. Br J Ind Med. 1980;37(4):337–362. doi:10.1136/oem.37.4.337
13. Kafaei Shirmanesh Y, Jones MD. Physical ability of people with rheumatoid arthritis and age-sex matched controls to use four commonly prescribed inhaler devices. Respir Med. 2018;135:12–14. doi:10.1016/j.rmed.2017.12.014
14. Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174–179. doi:10.1089/jamp.2012.0987
15. Prime D, de Backer W, Hamilton M, et al. Effect of Disease Severity in Asthma and Chronic Obstructive Pulmonary Disease on Inhaler-Specific Inhalation Profiles Through the ELLIPTA(R) Dry Powder Inhaler. J Aerosol Med Pulm Drug Deliv. 2015;28(6):486–497. doi:10.1089/jamp.2015.1224
16. Hamilton M, Leggett R, Pang C, Charles S, Gillett B, Prime D. In Vitro Dosing Performance of the ELLIPTA Dry Powder Inhaler Using Asthma and COPD Patient Inhalation Profiles Replicated with the Electronic Lung (eLungTM). J Aerosol Med Pulm Drug Deliv. 2015;28(6):498–506. doi:10.1089/jamp.2015.1225
17. Hira D, Koide H, Nakamura S, et al. Assessment of inhalation flow patterns of soft mist inhaler co-prescribed with dry powder inhaler using inspiratory flow meter for multi inhalation devices. PLoS One. 2018;13(2):e0193082. doi:10.1371/journal.pone.0193082
18. Howick J, Chalmers I, Glasziou P, et al. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). 2011; https://www.cebm.net/index.aspx?o=5653.
19. Ali M, Mazumder MK, Martonen TB. Measurements of electrodynamic effects on the deposition of MDI and DPI aerosols in a replica cast of human oral-pharyngeal-laryngeal airways. J Aerosol Med Pulm Drug Deliv. 2009;22(1):35–44. doi:10.1089/jamp.2007.0637
20. Al-Showair RAM, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395–2401. doi:10.1016/j.rmed.2007.06.008
21. Bagherisadeghi G, Larhrib EH, Chrystyn H. Real life dose emission characterization using COPD patient inhalation profiles when they inhaled using a fixed dose combination (FDC) of the medium strength Symbicort Turbuhaler. Int J Pharm. 2017;522(1–2):137–146. doi:10.1016/j.ijpharm.2017.02.057
22. Borgstrom L, Asking L, Lipniunas P. An in vivo and in vitro comparison of two powder inhalers following storage at hot/humid conditions. J Aerosol Med. 2005;18(3):304–310. doi:10.1089/jam.2005.18.304
23. Canonica GW, Arp J, Keegstra JR, Chrystyn H. Spiromax, a New Dry Powder Inhaler: dose Consistency under Simulated Real-World Conditions. J Aerosol Med Pulm Drug Deliv. 2015;28(5):309–319. doi:10.1089/jamp.2015.1216
24. Chapman KR, Fogarty CM, Peckitt C, et al. Delivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:
25. Cheng YS, Fu CS, Yazzie D, Zhou Y. Respiratory deposition patterns of salbutamol pMDI with CFC and HFA-134a formulations in a human airway replica. J Aerosol Med. 2001;14(2):255–266. doi:10.1089/08942680152484180
26. Chodosh S, Flanders JS, Kesten S, Serby CW, Hochrainer D, Witek TJ. Effective delivery of particles with the HandiHaler dry powder inhalation system over a range of chronic obstructive pulmonary disease severity. J Aerosol Med. 2001;14(3):309–315. doi:10.1089/089426801316970268
27. Ciciliani AM, Wachtel H, Langguth P. Comparing Respimat® Soft Mist™ Inhaler and DPI Aerosol Deposition by Combined In Vitro Measurements and CFD Simulations. Respir Drug Delivery. 2014;453–456.
28. Ciciliani AM, Wachtel H, Heussel CP, Langguth P. Evaluation of Respimat®Soft Mist™ Inhaler Based on In Vitro Measurements and CFD Simulations. Respir Drug Delivery Eur. 2015;1–5.
29. Colthorpe P, Voshaar T, Kieckbusch T, Cuoghi E, Jauernig J. Delivery characteristics of a low-resistance dry-powder inhaler used to deliver the long-acting muscarinic antagonist glycopyrronium. J Drug Assess. 2013;2(1):11–16. doi:10.3109/21556660.2013.766197
30. DeHaan WH, Finlay WH. In vitro monodisperse aerosol deposition in a mouth and throat with six different inhalation devices. J Aerosol Med. 2001;14(3):361–367. doi:10.1089/089426801316970321
31. Gillen M, Forte P, Svensson JO, et al. Effect of a spacer on total systemic and lung bioavailability in healthy volunteers and in vitro performance of the Symbicort (budesonide/formoterol) pressurized metered dose inhaler. Pulm Pharmacol Ther. 2018;52:7–17. doi:10.1016/j.pupt.2018.08.001
32. Jogi R, Lahelma S, Vahteristo M, Happonen A, Haikarainen J. In Vitro Flow Rate Dependency of Delivered Dose and Fine Particle Dose of Salmeterol/Fluticasone Propionate Easyhaler and Seretide Diskus with Patient Flow Rates Collected in a Randomized Controlled Trial. J Aerosol Med Pulm Drug Deliv. 2019;32(2):88–98. doi:10.1089/jamp.2018.1463
33. Melchor R, Biddiscombe MF, Mak VH, Short MD, Spiro SG. Lung deposition patterns of directly labelled salbutamol in normal subjects and in patients with reversible airflow obstruction. Thorax. 1993;48(5):506–511. doi:10.1136/thx.48.5.506
34. Meyer T, Brand P, Ehlich H, et al. Deposition of Foradil P in human lungs: comparison of in vitro and in vivo data. J Aerosol Med. 2004;17(1):43–49. doi:10.1089/089426804322994451
35. Rahmatalla MF, Zuberbuhler PC, Lange CF, Finlay WH. In vitro effect of a holding chamber on the mouth-throat deposition of QVAR (hydrofluoroalkane-beclomethasone dipropionate). J Aerosol Med. 2002;15(4):379–385. doi:10.1089/08942680260473452
36. Weers J, Ung K, Le J, et al. Dose emission characteristics of placebo PulmoSphere particles are unaffected by a subject’s inhalation maneuver. J Aerosol Med Pulm Drug Deliv. 2013;26(1):56–68. doi:10.1089/jamp.2012.0973
37. Wilson R, Templeton A, Leemereise C, et al. Safety, Tolerability, and Pharmacokinetics of a New Formulation of Nemiralisib Administered via a Dry Powder Inhaler to Healthy Individuals. Clin Ther. 2019;41(6):1214–1220. doi:10.1016/j.clinthera.2019.04.008
38. Worth Longest P, Hindle M. Evaluation of the Respimat Soft Mist Inhaler using a concurrent CFD and in vitro approach. J Aerosol Med Pulm Drug Deliv. 2009;22(2):99–112. doi:10.1089/jamp.2008.0708
39. Yang TT, Li S, Wyka B, Kenyon D. Drug delivery performance of the mometasone furoate dry powder inhaler. J Aerosol Med. 2001;14(4):487–494. doi:10.1089/08942680152744695
40. Zhang Y, Gilbertson K, Finlay WH. In vivo-in vitro comparison of deposition in three mouth-throat models with Qvar and Turbuhaler inhalers. J Aerosol Med. 2007;20(3):227–235. doi:10.1089/jam.2007.0584
41. Haidl P, Heindl S, Siemon K, Bernacka M, Cloes RM. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65–75. doi:10.1016/j.rmed.2016.07.013
42. Aalto E, Havu M, Kotaniemi J, et al. Comparison of terbutaline Turbuhaler and albuterol chlorofluorocarbon (CFC) inhaler in middle-aged and elderly patients with obstructive lung disease. Ann Allergy. 1992;69(1):
43. Broeders ME, Molema J, Hop WC, Folgering HT. Inhalation profiles in asthmatics and COPD patients: reproducibility and effect of instruction. J Aerosol Med. 2003;16(2):
44. Broeders MEAC, Molema J, Hop WCJ, Vermue NA, Folgering HTM. The course of inhalation profiles during an exacerbation of obstructive lung disease. Respir Med. 2004;98(12):1173–1179. doi:10.1016/j.rmed.2004.04.010
45. Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213–218. doi:10.1093/ageing/afl174
46. Newman SP, Pitcairn GR, Adkin DA, Vidgren MT, Silvasti M. Comparison of beclomethasone dipropionate delivery by easyhaler dry powder inhaler and pMDI plus large volume spacer. J Aerosol Med. 2001;14(2):217–225. doi:10.1089/08942680152484144
47. Brand P, Hederer B, Austen G, Dewberry H, Meyer T. Higher lung deposition with Respimat Soft Mist inhaler than HFA-MDI in COPD patients with poor technique. Int J Chron Obstruct Pulmon Dis. 2008;3(4):
48. Newman SP, Brown J, Steed KP, Reader SJ, Kladders H. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devices. Chest. 1998;113(4):957–963. doi:10.1378/chest.113.4.957
49. Steed KP, Towse LJ, Freundc B, Newman SP. Lung and oropharyngeal depositions of fenoterol hydrobromide delivered 1 from the prototype III hand-held multidose Respimat nebuliser. Eur J Pharm. 1997;5(2):55–61. doi:10.1016/S0928-0987(96)00016-4
50. Stuart BO. Deposition and clearance of inhaled particles. Environ Health Perspect. 1984;55:369–390. doi:10.1289/ehp.8455369
51. Lange CF, Finlay WH. Overcoming the adverse effect of humidity in aerosol delivery via pressurized metered-dose inhalers during mechanical ventilation. Am J Respir Crit Care Med. 2000;161(5):1614–1618. doi:10.1164/ajrccm.161.5.9909032
52. Horváth A, Balásházy I, Tomisa G, Farkas Á. Significance of breath-hold time in dry powder aerosol drug therapy of COPD patients. Eur J Pharm Sci. 2017;104:145–149. doi:10.1016/j.ejps.2017.03.047
53. Broeders ME, Molema J, Hop WC, Vermue NA, Folgering HT. The course of inhalation profiles during an exacerbation of obstructive lung disease. Respir Med. 2004;98(12):1173–1179.
54. Newman SP, Moren F, Pavia D, Little F, Clarke SW. Deposition of pressurized suspension aerosols inhaled through extension devices. Am Rev Respir Dis. 1981;124(3):317–320. doi:10.1164/arrd.1981.124.3.317
55. Newman SP, Clarke SW. Therapeutic aerosols 1–physical and practical considerations. Thorax. 1983;38(12):881–886. doi:10.1136/thx.38.12.881
56. De Backer W, Devolder A, Poli G, et al. Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients. J Aerosol Med Pulm Drug Deliv. 2010;23(3):137–148. doi:10.1089/jamp.2009.0772
57. Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ. Lung deposition of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone: a cross-over study in healthy volunteers. Chest. 2002;122(2):510–516. doi:10.1378/chest.122.2.510
58. Taylor RG, Pavia D, Agnew JE, et al. Effect of four weeks’ high dose ipratropium bromide treatment on lung mucociliary clearance. Thorax. 1986;41(4):
59. Vidgren M, Karkkainen A, Karjalainen P. Effect of extension devices on the drug deposition from inhalation aerosols. Int J Pharm. 1987;39:1107–1111. doi:10.1016/0378-5173(87)90204-3
60. Richards J, Hirst P, Pitcairn G, et al. Deposition and pharmacokinetics of flunisolide delivered from pressurized inhalers containing non-CFC and CFC propellants. J Aerosol Med. 2001;14(2):197–208. doi:10.1089/08942680152484126
61. Ashworth HL, Wilson CG, Sims EE, Wotton PK, Hardy JG. Delivery of propellant soluble drug from a metered dose inhaler. Thorax. 1991;46(4):245–247. doi:10.1136/thx.46.4.245
62. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;(9):CD000052.
63. Newman SP, Weisz AW, Talaee N, Clarke SW. Improvement of drug delivery with a breath actuated pressurised aerosol for patients with poor inhaler technique. Thorax. 1991;46(10):712–716. doi:10.1136/thx.46.10.712
64. Leach C. Effect of formulation parameters on hydrofluoroalkane-beclomethasone dipropionate drug deposition in humans. J Allergy Clin Immunol. 1999;104(6):S250–252. doi:10.1016/S0091-6749(99)70041-2
65. Leach CL, Davidson PJ, Boudreau RJ. Improved airway targeting with the CFC-free HFA-beclomethasone metered-dose inhaler compared with CFC-beclomethasone. Eur Respir J. 1998;12(6):1346–1353. doi:10.1183/09031936.98.12061346
66. Haussermann S, Acerbi D, Brand P, et al. Lung deposition of formoterol HFA (Atimos/Forair) in healthy volunteers, asthmatic and COPD patients. J Aerosol Med. 2007;20(3):331–341. doi:10.1089/jam.2007.0613
67. Kappeler D, Sommerer K, Kietzig C, et al. Pulmonary deposition of fluticasone propionate/formoterol in healthy volunteers, asthmatics and COPD patients with a novel breath-triggered inhaler. Respir Med. 2018;138:107–114. doi:10.1016/j.rmed.2018.03.029
68. Taburet AM, Schmit B. Pharmacokinetic optimisation of asthma treatment. Clin Pharmacokinet. 1994;26(5):396–418. doi:10.2165/00003088-199426050-00006
69. Fernandez Tena A, Casan Clara P. Deposition of inhaled particles in the lungs. Arch Bronconeumol. 2012;48(7):240–246. doi:10.1016/j.arbr.2012.02.006
70. Agertoft L, Pedersen S. Lung deposition and systemic availability of fluticasone Diskus and budesonide Turbuhaler in children. Am J Respir Crit Care Med. 2003;168(7):779–782. doi:10.1164/rccm.200302-200OC
71. Delvadia R, Hindle M, Longest PW, Byron PR. In vitro tests for aerosol deposition II: iVIVCs for different dry powder inhalers in normal adults. J Aerosol Med Pulm Drug Deliv. 2013;26(3):138–144. doi:10.1089/jamp.2012.0975
72. Hirst PH, Bacon RE, Pitcairn GR, Silvasti M, Newman SP. A comparison of the lung deposition of budesonide from Easyhaler, Turbuhaler and pMDI plus spacer in asthmatic patients. Respir Med. 2001;95(9):720–727. doi:10.1053/rmed.2001.1107
73. Leach CL, Bethke TD, Boudreau RJ, et al. Two-dimensional and three-dimensional imaging show ciclesonide has high lung deposition and peripheral distribution: a nonrandomized study in healthy volunteers. J Aerosol Med. 2006;19(2):117–126. doi:10.1089/jam.2006.19.117
74. Ciciliani AM, Langguth P, Wachtel H. In vitro dose comparison of Respimat((R)) inhaler with dry powder inhalers for COPD maintenance therapy. Int J Chron Obstruct Pulmon Dis. 2017;12:1565–1577. doi:10.2147/COPD.S115886
75. Vidgren M, KWkkiinen A, Karjalainen P, Paronen P, Nuutinen J. Effect of powder inhaler design on drug in the respiratory tract. Int J Pharm. 1988;42:211–216. doi:10.1016/0378-5173(88)90177-9
76. Mariotti F, Sergio F, Acerbi D, Meyer T, Herpich C. Lung Deposition of the Extrafine Dry Powder Fixed Combination Beclomethasone Dipropionate Plus Formoterol Fumarate via the NEXT DPI® in Healthy Subjects, Asthmatic and COPD Patients. Amsterdam: RS Annual Congress; 2011.
77. Newman SP, Moren F, Trofast E, Talaee N, Clarke SW. Deposition and clinical efficacy of terbutaline sulphate from Turbuhaler, a new multi-dose powder inhaler. Eur Respir J. 1989;2(3):247–252.
78. Thorsson L, Edsbacker S, Conradson T-B. Lung deposition of budesonide from Turbuhaler® is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J. 1994;7(10):1839–1844. doi:10.1183/09031936.94.07101839
79. Burnell PK, Small T, Doig S, Johal B, Jenkins R, Gibson GJ. Ex-vivo product performance of DiskusTMand TurbuhalerTMinhalers using inhalation profiles from patients with severe chronic obstructive pulmonary disease. Respir Med. 2001;95(5):
80. Dolovich MA. Influence of inspiratory flow rate, particle size, and airway caliber on aerosolized drug delivery to the lung. Respir Care. 2000;45(6):597–608.
81. Horváth A, Farkas Á, Szipőcs A, Tomisa G, Szalai Z, Gálffy G. Numerical simulation of the effect of inhalation parameters, gender, age and disease severity on the lung deposition of dry powder aerosol drugs emitted by Turbuhaler®, Breezhaler® and Genuair® in COPD patients. Eur J Pharm Sci. 2020;154:105508.
82. Brand P, Hederer B, Austen G, Dewberry H, Meyer T. Higher lung deposition with Respimat Soft Mist inhaler than HFA-MDI in COPD patients with poor technique. Int J Chron Obstruct Pulmon Dis. 2008;3(4):763–770.
83. Pitcairn G, Reader S, Pavia D, Newman S. Deposition of corticosteroid aerosol in the human lung by Respimat Soft Mist inhaler compared to deposition by metered dose inhaler or by Turbuhaler dry powder inhaler. J Aerosol Med. 2005;18(3):264–272.
84. Newman SP, Newhouse MT. Effect of add-on devices for aerosol drug delivery: deposition studies and clinical aspects. J Aerosol Med. 1996;9(1):55–70.
85. Wachtel H, Kattenbeck S, Dunne S, Disse B. The Respimat® Development Story: patient-Centered Innovation. Pulmonary Therapy. 2017;3(1):19–30.
86. Iwanaga T, Kozuka T, Nakanishi J, et al. Aerosol Deposition of Inhaled Corticosteroids/Long-Acting β2-Agonists in the Peripheral Airways of Patients with Asthma Using Functional Respiratory Imaging, a Novel Imaging Technology. Pulmonary Therapy. 2017;3(1):219–231.
87. Altshuler BYL, Palmes ED. Aerosols deposition in the human respiratory tract. Arch Environ Health. 1957;15:292–303.
88. Hochrainer D, Holz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18(3):273–282.
89. Zierenberg B. Optimizing the in vitro performance of Respimat. J Aerosol Med. 1999;12(Suppl 1):S19–24.
90. Tamura G. Comparison of the aerosol velocity of Respimat(R) soft mist inhaler and seven pressurized metered dose inhalers. Allergol Int. 2015;64(4):390–392.
91. Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032–1045.
92. Scichilone N, Spatafora M, Battaglia S, Arrigo R, Benfante A, Bellia V. Lung penetration and patient adherence considerations in the management of asthma: role of extra-fine formulations. J Asthma Allergy. 2013;6:11–21.
93. Janssens HM, Krijgsman A, Verbraak TF, Hop WC, de Jongste JC, Tiddens HA. Determining factors of aerosol deposition for four pMDI-spacer combinations in an infant upper airway model. J Aerosol Med. 2004;17(1):51–61.
94. Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172(12):1497–1504.
95. Newman SP, Steed KP, Reader SJ, Hooper G, Zierenberg B. Efficient delivery to the lungs of flunisolide aerosol from a new portable hand-held multidose nebulizer. J Pharm Sci. 1996;85(9):960–964.
96. Newman SP, Sutton DJ, Segarra R, Lamarca R, de Miquel G. Lung deposition of aclidinium bromide from Genuair, a multidose dry powder inhaler. Respiration. 2009;78(3):322–328.
97. Franssen FM, Wouters EF, Schols AM. The contribution of starvation, deconditioning and ageing to the observed alterations in peripheral skeletal muscle in chronic organ diseases. Clin Nutr. 2002;21(1):1–14.
98. Martinez FJ, Couser JI, Celli BR. Factors influencing ventilatory muscle recruitment in patients with chronic airflow obstruction. Am Rev Respir Dis. 1990;142(2):276–282.
99. Newhouse M, Sanchis J, Bienenstock J. Lung defense mechanisms (first of two parts). N Engl J Med. 1976;295(18):990–998.
100. Dolovich M, Ruffin RE, Roberts R, Newhouse MT. Optimal delivery of aerosols from metered dose inhalers. Chest. 1981;80(6 Suppl):911–915.
101. Al-Showair RA, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395–2401.
102. Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78–83. doi:10.1183/09031936.00024807
103. Kawamatawong T, Khiawwan S, Pornsuriyasak P. Peak inspiratory flow rate measurement by using In-Check DIAL for the different inhaler devices in elderly with obstructive airway diseases. J Asthma Allergy. 2017;10:17–21. doi:10.2147/JAA.S127580
104. Seheult JN, Costello S, Tee KC, et al. Investigating the relationship between peak inspiratory flow rate and volume of inhalation from a DiskusTM Inhaler and baseline spirometric parameters: a cross-sectional study. SpringerPlus. 2014;3(101597967):496. doi:10.1186/2193-1801-3-496
105. Taylor TE, Lacalle Muls H, Costello RW, Reilly RB. Estimation of inhalation flow profile using audio-based methods to assess inhaler medication adherence. PLoS One. 2018;13(1):e0191330. doi:10.1371/journal.pone.0191330
106. Warren S, Taylor G, Smith J, Buck H, Parry-Billings M. Gamma scintigraphic evaluation of a novel budesonide dry powder inhaler using a validated radiolabeling technique. J Aerosol Med. 2002;15(1):15–25. doi:10.1089/08942680252908548
107. Weiner P, Weiner M. Inspiratory muscle training may increase peak inspiratory flow in chronic obstructive pulmonary disease. Respir Int Rev Thoracic Dis. 2006;73(2):
108. Azouz W, Chetcuti P, Hosker H, Saralaya D, Chrystyn H. Inhalation characteristics of asthma patients, COPD patients and healthy volunteers with the Spiromax and Turbuhaler devices: a randomised, cross-over study. BMC Pulm Med. 2015;15(100968563):47. doi:10.1186/s12890-015-0043-x
109. Anderson P. Use of Respimat Soft Mist inhaler in COPD patients. Int J Chron Obstruct Pulmon Dis. 2006;1(3):251–259. doi:10.2147/copd.2006.1.3.251
110. Terry PD, Dhand R. Inhalation Therapy for Stable COPD: 20 Years of GOLD Reports. Adv Ther. 2020;37(5):1812–1828. doi:10.1007/s12325-020-01289-y
111. Lavorini F, Corrigan CJ, Barnes PJ, et al. Retail sales of inhalation devices in European countries: so much for a global policy. Respir Med. 2011;105(7):1099–1103. doi:10.1016/j.rmed.2011.03.012
112. Sanchis J, Gich I, Pedersen S. Aerosol Drug Management Improvement T Systematic Review of Errors in Inhaler Use: has Patient Technique Improved Over Time? Chest. 2016;150(2):394–406. doi:10.1016/j.chest.2016.03.041
113. Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am Rev Respir Dis. 1985;132(3):541–547. doi:10.1164/arrd.1985.132.3.541
114. Mak JC, Barnes PJ. Autoradiographic visualization of muscarinic receptor subtypes in human and guinea pig lung. Am Rev Respir Dis. 1990;141(6):1559–1568. doi:10.1164/ajrccm/141.6.1559
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
