Back to Journals » Infection and Drug Resistance » Volume 16
Lung Abscess Caused by Tannerella forsythia Infection: A Case Report
Received 9 August 2023
Accepted for publication 26 October 2023
Published 31 October 2023 Volume 2023:16 Pages 6975—6981
DOI https://doi.org/10.2147/IDR.S434494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Huiying Lv, Yawen Zhuang, Weijing Wu
Department of Respiratory and Critical Care Medicine, the Second Affiliated Hospital of Fujian Medical University, Quanzhou City, Fujian Province, People’s Republic of China
Correspondence: Weijing Wu, Department of Respiratory and Critical Care Medicine, the Second Affiliated Hospital of Fujian Medical University, No. 34, Zhongshan Bei Road, Lichen District, Quanzhou, Fujian Province, People’s Republic of China, Tel +8615159883230, Fax +86 595 22770258, Email [email protected]
Background: Tannerella forsythia is a gram-negative anaerobic bacterium commonly found in the oral cavity. It is among the common pathogenic bacteria associated with gingivitis, chronic periodontitis, and aggressive periodontitis. However, there is currently no literature discussing lung abscesses primarily caused by T. forsythia infection.
Presentation: This article presents the case of a 55-year-old male with a massive lung abscess. The patient underwent ultrasound-guided percutaneous drainage, and the sample was sent for pathogen metagenomic next-generation sequencing (mNGS) testing. The test indicated that the lung abscess was primarily caused by T. forsythia infection. A literature review was conducted to understand the characteristics of this pathogen as well as its clinical features and suitable treatment approaches.
Conclusion: Currently, there is no literature specifically mentioning T. forsythia as a primary pathogen causing lung abscesses. This anaerobic bacterium is commonly found in the oral cavity and is difficult to cultivate using routine culture methods. mNGS emerges as a value diagnostic method for identifying this pathogen. Treatment recommendations include drainage and antibiotic selection encompassing common periodontal pathogens such as red complex bacteria and Actinomyces.
Keywords: Tannerella forsythia, lung abscess, metagenomic next-generation sequencing, drainage
Background
Tannerella forsythia is a gram-negative anaerobic bacterium commonly found in the oral cavity. Together with Porphyromonas gingivalis and Treponema denticola, they are referred to as the “red complex” residing in dental plaques. This bacterium is commonly bacteria associated with gingivitis, chronic periodontitis, and aggressive periodontitis.1 Additionally, it is a frequent pathogen in peri-implantitis, an inflammatory condition around dental implants.2 In individuals with periodontal infection and a risk of aspiration, anaerobic bacteria from the gingival crevice can enter the lower respiratory tract and cause lung abscess formation.3 Cases of lung abscesses caused by common periodontal pathogens, such as P. gingivalis,4 have been reported in the literature. However, there is currently no literature regarding lung abscesses primarily caused by T. forsythia infection. This article presents a case in which T. forsythia was identified as the main pathogen in a lung abscess following dental implantation. The characteristics of this pathogen, its clinical features, and its treatment are discussed in the following literature review.
Case Report
Medical History
A 55-year-old male with type 2 diabetes was hospitalized for an investigation of a persistent cough and purulent sputum for 1 month. His medications included repaglinide, metformin, and acarbose for glycemic control and amlodipine for a history of hypertension. He has been a heavy alcohol consumer for over 30 years, averaging half a liter of spirits, and is an ex-smoker with an over 140-pack-year history, although he has been smoke-free for the past 10 years.
The cough and production of yellow purulent sputum started 12 days after a dental implantation. The patient denied chest tightness, shortness of breath, nasal congestion, rhinorrhea, and sore throat. There was no history of night sweats or hemoptysis. He did not experience abdominal distension or diarrhea. A chest CT scan performed at an external hospital revealed a large cystic lesion in the left lung (with a fluid level suggestive of abscess formation and local extension), thickening of the left lower pleura, and a small amount of fluid in the left pleural cavity. A diagnosis of a lung abscess was made, and the patient commenced a course of levofloxacin without symptom improvement. Subsequently, he sought medical attention at our hospital.
Physical Examination
The observations made on patient examination during presentation were unremarkable. The patient was alert and oriented. No jaundice was observed in the skin or mucous membranes. On dental examination, there were porcelain bridges on the upper right area involving the first premolar, second premolar, first molar, and second molar, with grade 1 mobility and tenderness. No redness, swelling, or purulent discharge was observed in the teeth, and there were no fistulas or sinuses. There was no palpable superficial lymph node enlargement. Decreased breath sounds were noted on the left side, while coarse breath sounds were heard on the right side. No significant dry or wet rales were auscultated. The cardiac borders were normal, and without enlargement. The abdomen was soft and flat, and the liver and spleen were not palpable below the rib cage. Neurological examination was unremarkable.
Investigations
Complete blood count: White blood cell count was 9.8 × 10−9/L, neutrophils were 7.31 × 10−9/L, and procalcitonin was 0.097 ng/mL. Pus analysis: White blood cell count was 242,368 × 10−6/L. The white blood cells in the pus increased significantly, and were positive for the Lee–White test (++). The total protein in the pus was 59.2 g/L. Oxygen and anaerobic cultures of the pleural fluid for 5 days showed no bacterial growth. Chest CT scan (Figure 1): There was a large cystic lesion in the left lung (with fluid level suggestive of abscess formation, and localized lung involvement), thickening of the left diaphragmatic pleura, and minimal pleural effusion on the left side.
Treatment and Prognosis
Based on the chest CT findings, empiric antibiotic treatment was commenced (meropenem at 8 hourly). Ultrasound-guided percutaneous chest tube (8 French) placement was performed to drain the abscess fluid (Figure 1). Pus mNGS (Table 1) revealed predominant T. forsythia (sequence count: 52,577; relative abundance: 85.48%), P. gingivalis (sequence count: 3985; relative abundance: 6.48%), Streptococcus milleri (sequence count: 1258; relative abundance: 2.05%), Schaalia cardiffensis (sequence count: 631; relative abundance: 1.03%), and Treponema socranskii (sequence count: 437; relative abundance: 0.71%). Pathological examination showed numerous neutrophils, few mesothelial cells, and lymphocytes in the smears and sediment (Figure 1). In the context of recent dental implantation, a diagnosis of a primary odontogenic lung abscess predominantly caused by T. forsythia was made. The patient’s cough and sputum improved after 1 week of continuous drainage and treatment with meropenem. A follow-up chest CT showed reduced fluid and the formation of empyema (Figure 2). The patient was provided with a 2-week course of moxifloxacin on discharge. During a telephone follow-up 2 weeks later, the patient reported no symptom recurrence. After 4 weeks, the patient’s chest ultrasound revealed a small amount of pleural effusion (Figure 3). The patient’s clinical symptoms did not worsen after 2 weeks of discontinuation of medication.
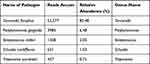 |
Table 1 The Result of mNGS |
 |
Figure 2 Follow-up chest CT after thoracic drainage and 1 week of meropenem anti-infective treatment. (A) Lung windows (B) Mediastinal windows. |
 |
Figure 3 Follow-up chest ultrasound indicated a small pleural effusion (as shown by the red arrow) after 4 weeks. |
Discussion
The current literature suggests that T. forsythia is a common agent of periodontal abscess,5 but available literature regarding abscesses caused by T. forsythia at other anatomical sites is limited. Animal experiments have demonstrated the ability of T. forsythia to induce skin abscesses.6–8 There are no reported cases in the literature describing lung abscesses caused primarily by T. forsythia. In this article, we present a case of lung abscess that developed after dental implantation. Following aspiration and drainage, high-throughput gene testing of the purulent fluid revealed T. forsythia as the predominant pathogen, along with coinfection by P. gingivalis, S. milleri, S. cardiffensis, and T. socranskii. The microbial profile of this infection is similar to the common pathogens associated with peri-implantitis,2 suggesting the lung abscess in this case was caused by an infection originating from the teeth.
T. forsythia has been challenging to detect using conventional culture techniques and is often overlooked.9 Research has shown that the culture detection rate of T. forsythia is low compared to PCR detection, suggesting that PCR techniques can be useful for epidemiological studies and clinical diagnosis of periodontal diseases.10 The low culture detection rate may be because the pathogen requires certain bacteria to provide nutrition or N-acetylmuramic acid to support its growth.11,12 In this case, pathogenic bacteria were not cultured from the purulent fluid, highlighting the importance of conducting high-throughput gene testing to determine the presence of dental-origin infection in patients with concomitant periodontal disease and lung abscess. This information can guide antibiotic selection.
Currently, mNGS is a very effective broad-spectrum pathogen screening method for cases where pathogen identification proves challenging. Several case reports have demonstrated that mNGS can assist in the clinical diagnosis of various infections, including leptospirosis,13 Scedosporium apiospermum,14 and Chlamydia psittaci;15 additionally, it contributes to clinical treatment by facilitating modifications to treatment protocols. In a large sample study on the diagnostic performance of mNGS in infectious diseases, it was concluded that mNGS outperformed traditional culture methods, especially in cases involving Mycobacterium tuberculosis, anaerobic bacteria, and fungi, and was less affected by prior antibacterial drug exposure;16 however, it also has some limitations. Given its unbiased and ultra-sensitive characteristics, an mNGS assay could produce a large number of sequences that match multiple microorganisms, which can be confusing. Moreover, it can produce false negative and false positive results and is expensive.17 In this case, we used antibiotics that covered the pathogens identified using mNGS, and we achieved good results. For patients whose pus cannot be cultured to detect pathogens, we recommend using the mNGS test.
T. forsythia may have an increased infection rate in immunocompromised individuals. A systematic review of 965 patients with HIV-associated periodontitis showed a high T. forsythia infection rate, reaching 51%, which was significantly higher than the rate of other common periodontal pathogens such as P. gingivalis and T. denticola.18 A study by Montevecchi et al showed that patients with diabetes and periodontal disease had a higher prevalence of T. forsythia than that of patients with periodontal disease without diabetes.19 Another study focusing on the salivary microbiota in type 2 diabetes found T. forsythia was enriched in type 2 diabetes.20 Therefore, it is suggested that T. forsythia may have a higher infection rate, increased virulence, and a greater tendency to act as a primary pathogen in immunocompromised individuals. However, further research is needed to confirm these observations.
The formation of abscesses in T. forsythia infections requires synergistic interactions with other oral pathogens. The growth of T. forsythia may be dependent on the nutritional support provided by other pathogens.11,12 Animal experiments have shown that when T. forsythia is co-inoculated with Fusobacterium nucleatum or P. gingivalis, significant abscess formation occurs, whereas single inoculation of F. nucleatum, P. gingivalis, or T. forsythia strains do not lead to significant abscess formation.7 Research by Yoneda et al has demonstrated a pathogenic synergistic effect between T. forsythia and P. gingivalis strains.8 Studies have also found a frequent co-detection of T. forsythia and P. gingivalis, and when cultured together, it was observed that the virulence and invasiveness of T. forsythia were enhanced. This may be attributed to the tissue-degrading enzymes produced by P. gingivalis, which exposes the extracellular matrix surface, allowing T. forsythia to adhere through its BspA protein, thus promoting the occurrence and progression of the disease.21
T. forsythia infection has also been implicated in the development of other diseases. Research has shown that T. forsythia and P. gingivalis can cause damage and destruction to host cells and tissues by producing various enzymes and toxins, thereby promoting the development of esophageal cancer.22 It has also been reported to be associated with the formation of atherosclerotic cardiovascular disease in relation to periodontal infections caused by T. forsythia and other oral pathogens.23
Currently, there is limited information on the antibiotic susceptibility of T. forsythia.24 An in vitro study showed that T. forsythia is highly sensitive (100%) to moxifloxacin, while the resistance rates to amoxicillin, azithromycin, and metronidazole were 25.6%, 21.1%, and 25.6%, respectively. Furthermore, the study demonstrated that other periodontal pathogens, such as P. gingivalis and Actinomyces, exhibited complete sensitivity to moxifloxacin.25 Some studies have reported resistance of T. forsythia to tetracycline and macrolide antibiotics.22,26,27 Previous research has indicated that the common pathogens in periodontitis and peri-implantitis are red complex bacteria and Actinomyces species.2,28 A study on antibiotic resistance in peri-implantitis revealed high resistance of periodontal pathogens, such as P. gingivalis, Aggregatibacter actinomycetemcomitans, T. forsythia, and Prevotella intermedia, to tetracycline, clindamycin, metronidazole, erythromycin, and azithromycin.29 In the present case of lung abscess following dental implantation, the main pathogen identified was T. forsythia, along with mixed infection of P. gingivalis, Actinomyces, and other bacteria. The pathogen profile was similar to that commonly observed in periodontal diseases and peri-implantitis. Therefore, antibiotic selection should primarily target the common pathogens associated with periodontitis. In this case, the patient was treated with meropenem and moxifloxacin, with significant improvement in symptoms observed after drainage of the purulent fluid, and radiological improvement on follow-up chest CT scans. The duration of treatment for lung abscesses caused by anaerobic bacteria is not clear at present, but the reported duration of treatment varies from 21 to 48 days, depending on the patient’s clinical symptoms and radiological response, with longer duration required for lung abscesses caused by Actinomyces and Nocardia.30,31 After percutaneous transthoracic catheter drainage for lung abscess, the patient’s symptoms improved significantly. We continued intravenous and oral anti-infective treatment for a total of 21 days. No new discomfort was reported during the follow-up. We think early puncture drainage is more important than a long course of anti-infection treatment for the mass lung abscess. This may shorten the duration of anti-infective treatment and protect the lungs from the constant attack of bacteria.
Most patients with lung abscesses can be treated with antibiotics. When antibiotic treatment proves ineffective, drainage or surgical treatment is required.32 Siraj O. Wali33 believed that percutaneous catheter drainage is a safe and effective method for treating lung abscesses, especially in cases where antibiotic treatment has failed. A meta-analysis34 of 194 patients with lung abscesses revealed that 166 (86.5%) patients showed improvement after percutaneous catheter drainage and 17 (8.8%) patients developed catheter-related complications, including pneumothorax, empyema, bronchopleural fistula, and hemothorax. These complications were associated with the use of large-bore catheters sized > 14 French. The authors of the study believe that percutaneous catheter drainage is an effective method for the treatment of lung abscesses with a low complication rate. In this case, the patient’s symptoms did not improve after anti-infective therapy, and a CT indicated a massive lung abscess. After consulting with a thoracic surgeon, we used ultrasound to guide tube drainage treatment with an 8-French caliber tube combined with systemic anti-infective treatment, and the patient’s symptoms improved significantly. When there was no more pus drainage, we pulled out the thoracic drainage tube and conducted a follow-up chest CT 2 days later, which revealed the formation of empyema. This is inconsistent with the results of the meta-analysis by Lee Jong Hyuk et al.34 The puncture drainage may form a fistula in the lung, and while it may cause complications, timely removal of pus is crucial because it helps protect the unaffected lung tissue. As the patient did not report further discomfort, we changed to moxifloxacin anti-infection treatment and discharged the patient for follow-up.
There are some limitations in this medical record study. We did not obtain the chest CT that was reviewed after the long treatment because the patient was concerned about radiation. In addition, because mNGS testing for periodontitis pathogens was not performed during treatment, it was not possible to confirm the presence of the same pathogens around the implanted teeth as in the lung.
Conclusion
To the best of our knowledge, this case report is the first report of a lung abscess primarily caused by T. forsythia infection. The limited previous reports about T. forsythia may be attributed to the fastidious nature of this anaerobic bacterium and its demanding cultivation requirements. T. forsythia is more likely to become pathogenic in immunocompromised people, and PCR or mNGS is an effective method to prove its infection. There is a lack of information regarding the antibiotic susceptibility of T. forsythia. When it causes a huge lung abscess, we advise early percutaneous catheter drainage and anti-infective treatment, which covers common periodontal pathogens such as red complex bacteria and Actinomyces. Moxifloxacin may be a favorable option to treat these pathogens.25
Ethics Approval and Informed Consent
The study was performed in accordance with the principles stated in the declaration of Helsinki and approved by the Second Affiliated Hospital of Fujian Medical University Ethics Committee (number:【2023】 (325)).
Consent for Publication
The patient provided informed consent for publication of the case. We have obtained the consent of the institution (the Second Affiliated Hospital of Fujian Medical University) to publish the case details.
Acknowledgments
We gratefully acknowledge the support of patients who involved in this study.
Funding
This work was sponsored by the Fujian Provincial Natural Science Foundation (2021J01259) and the Quanzhou Science and Technology Program of China (2022NS079).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mohanty R, Asopa SJ, Joseph MD, et al. Red complex: polymicrobial conglomerate in oral flora: a review. J Fam Med Prim Care. 2019;8(11):3480–3486. doi:10.4103/jfmpc.jfmpc_759_19
2. Pérez-Chaparro PJ, Duarte PM, Shibli JA, et al. The current weight of evidence of the microbiologic profile associated with peri-Implantitis: a systematic review. J Periodontol. 2016;87(11):1295–1304. doi:10.1902/jop.2016.160184
3. Chung G, Goetz MB. Anaerobic infections of the lung. Curr Infect Dis Rep. 2000;2(3):238–244. doi:10.1007/s11908-000-0041-9
4. Tanaka A, Kogami M, Nagatomo Y, et al. Subcutaneous abscess due to empyema necessitans caused by Porphyromonas gingivalis in a patient with periodontitis. IDCases. 2022;27:e01458. doi:10.1016/j.idcr.2022.e01458
5. Ozbek SM, Ozbek A. Real-time polymerase chain reaction of “red complex” (Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola) in periradicular abscesses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(5):670–674. doi:10.1016/j.tripleo.2010.07.001
6. Bird PS, Shakibaie F, Gemmell E, et al. Immune response to Bacteroides forsythus in a murine model. Oral Microbiol Immunol. 2001;16(5):311–315. doi:10.1034/j.1399-302x.2001.016005311.x
7. Takemoto T, Kurihara H, Dahlen G. Characterization of Bacteroides forsythus isolates. J Clin Microbiol. 1997;35(6):1378–1381. doi:10.1128/jcm.35.6.1378-1381.1997
8. Yoneda M, Hirofuji T, Anan H, et al. Mixed infection of Porphyromonas gingivalis and Bacteroides forsythus in a murine abscess model: involvement of gingipains in a synergistic effect. J Periodont Res. 2001;36(4):237–243. doi:10.1034/j.1600-0765.2001.036004237.x
9. Siqueira JF, Rôças IN, Moraes SR, et al. Direct amplification of rRNA gene sequences for identification of selected oral pathogens in root canal infections. Int Endod J. 2002;35(4):345–351. doi:10.1046/j.1365-2591.2002.00485.x
10. Lau L, Sanz M, Herrera D, et al. Quantitative real-time polymerase chain reaction versus culture: a comparison between two methods for the detection and quantification of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythensis in subgingival plaque samples. J Clin Periodontol. 2004;31(12):1061–1069. doi:10.1111/j.1600-051X.2004.00616.x
11. Dzink JL, Smith CM, Socransky SS. Development of a broth medium for Bacteroides forsythus. J Clin Microbiol. 1987;25(5):925. doi:10.1128/jcm.25.5.925-.1987
12. Wyss C. Dependence of proliferation of Bacteroides forsythus on exogenous N-acetylmuramic acid. Infect Immun. 1989;57(6):1757–1759. doi:10.1128/iai.57.6.1757-1759.1989
13. Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–2417. doi:10.1056/NEJMoa1401268
14. Xiao W, Han P, Xu Z, et al. Pulmonary scedosporiosis in a patient with acute hematopoietic failure: diagnosis aided by next-generation sequencing. Int J Infect Dis. 2019;85:114–116. doi:10.1016/j.ijid.2019.05.033
15. Gu L, Liu W, Ru M, et al. The application of metagenomic next-generation sequencing in diagnosing Chlamydia psittaci pneumonia: a report of five cases. BMC Pulm Med. 2020;20(1):65. doi:10.1186/s12890-020-1098-x
16. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl 2):S231–S240. doi:10.1093/cid/ciy693
17. Dongsheng H, Ziyang L, Rui L, et al. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019;45(5–6):668–685. doi:10.1080/1040841X.2019.1681933
18. Valian NK, Ardakani MT, Mahmoudi S. Microbiological study of periodontal disease in populations with HIV: a systematic review and meta-analysis. Clin Lab. 2023;69(5). doi:10.7754/Clin.Lab.2022.220738
19. Montevecchi M, Valeriani L, Gatto MR, et al. Subgingival pathogens in chronic periodontitis patients affected by type 2 diabetes mellitus: a retrospective case-control study. J Periodontal Implant Sci. 2021;51(6):409–421. doi:10.5051/jpis.2100180009
20. Cena JA, Reis LG, de Lima AK, Vieira Lima CP, Stefani CM, Dame-Teixeira N. Dame-Teixeira Naile. Enrichment of acid-associated microbiota in the saliva of type 2 diabetes mellitus adults: a Systematic Review. Pathogens. 2023;2(3):404.
21. Sharma A, Sojar HT, Glurich I, et al. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect Immun. 1998;66(12):5703–5710. doi:10.1128/IAI.66.12.5703-5710.1998
22. Bartosz M, Anna W, Klaudia Z, et al. Tannerella forsythia: the role of and in pathogenesis of esophageal cancer. Infect Agent Cancer. 2019;14(1):3. doi:10.1186/s13027-019-0220-2
23. Marroquin TY, Guauque-Olarte S. Integrative analysis of gene and protein expression in atherosclerosis-related pathways modulated by periodontal pathogens. Systematic review. Jpn Dent Sci Rev. 2023;59:8–22. doi:10.1016/j.jdsr.2022.12.001
24. Dahlen G, Preus HR. Low antibiotic resistance among anaerobic Gram-negative bacteria in periodontitis 5 years following metronidazole therapy. Anaerobe. 2017;43:94–98. doi:10.1016/j.anaerobe.2016.12.009
25. Ardila CM, Bedoya-García JA. Antimicrobial resistance of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia in periodontitis patients. J Glob Antimicrob Resist. 2020;22:215–218. doi:10.1016/j.jgar.2020.02.024
26. Rams TE, Dujardin S, Sautter JD, et al. Spiramycin resistance in human periodontitis microbiota. Anaerobe. 2011;17(4):201–205. doi:10.1016/j.anaerobe.2011.03.017
27. Lakhssassi N, Elhajoui N, Lodter JP, et al. Antimicrobial susceptibility variation of 50 anaerobic periopathogens in aggressive periodontitis: an interindividual variability study. Oral Microbiol Immunol. 2005;20(4):244–252. doi:10.1111/j.1399-302X.2005.00225.x
28. Ursu RG, Iancu LS, Porumb-Andrese E, et al. Host mRNA analysis of periodontal disease patients positive for Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans and Tannerella forsythia. Int J Mol Sci. 2022;23(17):9915. doi:10.3390/ijms23179915
29. Ardila CM, Vivares-Builes AM. Antibiotic resistance in patients with peri-Implantitis: a systematic scoping review. Int J Environ Res Public Health. 2022;19(23):15609. doi:10.3390/ijerph192315609
30. Kuhajda I, Zarogoulidis K, Tsirgogianni K, et al. Lung abscess-etiology, diagnostic and treatment options. Ann Transl Med. 2015;3(13):183. doi:10.3978/j.issn.2305-5839.2015.07.08
31. Takayanagi N, Kagiyama N, Ishiguro T, et al. Etiology and outcome of community-acquired lung abscess. Respiration. 2010;80(2):98–105. doi:10.1159/000312404
32. Egyud M, Suzuki K. Post-resection complications: abscesses, empyemas, bronchopleural fistulas. J Thorac Dis. 2018;10(suppl 28):S3408. doi:10.21037/jtd.2018.08.48
33. Wali SO. An update on the drainage of pyogenic lung abscesses. Ann Thorac Med. 2012;7(1):3–7. doi:10.4103/1817-1737.91552
34. Lee JH, Hong H, Tamburrini M, et al. Percutaneous transthoracic catheter drainage for lung abscess: a systematic review and meta-analysis. Eur Radiol. 2022;32(2):1184–1194. doi:10.1007/s00330-021-08149-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

