Back to Journals » Cancer Management and Research » Volume 11
LOX-1+ PMN-MDSC enhances immune suppression which promotes glioblastoma multiforme progression
Received 29 March 2019
Accepted for publication 6 June 2019
Published 2 August 2019 Volume 2019:11 Pages 7307—7315
DOI https://doi.org/10.2147/CMAR.S210545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
ErQing Chai,1,2,* Lan Zhang,3,* Changqing Li4
1Department of Neurosurgery, Gansu Provincial Hospital, Lanzhou 730000, People’s Republic of China; 2Cerebral Vascular Disease Center, Gansu Provincial Hospital, Lanzhou 730000, People’s Republic of China; 3Tuberculosis Prevention and Control Department, Gansu Province Center for Disease Control and Prevention, Lanzhou 730000, People’s Republic of China; 4Neurosurgery Department, Gansu University of Chinese Medicine, Lanzhou 730000, People’s Republic of China
*These authors contributed equally to this work
Background/aims: Patients with glioblastoma multiforme (GBM) that is the most common brain cancer in adults have a rather poor prognosis. The accumulation of immune suppressive myeloid-derived suppressor cell (MDSC) is negatively associated with clinical outcomes in various cancers. A recent study identified that lectin-type oxidized LDL receptor 1 (LOX-1) may serve as a specific marker of human polymorphonuclear neutrophil (PMN)-MDSC. Thus, herein we focused on exploring the role of LOX-1+ PMN-MDSC in GBM progression.
Methods: LOX-1, IFN-γ, dichlorodihydrofluorescein diacetate (DCFDA), CD15, CD4 and CD8 expression levels were examined by flow cytometry. ARG1 and iNOS expression levels in PMN were examined by quantitative real-time PCR. LOX-1 and CD15 expression levels in tumor tissue were determined by immunofluorescent microscopy. T cell proliferation was determined by 3H-thymidine incorporation.
Results: We identified a protumorigenic subset of PMN, which constitutively expressed LOX-1 and accumulated in the peripheral blood of GBM patients. Compared to LOX-1− PMN, the LOX-1+ PMN exhibited a PMN MDSC profile, with a significant increase in the expression of DCFDA, ARG1 and iNOS, and the capacity of inhibiting the CD3+ T cell proliferation in a dependent-ARG1/iNOS way. Additionally, we found that LOX-1+ PMN negatively correlated with effector immune cells in GBM patients, accumulated in GBM tissues, and was related to early recurrence and disease progression tightly.
Conclusion: Our study revealed that LOX-1+ PMN-MDSC inhibited the T cell proliferation to enhance immune suppression, which may play a key role in driving the GBM progression.
Keywords: MDSC, glioblastoma multiforme, immune suppression
Introduction
Glioblastoma multiforme (GBM) is the most common brain cancer in adults. Although surgical treatment, radiation, chemotherapy and immunotherapy for GBM patients have largely advanced in recent years, but the long-term prognosis of GBM patients remains poor, with a median survival of 14.6 months.1–6 High levels of immunosuppressive cytokines as well as the accumulation of Treg and myeloid-derived suppressor cells (MDSC) are two of major hallmarks of the GBM microenvironment,7–9 and they exert the accelerative effects on tumor progression. However, the precise roles in GBM progression are not fully understood yet.
Immune evasion is a major characteristic of cancer progression and a potent barrier to effective cancer immunotherapies.10 The accumulation of pathological MDSC with potent immunosuppressive activity usually occurs in cancer.11,12 Moreover, many studies suggested that the accumulation of immune suppressive MDSC was associated with poor prognosis in various cancers.13,14 Up to date, MDSC is divided into two large populations, including polymorphonuclear (PMN-MDSC) and monocytic cells (M-MDSC)15 In particular, PMN-MDSC is phenotypically and morphologically similar to neutrophils (PMN),16 and the most abundant population of MDSC in most types of cancers.17,18 Actually, it is always a puzzle to distinctly differentiate the PMN-MDSC form PMN in cancer tissues. Strikingly, a recent study reported that lectin-type oxidized LDL receptor 1 (LOX-1) may serve as a specific marker of human PMN-MDSC, and could be used to specifically identify PMN-MDSC.19 Therefore, in this study we focused on exploring the role of LOX-1+ PMN-MDSC in GBM progression.
Materials and methods
Processing of PB cells
Samples of PB were collected from patients Gansu Provincial Hospital. The study was approved by Institutional Review Boards of Gansu Provincial Hospital. All patients signed approved consent forms. Heparinized PB derived from GBM patients and the healthy subjects. Fifteen patients (13 men and 2 women) along with age and sex-matched controls were enrolled in this study (Table 1). Whole blood was enriched for PMNs using MACSxpress® Neutrophil Isolation Kit (Miltenyi) based on the protocol from the manufacturer. Cells were labeled using anti-Lox1-PE mAb (Biolegend) and then separated with anti-PE beads and MACS column (Miltenyi).
 |
Table 1 Demographic and baseline clinical characteristics of enrolled patients |
Mixed lymphocyte reaction
The isolation of CD4+ or CD8+ T cells from the PBMC of the same patients as LOX-1+ PMN was performed using human CD4+ or CD8+ T Cell Enrichment Column Kit (Miltenyi). PMNs were plated at different ratios with 105 T cells in a 96-well plate and then we added 1 μg/mL of soluble anti-CD3 (clone UCHT1; eBioscience) and 1Lμg/ml of anti-CD28 (clone CD28.2; eBioscience) into each plate. T cell proliferation was evaluated after 3-day culture using thymidine uptake. In parts of experiments, we added 1 μM NAC (Sigma) or 20 μM of Nω- hydroxy-nor-arginine (nor-NOHA; sigma) into the culture condition to block ROS or arginase I activity, respectively.
RNA isolation and quantitative real-time PCR
The isolation of total RNA from PMN was performed using TRIzol reagent (Takara, Dalian, People's Republic of China). Quantitative RT-PCR was conducted using Power SYBR Green PCR Master mix (Applied Biosystems, Shanghai, People's Republic of China) in the Bio-Rad CFX96 Real-Time System. The relative amount of mRNA was estimated using the comparative threshold cycle method, in which we chose β-actin as the reference gene. To analyze the gene expression, we used the following primers: ARGI–5ʹ-CTTGTTTCGGACTTGCTCGG-3ʹ; 5ʹ-CACTCTATGTATGGGGGCTTA-3ʹ, β-actin-5ʹ-CACGAAACTACCTTCAACTCC-3ʹ; 5ʹ- CATACTCCTGCTTGCTGATC-3ʹ, iNOS–5ʹ- CAGCGGGATGACTTTCCAA-3ʹ; 5ʹ- AGGCAAGATTTGGACCTGCA-3ʹ.
Immunofluorescent microscopy
Samples of cancer and non-cancer tissues were collected from patients Gansu Provincial Hospital. The study was approved by Institutional Review Boards of Gansu Provincial Hospital. All patients signed the approved consent forms. Cancer and non-cancer tissues were obtained from 20 GBM patients (Table 2). Immunofluorescent staining of GBM tissues was carried out using a two-step protocol. In as brief, sample sections were incubated at 4°C overnight using anti-human CD15 (CST), and LOX-1 (Life Technologies). The sections were then incubated for 30 mins at 37°C with a mixture of primary-antibody matched fluorescently labeled secondary antibodies.
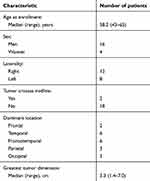 |
Table 2 Baseline features of patients with glioblastoma |
Flow cytometry
FITC-, PE-or APC-conjugated mouse anti-human CD45, CD15, CD8, CD4, IFN-γ, DCFDA and DAPI from BioLegend (San Diego, CA, USA) were applied for flow cytometry assays. The cells were collected and resuspended in 100 μL of PBS containing 0.1% BSA, and then stained using specific Abs for 30 mins over ice. The cells were washed using PBS containing 0.1% NaN3 and 0.5% BSA, and then fixed in 1% paraformaldehyde solution. Analyses were fulfilled using FACScan and CellQuest software (BD Biosciences Franklin Lakes, NJ, USA). To perform the intracellular staining, we added brefeldin A (5 mg/mL; Sigma-Aldrich) at the last 3 hrs. The cells were collected, washed and stained with monoclonal-specific antibodies for CD4 and CD8 for 20 mins over ice. After that, the cells were washed for two times in PBS, fixed and then permeabilized with fixation/permeabilization solution (eBioSciences, San Diego, CA, USA) for 15 mins at room temperature. Then, the cells were washed again and stained using monoclonal antibodies against IFN-γ for 20 mins at room temperature.
Statistics
Statistical analysis was conducted using a two-tailed Student’s t-test or Mann–Whitney test when the distribution of variables was determined. Correlations between parameters were estimated using Pearson’s correlation analysis. Statistical differences were considered as significant when P-value was <0.05. All statistical calculations were performed using GraphPad Prism 5 software (GraphPad Software Inc.). *P<0.05, **P<0.01, ***P<0.001
Results
The upregulation of LOX-1+ PMN frequency in GBM patients
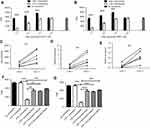
We collected and labeled cells in peripheral blood (PB) directly using leucocyte antibody CD45 and granulocyte-specific CD15 antibody, removed the dead cell interference with DAPI and then evaluated the LOX-1 expression levels among all CD45+CD15+ cells (Figure 1A). The frequency of LOX-1+ cells among all PMN in healthy donors was very low (0.58±0.09, N=15), while in GBM patients it increased to 6.88% (Figure 1B and C).
The identification of LOX-1+ PMN as a population of PMN-MDSC in GBM patient PB
The PMN-MDSC is featured by their capacity of suppressing T cell function. LOX-1− and LOX-1+ PMN were sorted directly from PB of GBM patients and used in T cell suppression assay. As shown in Figure 2A, LOX-1+ PMN significantly inhibited the CD4+ T cell proliferation compared to LOX-1− PMN. The similar results were observed in CD8+ T cell suppression assay (Figure 2B). The dichlorodihydrofluorescein diacetate (DCFDA) expression was determined using flow cytometry to assess the ROS production, and the results showed that LOX-1+ PMN had significantly higher level of ROS production than LOX-1− PMN (Figure 2C). The expressions of ARG1 and iNOS were evaluated by qPCR and from the results we found that iNOS expression level in PMN was much lower than ARG1 (Figure 2D), and there were significantly higher levels of ARG1 and iNOS expression in LOX-1+ PMN-MDSC than in LOX-1− PMN (Figure 2E). Moreover, our results showed that both N-acetylcysteine (NAC) and catalase significantly abrogated the suppressive activity of LOX-1+ PMN ( Figure 2F and G).Conversely, ARG1 inhibitor Nor-NOHA could abolish the suppressive activity of these cells (Figure 2F and G). Thus, these results confirmed that LOX-1+ PMN indeed represented a population of PMN-MDSC.
The inverse correlation of LOX-1+ PMN with effector immune cells in GBM patient PB
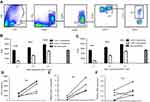
To determine the immune suppression function of LOX-1+ PMN in immune cells, we determine the association between the proportion of LOX-1+ PMN and effector T cells in GBM patient PB. The data revealed that GBM patients with higher proportion of LOX-1+ PMN had lower proportion of IFN-γ+ CD8+ or CD4+ T cells, whereas those with lower proportion of LOX-1+ PMN had higher proportion of IFN-γ+ CD8+ or CD4+ T cells (Figure 3A). The correlation analysis data showed that the proportion of LOX-1+ PMN was negatively correlated with IFN-γ+ CD8+ or CD4+ T cells (Figure 3B and C).
LOX-1 as a specific biomarker for a subset of PMN-MDSC in GBM tissues
We collected and labeled cells in cancer tissue directly using leucocyte antibody CD45 and granulocyte-specific CD15 antibody, removed the dead cell interference with DAPI and then evaluated the LOX-1 expression levels among all CD45+CD15+ cells. As shown in Figure 4A, GBM tissue had abundant LOX-1+ PMN. Using the identical method, we sorted LOX-1− and LOX-1+ PMN directly from the tumor tissue of GBM patients and evaluated the suppressive activity of these cells. As the results showed, LOX-1+ PMN inhibited the CD4+ or CD8+ T cell proliferation in comparison with LOX-1− PMN (Figure 4B and C). Additionally, we found that LOX-1+ PMN had significantly higher level of ROS production (Figure 4D), as well as higher expression of ARG1 and iNOS than LOX-1− PMN (Figure 4E and F).
The accumulation of LOX-1+ PMN in GBM tissues and its association with early recurrence and disease progression
We performed immuno-fluorescence staining using LOX-1 and CD15 antibody to assess the LOX-1 expression levels of PMN in 23 GBM specimens (paired non-tumor tissues, and intra-tumor tissues). The results showed that LOX-1+ PMN substantially accumulated in tumor tissues (Figure 5A and B) compared to no-tumor tissues. Importantly, using 6 months as the cutoff, GBM patients with a higher frequency of LOX-1+ PMN had early disease recurrence (Figure 5C). Thus, these findings indicated that LOX-1 selectively enriched in GBM tissues, which may contribute to early recurrence and disease progression in GBM patients.
Discussion
In this study, we identified a protumorigenic subset of PMN, which constitutively expressed LOX-1 and accumulated in the PB of GBM patients. Compared to LOX-1− PMN, LOX-1+ PMN highly expressed DCFDA, ARG1 and iNOS, and meanwhile could inhibit the CD3+ T cell proliferation in a dependent-ARG1/iNOS way. Additionally, we found that LOX-1+ PMN negatively correlated with effector immune cells in GBM patients, accumulated in GBM tissues, and was related to early recurrence and disease progression closely.
MDSC is a heterogeneous myeloid cell population including macrophage, granulocyte and other cells that express both Gr-1 and CD11b in mice.20 However, human MDSC appears to be more heterogeneous and has not been well described due to lack of specific markers.21 Generally, human MDSC does not express Lin and HLA-DR, but express CD11b and CD3322,23,24 and can be classified into PMN-MDSC and M-MDSC.25 A recent study revealed that LOX-1 may be a specific marker of human PMN-MDSC and LOX-1+ PMN was identified in various cancers including melanoma, colon cancer, non-small cell lung cancerand head and neck cancer.19 Similarly, we also found that the LOX-1+ PMN existed in GBM patient PB. Moreover, it was observed that the LOX-1+ PMN highly expressed DCFDA, ARG1 and iNOS, and could inhibit CD3+ T cell proliferation dependent on ARG1 and iNOS, as well as negatively correlated with effector immune cells in GBM patients. Thus, it may be reasonable to deduce that LOX-1+ PMN is an unrecognized immunosuppressive PMN-MDSC population in GBM patients.
High levels of immunosuppressive cytokines and the accumulation of Treg and MDSC are two major hallmarks of the GBM microenvironment.7–9 In gliomas, Treg infiltration is higher in GBM compared to other grade astrocytomas.7,26,27 Increased level of peripheral MDSC has been observed in GBM patients, and monocytes from healthy donors acquire MDSC characteristics when treated with conditioned media from GBM cell lines.22,28 In this study, we firstly identified an unrecognized immunosuppressive PMN-MDSC population, LOX-1+ PMN, and investigated the biological function and clinical relevance of those cells in GBM patients. Our study showed that LOX-1+ PMN was enriched in both GBM PB and tissues GBM patients, exhibited a PMN-MDSC profile, and negatively correlated with prognosis of GBM patients. Thus, we deduced that LOX-1+ PMN-MDSC may promote GBM progression through LOX-1+ PMN-induced enhancing immune suppression.
Recent studies have demonstrated that malignant lesions polarized macrophages and neutrophils within the tumor microenvironment and redirected them toward tumor-promoting phenotypes.25–27 For the majority of solid tumors, staging and prognosis are based upon direct tumor biopsies, which does not take the key information about the tumor microenvironment into account.28 In addition to surgical treatment, currently many other therapies including radiation therapy, chemotherapy and/or targeted therapy are also often utilized to deal with cancer patients alone or with a combination of surgery,5,29,30 which may affect the tumor microenvironment dynamically and extensively. Although neoadjuvant anti-PD-1 immunotherapy has been considered to improve prognosis in GBM patients,29 quite a part of cancer patients fail to respond to or quickly show resistance to this therapy,31 which may be associated with the reestablishment of the tumor microenvironment. In this study, we found that LOX-1+ PMN could inhibit T cell function, and was enriched in GBM tissues, suggesting that targeting LOX-1+ PMN may restore immune function in GBM patients, and probably improve the therapeutic effectiveness of neoadjuvant anti-PD-1 immunotherapy.
In summary, we identified a protumorigenic subset of PMN, which constitutively expressed LOX-1 and accumulated in the PB of GBM patients. Compared to LOX-1− PMN, the LOX-1+ PMN highly expressed DCFDA, ARG1 and iNOS, and restricted the CD3+ T cell proliferation in a dependent-ARG1/iNOS manner. Additionally, we found that LOX-1+ PMN negatively correlated with effector immune cells in GBM patients, accumulated in GBM tissues, and was related to early recurrence and disease progression. Therefore, our findings revealed that LOX-1+ PMN MDSC inhibited the T cell proliferation to enhance immune suppression, which may play a key role in driving the GBM progression.
Acknowledgments
This work is supported by the grants from the Natural Science Foundation of Gansu province (18JR3RA306) and the Science and Technology Bureau Foundation of Lanzhou city (2017-RC-57).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Minniti G, De Sanctis V, Muni R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88(1):97–103. doi:10.1007/s11060-008-9538-0
2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi:10.1056/NEJMoa043330
3. Poulsen HS, Urup T, Michaelsen SR, Staberg M, Villingshoj M, Lassen U. The impact of bevacizumab treatment on survival and quality of life in newly diagnosed glioblastoma patients. Cancer Manag Res. 2014;6:373–387. doi:10.2147/CMAR.S39306
4. Ren Z, Liang J, Zhang P, Chen J, Wen J. Inhibition of human glioblastoma cell invasion involves PION@E6 mediated autophagy process. Cancer Manag Res. 2019;11:2643–2652. doi:10.2147/CMAR.S200151
5. Syed M, Liermann J, Verma V, et al. Survival and recurrence patterns of multifocal glioblastoma after radiation therapy. Cancer Manag Res. 2018;10:4229–4235. doi:10.2147/CMAR.S165956
6. Wang J, Liang H, Sun M, et al. Delta-6-desaturase inhibitor enhances radiation therapy in glioblastoma in vitro and in vivo. Cancer Manag Res. 2018;10:6779–6790. doi:10.2147/CMAR.S185601
7. El Andaloussi A, Lesniak MS. CD4+ CD25+ FoxP3+ T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. J Neurooncol. 2007;83(2):145–152. doi:10.1007/s11060-006-9314-y
8. Perng P, Lim M. Immunosuppressive mechanisms of malignant gliomas: parallels at non-CNS sites. Front Oncol. 2015;5:153. doi:10.3389/fonc.2015.00153
9. Crane CA, Ahn BJ, Han SJ, Parsa AT. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro-oncology. 2012;14(5):584–595. doi:10.1093/neuonc/nos014
10. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
11. Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. doi:10.1146/annurev-med-051013-052304
12. Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol. 2013;91(8):493–502. doi:10.1038/icb.2013.29
13. Messmer MN, Netherby CS, Banik D, Abrams SI. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunol Immunother. 2015;64(1):1–13. doi:10.1007/s00262-014-1639-3
14. Wang PF, Song SY, Wang TJ, et al. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: A meta-analysis of 40 studies. Oncoimmunology. 2018;7(10):e1494113. doi:10.1080/2162402X.2018.1490854
15. Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi:10.4049/jimmunol.181.8.5791
16. Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012;144(3):250–268. doi:10.1016/j.clim.2012.06.003
17. Barrera L, Montes-Servin E, Hernandez-Martinez JM, et al. Levels of peripheral blood polymorphonuclear myeloid-derived suppressor cells and selected cytokines are potentially prognostic of disease progression for patients with non-small cell lung cancer. Cancer Immunol Immunother. 2018;67(9):1393–1406. doi:10.1007/s00262-018-2196-y
18. Romano A, Parrinello NL, La Cava P, et al. PMN-MDSC and arginase are increased in myeloma and may contribute to resistance to therapy. Expert Rev Mol Diagn. 2018;18(7):675–683. doi:10.1080/14737159.2018.1470929
19. Condamine T, Dominguez GA, Youn JI, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1:2. doi:10.1126/sciimmunol.aah6817
20. Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16(1):53–65. doi:10.1016/j.semcancer.2005.07.005
21. Millrud CR, Bergenfelz C, Leandersson K. On the origin of myeloid-derived suppressor cells. Oncotarget. 2017;8(2):3649–3665. doi:10.18632/oncotarget.12278
22. Raychaudhuri B, Rayman P, Ireland J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro-oncology. 2011;13(6):591–599. doi:10.1093/neuonc/nor042
23. Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11(7):802–807. doi:10.1016/j.intimp.2011.01.003
24. Kong YY, Fuchsberger M, Xiang SD, Apostolopoulos V, Plebanski M. Myeloid derived suppressor cells and their role in diseases. Curr Med Chem. 2013;20(11):1437–1444.
25. Solito S, Falisi E, Diaz-Montero CM, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118(8):2254–2265. doi:10.1182/blood-2010-12-325753
26. Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14(16):5166–5172. doi:10.1158/1078-0432.CCR-08-0320
27. Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi:10.1158/0008-5472.CAN-05-3773
28. Fujita M, Kohanbash G, Fellows-Mayle W, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71(7):2664–2674. doi:10.1158/0008-5472.CAN-10-3055
29. Chen Z, Xu N, Zhao C, Xue T, Wu X, Wang Z. Bevacizumab combined with chemotherapy vs single-agent therapy in recurrent glioblastoma: evidence from randomized controlled trials. Cancer Manag Res. 2018;10:2193–2205. doi:10.2147/CMAR.S173323
30. Hatoum A, Mohammed R, Zakieh O. The unique invasiveness of glioblastoma and possible drug targets on extracellular matrix. Cancer Manag Res. 2019;11:1843–1855.
31. O’Donnell JS, Hoefsmit EP, Smyth MJ, Blank CU, Teng MWL. The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clin Cancer Res. 2019. doi:10.1158/1078-0432.CCR-18-2641
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.