Back to Journals » International Journal of General Medicine » Volume 15
Lumbar Spinal Involvement in Calcium Pyrophosphate Dihydrate Disease: A Systematic Literature Review
Authors Ben Tekaya A, Nacef L, Bellil M, Saidane O, Rouached L, Bouden S, Tekaya R , Mahmoud I, Abdelmoula L
Received 10 February 2022
Accepted for publication 27 April 2022
Published 6 October 2022 Volume 2022:15 Pages 7639—7656
DOI https://doi.org/10.2147/IJGM.S360714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Aicha Ben Tekaya,1,2 Lilia Nacef,1,2 Mehdi Bellil,2,3 Olfa Saidane,1,2 Leila Rouached,1,2 Selma Bouden,1,2 Rawdha Tekaya,1,2 Ines Mahmoud,1,2 Leila Abdelmoula1,2
1Rheumatology Department, Charles Nicolle Hospital, Tunis, Tunisia; 2Faculty of Medicine of Tunis, University Tunis El Manar, Tunis, Tunisia; 3Orthopedic Department, Charles Nicolle Hospital, Tunis, Tunisia
Correspondence: Aicha Ben Tekaya, Rheumatology Department, Charles Nicolle Hospital, Tunis, Tunisia, Tel +216 97850485, Email [email protected]
Background: Calcium-pyrophosphate-dihydrate-disease (CPPD) is a crystal-induced arthropathy. The lumbar-spinal involvement is rare and often under-diagnosed. This study aimed to report the case of a lumbar spine CPPD involvement and to perform a systematic review of clinical, imaging features of lumbar involvement in CPPD patients, and treatments that have been implemented.
Methods: This systematic review was conducted in accordance with the Preferred-Reporting-Items-for-Systematic-Reviews and Meta-Analyses (PRISMA) guidelines.
Results: One hundred and sixty-seven articles met the search criteria using electronic databases searches. We retained 28 articles (20 case reports, 2 case series, 1 family survey, 4 retrospective studies, and 1 prospective study) involving a total of 62 patients. The age ranged between 39 and 89 years old. Among patients with lumbar spine CPPD, 32 were women. The duration of symptoms varied between one day and 8 years. The affection has been discovered during back pain in most cases. In 5 studies, the diagnosis was made on histological specimens of patients operated on for another pathology. X-ray showed calcifications in 2 cases. CT-scan detected calcium deposit in 7 cases. MRI showed lesions going from the increased signal of the disk, to calcified or not-cystic lesion of the facet joints, an intramedullary mass mimicking a schwannoma. Histological examination established the diagnosis of CPPD in 21 patients in all studies. Medical treatment included NSAIDs, Colchicine, Interleukin-1-receptor-antagonist, and antibiotics. Surgery was performed on 13 patients and allowed to establish the histological diagnosis.
Conclusion: In the case of inflammatory back pain in elderly subjects, without an infectious gateway, diagnosis of CPPD should be considered, especially for patients with a history of spinal surgery or degenerative radiography changes. CT scan is more sensitive than conventional radiographs. The discovertebral biopsy is the Gold-Standard and should be performed whenever the diagnosis was uncertain. Treatment includes the medical and surgical components.
Keywords: chondrocalcinosis, calcium pyrophosphate dihydrate disease, spine, radiculopathy, sciatica
Background
Calcium pyrophosphate dihydrate disease (CPPD) is a pathology defined by a deposit of crystals of calcium pyrophosphate dihydrate in the joints. Spinal CPPD is present in different clinical pictures depending on its location. The location of the cervical spine is best known as crowned dens syndrome, but the locations can affect all parts of the spine. Clinical presentation could be acute, subacute, or chronic spinal pain. It can even mimic infectious spondylodiscitis in its spinal form. But often, it is of radiological discovery, where the deposition of CPPD crystals occurs within the hyaline cartilage and especially within fibrocartilages.1 Although CPPD has several radiographic features in common with primary osteoarthritis, the axial location of the calcifications is an important criterion of distinction.2
Thus, the clinical pictures can be diverse and mislead the clinician in his diagnosis. It is therefore important to know the different presentations of this pathology.
This study aimed to report the case of a lumbar spine CPPD location and to review clinical, imaging features of lumbar involvement in CPPD patients, and treatments that have been implemented.
Case Report
Written informed consent has been provided and signed by the patient to have the case details and any accompanying images published. A 73-year-old patient, with a history of multiple comorbidities, presented with chronic knee and shoulders pain. X-rays revealed calcifications of the cartilages and entheses in the knees, shoulders, and pelvis, as well as symphysitis. We made the diagnosis of CPPD and the patient was put on colchicine for 15 days with clinical improvement. Six months later, she complained of non-inflammatory L5 sciatica associated with neuropathic pain (DN4 score was 6/10), without signs of impingement. Plain radiography showed levoscoliosis and nonspecific degenerative findings (disc space narrowing, osteophyte formation). Lumbar CT scan disclosed calcifications of the interspinous ligaments, yellow ligament, anterior and posterior disc calcifications, and a focal median and left paramedian protrusion at the L5-S1 level, with cranial migration of 9mm (Figures 1–3). The patient was put on colchicine (1mg daily) and rehabilitation. Symptoms were decreasing and pain relief was reported. At 36 months of follow-up, she still complained of sciatica flare-ups.
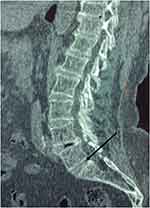 |
Figure 1 A CT-scan sagittal image shows linear calcification into the L3-L4, L4-L5 and L5-S1 (Arrow) intervertebral discs. |
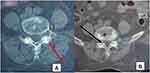 |
Figure 2 Axial CT-scan image of the lumbar spine, exhibiting calcification of the interapophyseal joint cartilage (Red Arrow) in (A) and ligamentum flavum (Black Arrow) in (B). |
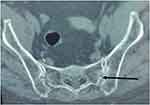 |
Figure 3 AxialCT-scan image of the pelvis showing linear bilateral calcification of the sacroiliac joint (Head arrow). |
Literature Review
Methods
Database and Search Strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
The search of articles was performed in PubMed, Google Scholar, Medline, Scopus, and Cochrane library databases. The following keywords were used: (“Chondrocalcinosis” OR “Calcium Pyrophosphate Dihydrate Disease”) AND (“Spine” OR “Osteoarthritis, Spine” OR “radiculopathy” OR “sciatica” OR “lumbar vertebrae”). Keywords referred to medical subject heading (MeSH). All references list of the retained papers were also screened manually for additional eligible studies. Titles, abstracts, and full reports of the identified articles were systematically screened.
Study Selection Criteria
Papers screened for eligibility were case reports, meta-analysis, systematic reviews, letters, cross-sectional, prospective and retrospective studies published in English or French that incorporate CPPD of the lumbar spine. Articles that treated other joints, cervical and thoracic spine were not included. Studies of more than 20 years were excluded.
Data Extraction and Quality Assessment
All selected articles were reviewed by two authors. Each article was analyzed through a critical reading platform: Fichas de LecturaCritica 3.0 to determine whether it fulfilled or not validity criteria. All data were extracted using a standardized template: title, author, type of the study, year of publication, and study population. For case reports, clinical presentation, imaging, biological findings, and treatment were mentioned. Only studies rated “High” or “Average” were selected. For cohort studies, we used the Newcastle Ottawa Scale (NOS). For case series and case reports, we used the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports.
Statistical Analysis
We used descriptive statistics to report data.
Results
Flow Chart of the Study
A total of 167 articles met the search criteria using electronic databases searches (PubMed, Google Scholar, Scopus, and Cochrane library databases manual research, references lists). We identified 110 studies after screening titles and abstracts and removing duplicates. After excluding the cervical and thoracic location and assessing the value quality of the studies, we retained 28 articles (20 case reports, 2 case series, 1 family survey, 4 retrospective studies, and 1 a prospective study) involving totally 62 patients. Among the studies selected, 4 screening studies with asymptomatic patients were performed on histological specimens (Figure 4).3–6 Detailed characteristics of all studies included are summarized in Table 1.
 |  |  |  |  |  |  |
Table 1 Overview of the Studies on Lumber CPPD |
 |
Figure 4 Flow chart of the study. |
Characteristics of the Patients
The age ranged between 39 and 89 years old. Among patients with lumbar spine deposits of CPPD, 32 were women.7–21 In the other studies, the epidemiological characteristics such as age and gender were not mentioned, as they concerned histological specimens of operated discs.3–6 A history of CPPD was present in 40 patients.17,20,22,23 Five patients had more than two comorbidities including diabetes, hypertension, coronary artery disease, hypothyroidism, gout, prostate cancer, stomach, and cervical tumor.8,11,14,18,24,25
Clinical Features
Duration of Symptoms and Background Circumstances
- The duration of symptoms varied between one day (acute back pain)10,14,15,22,26–28 and 8 years.9 The affection has been discovered during back pain in 22 cases.7,11,12,15–19,21,29,30 Sciatica has been observed in 13 patients.12,15–19,21,26,29,30 Neurological signs revealed the disease in 9 cases15–19,26,29,31,32 and consisted in dorsiflexion weakness,15–17,19,26 stiffness,31 hypesthesia,32 paresthesia,15,33 foot drop and numbness18 with residual urine sensation,29 urinary retention,15 and abnormal tendon reflexes.15 Fever was present in 6 cases.7,10,14,25,27,33 In two cases, the symptomatology led to a misdiagnosis of a urinary tract infection.10,27
- In 5 studies, the diagnosis was made on histological specimens of patients operated on for degenerative spine, canal stenosis, or disc disease.3–6,20 Moshrif et al diagnosed 24.3% of lumbar CPPD among 152 patients with a history of peripheral CPPD (knees, wrists, shoulders, hip, and pubic symphysis).20 Gruber et al found 14.69% lumbar CPPD among patients operated for herniated discs, degenerative disc disease, and recurrent disc herniation.20 In the study conducted by Yayama et al, 62/206 (30.1%) of the discs taken from patients operated on for asymptomatic narrow lumbar spine, and 37/64 (57.8%) in patients operated on for spondylolisthesis showed deposits of calcium pyrophosphate.5 Ariyawatakul et al led a retrospective study on lumbar spinal stenosis patients who had undergone a decompressive laminectomy. Histological examination identified eighteen patients among 34 that had CPPD.4
Diagnostic Method
Imaging
- Among the 62 patients, plain radiographs (X-ray) have been required in 13 cases,8,10–14,17–20,24,26–28,30 and showed calcifications in 2 cases.8,28 X-rays have mainly allowed diagnosing an underlying pathology such as degenerative spinal disease, spondylolisthesis, collapse fractures, or indirect signs of the narrow spinal canal.
- A CT scan has been prescribed in 15 cases,9,11–13,15–18,20,22,25–29 and has detected calcium deposit in 7 of them (61%).11–13,17,20,22,28 The lesions observed on CT scan were: calcium deposit in intervertebral joints,13,22,28,29 calcified cystic lesion,9,11 interspinous bursitis,13 calcification of the ligamentum flavum and the intervertebral disc,20 or degenerative joint disease.16 The CT scan was useful for performing a CT-guided biopsy for the histological diagnosis of the lesion.7,16,25 In two of the five cases where X-ray and CT scans were available, CT scan showed calcifications while plain radiographs were normal.11,13
- MRI: The elementary lesions observed in the MRI images were: increased signal within the intervertebral disk on the T1 and T2-weighted sequences,26 an increased signal of the lumbar facet joints compatible with inflammation,7,13,16,25,30 degenerative disc disease,8,14,24 calcified or not cystic lesion at the side of the facet joints,9,11,19,26,27 intramedullary mass mimicking a schwannoma,12,24 extradural heterogeneous mass-like lesion in the anterior epidural space.29 Spondylodiscitis/osteomyelitis was observed in 7 cases;7,10,16,21,22,25,28 and was associated to an image of epidural phlegmon in one case,26 Gadolinium-enhanced T1 hypersignal in two cases,13,27 intradural calcified lesions with no post-contrast enhancement in one case.17 The image of an abscess was found in 2 cases.22,25 A cord compression was reported in 6 cases.8,15,18,19,29,30 MRI showed calcifications or a chalky material in 4 cases.9,11,17,18
Histological Examination
Biopsy and Needle Aspiration
A disco vertebral biopsy was performed to eliminate spondylodiscitis based on the MRI features in 3 patients.7,16,25 A percutaneous soft tissue biopsy was performed in 3 cases.22,26,27 An aspiration of the facet joint effusion and the cerebrospinal fluid for bacteriological examination in 2 cases.14,15 Histological examination established the diagnosis of CPPD in 21 patients among all studies including the retrospective ones.8,9,11–19,21,22,24,25,27–30
Treatment
Medical Treatment
NSAIDs were prescribed in 5 cases7,10,13,16,27 with clinical and biological improvement. Colchicine was used in 2 cases and the symptomatology disappeared under treatment.16,22 Interleukin-1 receptor antagonist (Anakinra) was prescribed in one patient with clinical and biological improvement.28 Antibiotics (Ceftriaxone) were attempted in one case even if no microorganism was found, and the patient improved significantly.25 A low-dose steroid (Decadron) (6 mg every 6 hours, stopped at day 7) has been used in one case.16
Surgical Treatment
Surgery was attempted in 13 patients.8,9,11,12,17–19,21,24,26,29,30 In all cases, surgery was performed for an etiology other than CPPD: either for decompression, tumor resection, or laminectomy. Histological examination was performed on surgery specimens in patients who underwent surgical interventions for different pathologies. CPPD was found after tumor resection of a schwannoma-like mass,12,24 surgical decompression of a herniated disc8 or a compressing mass9,11,19,29 or radiographic bony destruction with sterile culture on biopsy,26 neurological symptoms requiring a laminectomy,17,18 spinal stenosis caused by a posterior bony protrusion from a collapsed vertebral body18 or a worsening discitis despite well-conducted medical treatment.21 Improvement after surgery was observed in 8 patients8,11,12,17–19,26,29 and not mentioned in one patient.24 A therapeutic failure was observed in two cases.21,30 In one case, after the failure of surgical decompression, a second surgery was performed (distal L4 and proximal L5 laminotomies followed by flavectomy and medial facetectomy to expose the cyst.).22
Discussion
This review aimed to evaluate the involvement of the lumbar spine in CPPD. Most of the studies included were case reports (n = 20), suggesting that this pathological feature is still uncommon. However, through this review, we were able to show that the axial CPPD is not only rare, but also underdiagnosed, regarding the non-specificity of clinical signs, and the variable performance of imaging techniques.
In our review, spinal deposition of calcium pyrophosphate crystals was reported in 62 patients. The diagnosis of axial CPPD was made in the majority of cases postoperatively, on histopathological specimens in patients who initially consulted for chronic lower back pain. In those studies, the prevalence of spinal CPPD varied between 14.7% and 57.8%. Most of the patients were women, aged between 39 and 89.
A cross-sectional study involving 2157 cases of CPPD in US veterans reported a point prevalence of 5.2 per 1000, all joints included, with an average age of 68 years and 95% of male prevalence.34 However, to our knowledge, the prevalence of lumbar spine CPPD has not been reported.
Among the case reports, most authors reported that a chronic symptom of CPPD in the lumbar spine which was lower back pain and radiculopathy due to compression of spinal nerves. CPPD of the lumbar spine was usually asymptomatic. Many of the patients with CPPD were elderly and have unrelated degenerative disease of the spine. However, CPPD can produce severe degenerative disk disease, often involving multiple levels.35 The thoracic and lumbar regions are often affected, especially at the L2-L3 disc level.36
As the clinical features are not specific, imaging is required for diagnosis. The radiographic presentation of spinal CPPD can vary from simple calcium deposit to spinal masses, tophi, and hematomas causing compressive phenomena. Its diagnosis can be challenging because of this radiographical ambiguity and its relationship to osteoarthritis.21 In this review, conventional radiographs showed disc calcifications in only 2 out of 13 patients. Studies have shown that arthropathy can precede radiographically detectable cartilage calcification, and the calcification may not always be dense enough to be visualized on conventional radiographs, or it may be difficult to identify if the joint is severely damaged.37 In one study of 3228 patients undergoing knee arthroscopy, a radiographic diagnosis ofkneeCPPDwasmade in only 4% of patients with pathologically proved CPPD crystal deposition.38
A systematic literature review and meta-analysis supported the high specificity and low sensitivity of conventional radiography in the diagnosis of CPPD and showed that ultrasound was more sensitive and less specific than plain radiographs in detecting CPPD.39
When the conventional radiography is non-effective, a CT scan may help to detect calcifications. In this review, CT scan showed calcifications while plain radiographs were normal in only two cases, but this can be explained by the fact that X-ray was not performed or not mentioned in 13 cases. In the study led by Moshrif et al, CT scan was more sensitive than conventional radiography in detecting intervertebral disc and vertebral ligament calcification.20
MRI showed calcifications or a chalky material in 4 cases.9,11,17,18 It has been reported that MRI was poorly sensitive to detect calcium deposits because CPP-containing tissues seem isointense to neural tissue on T1 weighted images and iso to hyperintense on T2 weighted sequences but were able to reveal endplate and disc inflammation.20 Our review showed that MRI misled the diagnosis in several situations. Indeed, the hyper signal related to the edema and the juxta-articular collections related to calcified cysts were confused with an infection and epidural abscesses. For example, in the case described by Petit et al, MRI showed short-tau inversion recovery (STIR) hyper signal on the lumbar facet joints.7 Grobost et al reported the appearance of L4-L5 spondylodiscitis, with epidural abscesses, Gadolinium-enhanced signal in L4-L5 zygapophysial joints, and intervertebral lumbar disks.22 In the case reported by Mikhael et al, MRI showed an increased signal within the L5-S1 intervertebral disk and the endplates of the L5 and S1 vertebrae on the T1 and T2-weighted sequences.26 Pazkad et al showed an increased signal consistent with inflammation.25 In this study, CPPD was complicated by authentic osteomyelitis and the patient improved under antibiotics.25 Another misleading image on MRI was compressive mass, which corresponds to a calcified cyst. MRI detected a cystic lesion at the side of the facet joints in 5 cases,9,11,19,26,27 an intramedullary mass mimicking a schwannoma in 2 cases,12,24 and an extradural heterogeneous mass-like lesion in the anterior epidural space in one case.29
It has been demonstrated that subchondral cysts are among the hallmarks of this arthropathy. The cysts usually are larger, more numerous, and more widespread than those in osteoarthritis. Cysts may form before cartilage loss is evident.35 This proves the diagnostic difficulty in front of a compressive mass on MRI when calcifications are not visible. In this case, the CT scan may be an alternative before proceeding with the biopsy.
The discovertebral biopsy, despite its invasive character,allowed to make the diagnosis in almost 21 patients.3,5–9,11,12,14,16–19,21,22,24–26,29,31 It often showed a fibrous tissue with positive birefringent crystal deposits.
CPPD crystals may be deposited in the ligamentum flavum and posterior longitudinal ligament, leading to myelopathy, cord compression, and spinal stenosis.40,41
In our review, surgery was attempted in all cases where there was a compressive mass, a degenerative spine with a surgery indication, or in the event of failure of medical treatment, and was always efficient.8,9,11,12,17–19,29,31–33,42 It consisted of a decompressive surgery in the majority of cases. As for pharmacological treatment, it consisted of NSAIDs, Colchicine, and Interleukin-1 inhibitor (Anakinra). Studies that have looked at the treatment of axial CPPD have compared it to that of gout. As both crystal diseases are mediated by IL-1-driven processes, the therapeutic intervention first targets acute inflammation consisting of Colchicine, NSAIDs, and glucocorticoids.43
In a systematic review of the literature summarizing the management of CPPD, intravenous Colchicine demonstrated efficacy in pain and seizure reduction. Methotrexate was effective over an average duration of 7.4 weeks of treatment. Hydroxychloroquine was efficient in 85% of patients, with 50% responding within a month. Significant improvement in pain and objective assessment was observed with Magnesium Carbonate supplementation, with a pronounced placebo effect. Anakinra efficiency was also reported. ACTH analogues led to the resolution of pseudogout attacks within 4.2 days. Tocilizumab improved disease activity in all patients after 3 months-treatment but had some adverse effects.43
Clinical experience supports the effectiveness of NSAIDs in CPPDarthritis. However, to our knowledge, there are no clinical trials of NSAIDs in this condition. Despite this, they are recommended as one of the first-line treatments for acute CPP crystal arthritis.44 NSAIDs should be prescribed with caution, given the risk of toxicity and since patients with CPPD are usually elderly with multiple comorbidities. Low-dose steroids have been used in one case.16 This is particularly relevant in elderly individuals with comorbidities. No recent data has added to our knowledge of the use of Glucocorticoids in CPPD.44
The major advance in the management of CPP crystal arthritis over the last decade has been the use of biological agents. Martinon et al demonstrated that CPP crystals are capable of activating the inflammasome NLR-P3- caspase1-interleukin-1beta (IL-1b) pathway present in innate immune cells such as neutrophils.45 Three IL-1b blocking agents are currently authorized for humans by the FDA: a monoclonal antibody against IL-1b (canakinumab); a recombinant IL-1 receptor antagonist (anakinra); and a dimeric fusion protein of IL-1 receptor and IL-1 receptor accessory protein (rilonacept).
Anakinra use was described in cervical and thoracic spine CPPD, in association with Colchicine is a 3-days course and the patient improved.33 In peripheral arthritis, Anakinra showed efficiency in decreasing pain and was well-tolerated.46–49
In our review, it was used in only one study, with a clinical and biological improvement.28
Conclusion
CPPD of the lumbar spine is an underdiagnosed pathology due to its nonspecific clinical features and its radiographic ambiguity, often associated with degenerative changes.
Through this review, we tried to identify the most frequent clinical and imaging features, but also the misleading clinical signs and diagnostic pitfalls of this disease (Table 2). In case of inflammatory back pain in elderly subjects, without an infectious gateway, the diagnosis of CPPD should be systematically considered, especially if the patient has a history of spinal surgery or degenerative changes on standard radiography. The absence of calcifications on conventional radiographs does not rule out CPPD. The CT scan is more sensitive than conventional radiographs to visualize calcifications and should be requested in case of doubt.
 |
Table 2 Summary of the Most Common and Misleading Clinical and Imaging Features in CPPD |
When MRI is indicated, because of neurological or infectious signs, the diagnosis of CPPD is still appropriate, even if the images are in favor of spondylodiscitis or osteomyelitis, since it can masquerade calcifications. Lumbar spine CPPD can even mimic a compressive cystic mass. In these two cases, the discovertebral biopsy is the Gold Standard and should be performed whenever the diagnosis is uncertain. It shows, in most cases, deposits of birefringent crystals in polarized light. The treatment is not well-established and includes a medical and a surgical component. As for the pharmacological treatment, NSAIDs (to be used with caution), or low-dose glucocorticoids could be efficient. Interleukin 1 inhibitors are an innovative alternative given their mechanism of action. Finally, surgery is useful when CPPD is associated with degenerative phenomena, compressive cysts or if it is responsible for spine stenosis.
Disclosure
The authors report no conflict of interest in this work.
References
1. Parperis K, Carrera G, Baynes K, et al. The prevalence of chondrocalcinosis (CC) of the acromioclavicular (AC) joint on chest radiographs and correlation with calcium pyrophosphate dihydrate (CPPD) crystal deposition disease. Clin Rheumatol. 2013;32:1383–1386. doi:10.1007/s10067-013-2255-x
2. Rosenthal AK, Ryan LM, Campion EW. Calcium pyrophosphate deposition disease. N Engl J Med. 2016;374(26):2575–2584. doi:10.1056/NEJMra1511117
3. Gruber HE, Norton HJ, Sun Y, Hanley EN. Crystal deposits in the human intervertebral disc: implications for disc degeneration. Spine J. 2007;7:444–450. doi:10.1016/j.spinee.2006.08.015
4. Ariyawatkul T, Pichaisak W, Chavasiri C, Vamvanij V, Wilartratsami S, Luksanapruksa P. The role of calcium pyrophosphate dihydrate deposition in the postoperative outcome of lumbar spinal stenosis patients. Asian Spine J. 2019;13:1001–1009. doi:10.31616/asj.2018.0280
5. Yayama T, Baba H, Furusawa N, et al. Pathogenesis of calcium crystal deposition in the ligamentum flavum correlates with lumbar spinal canal stenosis. Clin Exp Rheumatol. 2005;23(5):637.
6. Pytel P, Wollmann RL, Fessler RG, Krausz TN, Montag AG. Degenerative spine disease: pathologic findings in 985 surgical specimens. Am J Clin Pathol. 2006;125:193–202. doi:10.1309/89FVRT04EGBVEUD9
7. Petit H, Marcellin L, Chatelus E. Lumbar spine chondrocalcinosis. J Rheumatol. 2017;44:1288–1289. doi:10.3899/jrheum.161452
8. Baty V, Prost B, Jouvet A, Laurent J, Vallée B. Acute spinal cord compression and calcium pyrophosphate deposition disease. J Neurosurg. 2003;99:240. doi:10.3171/spi.2003.99.2.0240
9. Gadgil AA, Eisenstein SM, Darby A, Cassar Pullicino V. Bilateral symptomatic synovial cysts of the lumbar spine caused by calcium pyrophosphate deposition disease: a case report. Spine. 2002;27:E428–E431. doi:10.1097/00007632-200210010-00024
10. Ujihara T, Yamamoto K, Kitaura T, et al. Calcium pyrophosphate deposition disease involving a lumbar facet joint following urinary tract infection. Intern Med. 2019;58:1787–1789. doi:10.2169/internalmedicine.2099-18
11. Bin Mohamed Namazie MR, Fosbender MR. Calcium pyrophosphate dihydrate crystal deposition of multiple lumbar facet joints: a case report. J Orthop Surg. 2012;20:254–256. doi:10.1177/230949901202000225
12. Amouzougan A, Vassal F, Peoc’h M, Marotte H, Thomas T. Calcium pyrophosphate deposition disease arthropathy–related sciatica. Arthritis Rheumatol. 2019;71:2099. doi:10.1002/art.41099
13. Wendling D, Martin M, Guillot X, Prati C. Interspinous bursitis and chondrocalcinosis. Joint Bone Spine. 2012;79:516. doi:10.1016/j.jbspin.2012.04.003
14. Fujishiro T, Nabeshima Y, Yasui S, Fujita I, Yoshiya S, Fujii H. Pseudogout attack of the lumbar facet joint: a case report. Spine. 2002;27:E396–E398. doi:10.1097/00007632-200209010-00028
15. Cameron CR, Burgess CD. Recurrent back pain and fevers. Med J Aust. 2007;186:208–209. doi:10.5694/j.1326-5377.2007.tb00864.x
16. Greca I, Ben Gabr J, Perl A, Bryant S, Zaccarini D. Trauma induced calcium pyrophosphate deposition disease of the lumbar spine. Case Rep Rheumatol. 2020;2020:1–5. doi:10.1155/2020/3218350
17. Cacciotti G, Novegno F, Fiume D. Calcium pyrophosphate dihydrate deposition disease of the filum terminale. Eur Spine J. 2013;22:501–505. doi:10.1007/s00586-013-2723-7
18. Lam H, Cheung K, Law S, Fung K. Crystal arthropathy of the lumbar spine: a report of 4 cases. J Orthop Surg. 2007;15:94–101. doi:10.1177/230949900701500122
19. Mahmud T, Basu D, Dyson PHP. Crystal arthropathy of the lumbar spine: a series of six cases and a review of the literature. J Bone Joint Surg Br. 2005;87-B:513–517. doi:10.1302/0301-620X.87B4.15555
20. Moshrif A, Laredo JD, Bassiouni H, et al. Spinal involvement with calcium pyrophosphate deposition disease in an academic rheumatology center: a series of 37 patients. Semin Arthritis Rheum. 2019;48:1113–1126. doi:10.1016/j.semarthrit.2018.10.009
21. Loizidis G, Stern J, Baker JF. When calcium pyrophosphate deposition disease masquerades as spinal infection. J Clin Rheumatol. 2019;25:e118–e122. doi:10.1097/RHU.0000000000000727
22. Grobost V, Vayssade M, Roche A, Kemeny J-L, Soubrier M. Axial calcium pyrophosphate dihydrate deposition disease revealed by recurrent sterile spondylodiscitis and epidural abscess. Joint Bone Spine. 2014;81:180–182. doi:10.1016/j.jbspin.2013.07.007
23. Béjia I, Rtibi I, Touzi M, Zrour S, Younes M, Naceur B. Familial calcium pyrophosphate dihydrate deposition disease. A Tunisian Kindred. Joint Bone Spine. 2004;71:401–408. doi:10.1016/j.jbspin.2003.10.012
24. Reis GF, Perry A. A 67-year-old man with a lumbar spine lesion: correspondence. Brain Pathol. 2014;24:547–548. doi:10.1111/bpa.12201
25. Pakzad K, Yang YJ, Ambrose JL, Landas SK. Diagnosis of calcium pyrophosphate dihydrate deposition disease by fine needle aspiration biopsy. Acta Cytol. 2002;46:46–49. doi:10.1159/000326715
26. Mikhael MM, Chioffe MA, Shapiro GS. Calcium pyrophosphate dihydrate crystal deposition disease (pseudogout) of lumbar spine mimicking osteomyelitis-discitis with epidural phlegmon. Am J Orthop. 2013;42(8):E64–E67.
27. Hakozaki M, Sekine T, Otani K, Konno S. Acute pseudogout lumbar discitis resembling acute pyelonephritis in an elderly woman. Intern Med J. 2019;49:1048–1050. doi:10.1111/imj.14390
28. Kleyer A, Knitza J, Schett G, Manger B. Calcium pyrophosphate deposition disease induced inflammatory back pain. Rheumatology. 2019;kez225. doi:10.1093/rheumatology/kez225
29. Lee J, Cho K-T, Kim E-J. Cauda equina syndrome caused by pseudogout involving the lumbar intervertebral disc. J Korean Med Sci. 2012;27:1591. doi:10.3346/jkms.2012.27.12.1591
30. Ogawa Y, Nagatsuma M, Kubota G, et al. Acute lumbar spinal pseudogout attack after instrumented surgery. Spine. 2012;37:E1529–E1533. doi:10.1097/BRS.0b013e31826b7977
31. Muthukumar N, Karuppaswamy U, Sankarasubbu B. Calcium pyrophosphate dihydrate deposition disease causing thoracic cord compression: case report. Neurosurgery. 2000;46:222–225. doi:10.1093/neurosurgery/46.1.222
32. Paolini S, Ciappetta P, Guiducci A, Principi M, Missori P, Delfini R. Foraminal deposition of calcium pyrophosphate dihydrate crystals in the thoracic spine: possible relationship with disc herniation and implications for surgical planning. J Neurosurg. 2005;2:75–78. doi:10.3171/spi.2005.2.1.0075
33. Bridges KJ, Bullis CL, Wanchu A, Than KD. Pseudogout of the cervical and thoracic spine mimicking infection after lumbar fusion: case report. J Neurosurg. 2017;27:145–149. doi:10.3171/2016.12.SPINE16979
34. KleiberBalderrama C, Rosenthal AK, Lans D, Singh JA, Bartels CM. Calcium pyrophosphate deposition disease and associated medical comorbidities: a national cross-sectional study of US veterans: CPDD in US veterans. Arthritis Care Res. 2017;69:1400–1406. doi:10.1002/acr.23160
35. Steinbach LS, Resnick D. Calcium pyrophosphate dihydrate crystal deposition disease: imaging perspectives. Curr Probl Diagn Radiol. 2000;29:206–229. doi:10.1016/S0363-0188(00)90014-8
36. Richards AJ, Hamilton EBD. SPINAL CHANGES IN IDIOPATHIC CHONDROCALCINOSIS ARTICULARIS. Rheumatology. 1976;15:138–142. doi:10.1093/rheumatology/15.3.138
37. Martel W, McCarter DK, Solsky MA, et al. Further observations on the arthropathy of calcium pyrophosphate crystal deposition disease. Radiology. 1981;141:1–15. doi:10.1148/radiology.141.1.6270724
38. Fisseler-Eckhoff A, Mu¨ller KM. Arthroscopy and chondrocalcinosis. Arthroscopy. 1992;8:98–104. doi:10.1016/0749-8063(92)90142-X
39. Cipolletta E, Filippou G, Scirè CA, et al. The diagnostic value of conventional radiography and musculoskeletal ultrasonography in calcium pyrophosphate deposition disease: a systematic literature review and meta-analysis. Osteoarthr Cartil. 2021;29:619–632. doi:10.1016/j.joca.2021.01.007
40. Wells CR, Morgello S, DiCarlo E. Cervical myelopathy due to calcium pyrophosphate dihydrate deposition disease. J Neurol Neurosurg Psychiatry. 1991;54:658–659. doi:10.1136/jnnp.54.7.658
41. Salcman M, Khan A, Symonds DA. Calcium pyrophosphate arthropathy of the spine. Neurosurgery. 1994;34:915–918. doi:10.1097/00006123-199405000-00022
42. Muthukumar N, Karuppaswamy U. Tumoral calcium pyrophosphate dihydrate deposition disease of the ligamentum flavum. Neurosurgery. 2003;53:103–109. doi:10.1227/01.NEU.0000068861.47199.A8
43. Reuss-Borst M, Tausche A-K. Gicht und Calciumpyrophosphat-Dihydrat-Arthropathie („Pseudogicht“) – ein Update. Dtsch med Wochenschr. 2018;143:1157–1166. doi:10.1055/a-0504-5684
44. Andrés M, Sivera F, Pascual E. Therapy for CPPD: options and Evidence. Curr Rheumatol Rep. 2018;20:31. doi:10.1007/s11926-018-0739-z
45. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi:10.1038/nature04516
46. Dumusc A, Pazar Maldonado B, Benaim C, Zufferey P, Aubry-Rozier B, So A. Anakinra compared to prednisone in the treatment of acute CPPD crystal arthritis: a randomized controlled double-blinded pilot study. Joint Bone Spine. 2021;88:105088. doi:10.1016/j.jbspin.2020.105088
47. Ben Tekaya A, Boukriba S, Fendri A, et al. Endothelial dysfunction and increased carotid intima-media thickness in patients with spondyloarthritis without traditional cardiovascular risk factors. RMD Open. 2022;8(2):e002270. doi:10.1136/rmdopen-2022-002270.4
48. Ben Tekaya A, Ben Hammamia M, Tekaya R, Ben Mrad M, Denguir R, Abdelmoula L. Endovascular repair of inflammatory aortic aneurysm. Tunis Med. 2017;95(12):229-232
49. Tekaya R, Ben Tekaya A, Sfar I, et al. Interleukin-1 gene polymorphisms in axial spondyloarthritis Tunisian patients. Tunis Med. 2020;98(12):986-991.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
