Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Lower Limb Arterial Ischemia: An Independent Risk Factor of Sudomotor Dysfunction in Type 2 Diabetes
Authors Lv Y, Yang Z, Xiang L, Yu M, Zhao S, Zhang X, Li R
Received 11 January 2023
Accepted for publication 17 March 2023
Published 28 March 2023 Volume 2023:16 Pages 883—891
DOI https://doi.org/10.2147/DMSO.S402797
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Yuhuan Lv, Zheng Yang, Linyu Xiang, Meng Yu, Subei Zhao, Xiaoru Zhang, Rong Li
Department of Endocrinology, the First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, People’s Republic of China
Correspondence: Rong Li, The Department of Endocrinology, the First Affiliated Hospital of Chongqing Medical University, YouYi Road 1#, Yuzhong District, Chongqing, People’s Republic of China, Email [email protected]
Background: As an early manifestation of diabetic peripheral neuropathy (DPN), sudomotor dysfunction significantly increases the risk of diabetic foot ulcer. The pathogenesis of sudomotor dysfunction is still unclear. Lower limb ischemia may be related to sudomotor dysfunction, but few studies have explored it. The purpose of this study is to explore the relationship between sudomotor function and comprehensive lower limb arterial ischemia including large arteries, small arteries and microvascular in type 2 diabetes mellitus (T2DM).
Patients and Methods: 511 T2DM patients were enrolled in this cross-sectional study. Sudomotor function was assessed qualitatively and quantitatively by Neuropad. Lower limb arterial ischemia was defined as any abnormality of the ankle brachial index (ABI), toe brachial index (TBI) or transcutaneous oxygen tension (TcPO2).
Results: In this study, 75.1% of patients had sudomotor dysfunction. Compared with normal sudomotor function, patients with sudomotor dysfunction had a higher incidence of lower limb arterial ischemia (51.2% vs 36.2%, p = 0.004). Similarly, compared with the non-arterial ischemia group, the proportion of sudomotor disorders was higher in the arterial ischemia group (p = 0.004). Low TBI and low TcPO2 groups also had a higher proportion of sudomotor disorders (all p < 0.05).Compare with normal groups, low ABI, low TBI, and low TcPO2 groups had lower Slop4 which quantitatively reflecting Neuropad discoloration. Arterial ischemia was an independent risk factor for sudomotor dysfunction [OR = 1.754, p = 0.024]. Low TcPO2 also independently increased the risk of sudomotor disorders [OR = 2.231, p = 0.026].
Conclusion: Lower limb arterial ischemia is an independent risk factor of sudomotor dysfunction. Especially below the ankle (BTA) small arteries and microvascular ischemia may also be involved in the occurrence of sudomotor disorders.
Keywords: sudomotor function, lower limb arterial ischemia, ankle brachial index, toe brachial index, transcutaneous oxygen tension
Introduction
Diabetic peripheral neuropathy (DPN) is one of the common complications of diabetes,1 and the most frequently observed form of DPN is distal symmetric polyneuropathy (DSPN).2 Sudomotor dysfunction, which is caused by impairment of unmyelinated sympathetic nerve fibers, could be detected in the early stage of DSPN.3 Notably, the anhidrosis, dryness, and fissures induced by sudomotor dysfunction are related to a high risk of diabetic foot ulcers (DFU). A cross-sectional study4 confirmed that sudomotor dysfunction assessed by sympathetic skin response (SSR) was associated with an almost 15 times higher risk of foot ulceration in comparison with normal subjects. Another 6-year follow-up study5 of 308 patients without DFU showed that the sudomotor dysfunction assessed by Neuropad increased the risk of DFU by nearly 3 times. Therefore, early detection and intervention of the sudomotor dysfunction are particularly significant for reducing the occurrence of DFU, even if the patients without overt neuropathic symptoms.
Lower limb ischemia is an important cause of DPN. Prolongation of distal motor latencies, decrease of motor and sensory nerve conduction velocities were found in 25 patients with chronic lower limb ischemia.6 Ziegler et al7 used the Michigan Neuropathy Screening Instrument (MNSI) to determine the presence of diabetes polyneuropathy and found that peripheral arterial disease (PAD) was an independent risk factor for polyneuropathy.
However, few studies have focused on the relationship between lower limb ischemia and sudomotor dysfunction caused by autonomic nerve damage. Argyriou8 et al discovered that significantly abnormal SSR in 25 patients with PAD presenting the Fontaine stage IIa compared with healthy subjects. Chahal et al9 revealed that sudomotor dysfunction was closely related to PAD evaluated by ankle brachial index (ABI) in 36 type 2 diabetic patients. Existing studies had small sample size, weak statistical power, and only included patients with major artery ischemia with decreased ABI.
The ABI is considered a diagnostic criterion for PAD, but it is susceptible to arterial calcification and only reflects the larger arteries ischemia below the knee (BTK).10 Other point-of-care devices (POCDs) such as toe brachial index (TBI) and transcutaneous oxygen tension (TcPO2) made up for the shortcomings of ABI. TBI is less affected by arterial calcification, and it reflects the ischemia of the distal small arteries below the ankle (BTA),11,12 which has been reported to be related to the occurrence of DFU.13 TcPO2 directly reflects tissue oxygenation and microvascular ischemia, which can distinguish between asymptomatic ischemic and non-ischemic states.14 The Neuropad is a non-invasive and convenient POCDs that evaluates the sweat gland secretion function.15 The sudomotor function can be evaluated qualitatively and quantitatively by using Neuropad. Consequently, the objective of this research is to explore the correlation of sudomotor function assessed by Neuropad with comprehensive lower limb arterial ischemia assessed by ABI, TBI and TcPO2 in type 2 diabetes mellitus (T2DM).
Materials and Methods
Study Population
511 inpatients were enrolled in this cross-sectional study at the First Affiliated Hospital of Chongqing Medical University. Inclusion criteria: 1) patients with the diagnosis of T2DM according to the American Diabetes Association (ADA) standards and 2) patients aged 18 to 80 years. Exclusion criteria included: 1) taking drugs that affect autonomic nerve function, such as β receptor blockers and antiarrhythmic drugs; 2) diabetic foot ulcers; 3) extensive foot skin lesions, plantar hyperkeratosis, and desquamation; 4) renal failure with estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 and hepatic failure with liver enzymes three times higher than normal; 5) acute and chronic infection; 6) malignant tumor; 7) cervical and lumbar diseases; 8) thyroid diseases; 9) mental and psychological illness; 10) taking medications that impact vascular function;11) serious arrhythmia and cardiac failure;12) acute thrombosis. This study was approved by the Research Ethics Committee of Chongqing Medical University, and was conducted in accordance with the Declaration of Helsinki. All participants have signed informed consent prior to the survey.
Clinical and Laboratory Assessment
Historical and anthropometric data were collected (including age, sex, duration of diabetes, body mass index (BMI), blood pressure, history of hypertension, smoking and drinking). For the routine biochemical variables, glycosylated hemoglobin (HbA1c) level was assessed by high-pressure liquid chromatography (Trinity Biotech, PremierHb9210, Ireland). The fasting serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured by an enzymatic assay (Wako Diagnostics, Tokyo, Japan). The thyroid-stimulating hormone (TSH) was measured by chemiluminescence (Modular DDP, Roche). The serum creatinine (Scr) was determined by enzymatic methods (Roche Diagnostic, Mannheim, Germany). The estimated glomerular filtration rate (eGFR) level was calculated using the simplified Modification of Diet in Renal Disease (MDRD) equation. The urinary albumin and creatinine concentrations were measured by the turbidimetric immunoassay and the enzymatic colorimetric method on an automatic analyzer (Hitachi 7600, Tokyo, Japan). The urinary albumin/creatinine ratio (ACR) was calculated as the urinary albumin (mg)/creatinine (g).
Peripheral Vascular Examination and Definition of Arterial Ischemia
ABI and TBI were evaluated using an automatic device (OMRON HBP-8000, Tokyo, Japan). The ABI is the ratio of the ankle artery pressure to the brachial artery systolic pressure, while the TBI is the ratio of the toe artery pressure to the brachial artery systolic pressure. Participants were rested in a supine position at a room temperature of 24 to 26°C, and then the brachial systolic pressure, ankle pressure, and toe blood pressure were measured simultaneously. The TcPO2 (PERIMED PF6000, Sweden) was measured in the forefoot at the dorsum of first intermetatarsal space with the transducer heated to 45°C when the patient was in the supine position. All measurement results were selected on the right side for analysis.
A low ABI was deemed to be an ABI <0.9,16 and a low TBI was <0.75.17 It was considered as a low TcPO2 if the TcPO2 was < 40 mmHg.18 We adopted an ABI < 0.9 or TBI < 0.75 or TcPO2< 40 mmHg as lower limb arterial ischemia.
Neuropad Test Procedure and Definition of Sudomotor Function Indicators
The Neuropad (miro Verbandstoffe GmbH, Germany) is an adhesive patch that assesses plantar sweat production by a chemical reaction manifested. Patients removed their shoes and socks, and rested in a supine position for at least ten minutes at room temperature 25°C. The patch was applied between the first and second metatarsal heads on the sole of the right foot, avoiding areas of hyperkeratosis. The Neuropad color change was assessed at 10 min. Sudomotor dysfunction was considered if the Neuropad patch remained blue after 10 minutes of application to the plantar surface of the foot, or if the patch was patchy but not homogeneous pink.19
In addition, we also quantitatively evaluated the Neuropad response. The Sudocheck (Norsda Medical instruments, China), a handheld chromatic aberration analyzer, was used to detect the chromatic aberration value of the patch pasted on the foot per minute. After the examination, the chromatic aberration value was exported to NORSDA-NCM-PC software, and the chromatic aberration curve was drawn. The change rate of the chromatic aberration value was calculated per minute using the SLOPE function. According to our previous study (NCT05347420), the slope of the chromatic aberration curve in the first 4 minutes (Slop4) was selected to represent the sudomotor function because it has the best diagnostic efficacy for DSPN. A higher slope indicated faster discoloration and a better sudomotor function.
Statistical Analyses
Continuous variables are shown as means ± SDs in the case of normally distributed parameters or medians (interquartile range) in case of non-normally distributed parameters. Categorical variables are represented as frequency (percentage). The data were compared between the two groups using the Student’s t-test for normal distributions and the Mann–Whitney U-test for non-normal distributions. The Chi-Square test was used to analyze the categorical date. The three groups were compared using a one-way ANOVA. Both a binary logistic regression analysis and a multivariate linear logistic regression analysis were used to analyze the associations between the sudomotor function and arterial ischemia. A two-sided p < 0.05 was considered statistically significant. The statistical analyses were performed using IBM SPSS version 24.0.
Results
Enrolled Patients
A total of 511 T2DM inpatients were analyzed in this study. Among them, 384(75.1%) were identified to have sudomotor dysfunction and 127(24.9%) were not. 242(47.4%) patients had lower limb arterial ischemia, including 7(2.4%) cases with low ABIs, 191(66.6%) cases with low TBIs, and 89 (31.0%) cases with low TcPO2s.
Characteristics of the Population and Comparison Between Patients with and without Sudomotor Dysfunction
Compared with normal sudomotor function, patients with sudomotor dysfunction had a higher incidence of lower limb arterial ischemia (51.2 vs 36.2%, p = 0.004). Additionally, the ages, duration of diabetes and Scr were higher (all p < 0.05), while the diastolic pressures and eGFR (all p < 0.05) were lower in the sudomotor dysfunction group (Table 1). There were no significant differences in sex, systolic pressure, BMI, HbA1c, TSH, TC, TG, LDL, HDL, or ACR between the two groups.
 |
Table 1 Primary Characteristics of Patients with and without Sudomotor Dysfunction |
Comparison of Groups Based on the Presence or Absence of Lower Limb Arterial Ischemia
Compared with the non-arterial ischemia group, the ABIs, TBIs, and TcPO2s were significantly lower in the arterial ischemia group (all p < 0.001). The prevalence of sudomotor dysfunction (80.9 vs 69.9%, p = 0.004) was significantly higher in the patients with arterial ischemia than those without arterial ischemia. In addition, the index that quantitatively reflects the sudomotor function, Slop4, was lower in the lower limb arterial ischemia patients than that in the control patients (8.08 ± 4.84 vs 10.42 ± 5.32, p < 0.001) (Table 2).
 |
Table 2 Comparison of Subjects Without vs with Lower Limb Arterial Ischemia |
Comparison of the Sudomotor Function Among the Different Types of Arterial Ischemia
Qualitative sudomotor function:
Compared with the normal TBI group, the low TBI group had a higher incidence rate of sudomotor dysfunction (80.6 vs 71.8%, p = 0.025). Similarly, the low TcPO2 group also had a higher incidence rate of sudomotor dysfunction compared with the control group (84.3 vs 71.8%, p = 0.016) (Table 3).
 |
Table 3 Sudomotor Function Data in the Different Types of Arterial Ischemia |
Quantitative sudomotor function:
The low ABI, low TBI, and low TcPO2 groups had lower Slop4 compared with the normal ABI, TBI, and TcPO2 groups (all p < 0.05) (Table 3).
Binary Logistic Regression Analysis of Sudomotor Function as a Categorical Variable
Using the sudomotor dysfunction as the dependent variable, the univariate logistic regression analysis (model crude) demonstrated that patients with lower limb arterial ischemia possessed an increased risk of sudomotor dysfunction [OR = 1.836, 95% CI: 1.214, 2.776, p = 0.004] (Table 4). In addition, for different types of arterial ischemia, low TBI and low TcPO2 also increased the risk of sudomotor dysfunction [OR=1.636, 95% CI: 1.069, 2.524, p = 0.026; OR=2.105 95% CI: 1.142, 3.880, p = 0.017]. According to the multivariate logistic regression analysis, after gender, age, BMI, duration of diabetes, diastolic pressures, HbA1c, eGFR, TG, ACR, hypertension, alcohol consumption, and smoking were adjusted, the lower limb arterial ischemia was an independent risk factor for sudomotor disorders (model 1 and model 2) (all p < 0.05). Similarly, a low TcPO2 also independently increased the risk of sudomotor disorders (model 1 and model 2) (p < 0.05).
 |
Table 4 Different Lower Limb Ischemia Indicators on Risk of Sudomotor Dysfunction |
Multiple Regression Analysis of Sudomotor Function as a Continuous Variable
Using the quantitative index Slop4 of sudomotor function as the dependent variable, we separately analyzed the linear correlations between the three indicators of arterial ischemia and the Slop4 (Table 5). After adjusting for the gender, age, BMI, duration of diabetes, diastolic pressures, HbA1c, eGFR and TG, the Slop4 was positively associated with the TBI (β = 0.091, p = 0.047) and TcPO2 (β = 0.107, p = 0.022). Each model has no collinearity problem (VIF < 5). However, the ABI did not show a relationship with the Slop4 (β = 0.059, p = 0.200).
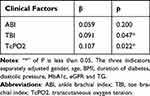 |
Table 5 Association of Different Types of Arterial Ischemia with Slop4 by Multivariate Linear Regression |
Association of the Sudomotor Function with the Severity of the Different Arterial Ischemia
The population was stratified according to the tertile of the ABI, TBI, and TcPO2. The Slop4 was significantly decreased in subjects in the lower TBI and TcPO2 tertile (p < 0.05) (Figure 1B and C). There was no significant difference of the Slop4 among the tertile of the ABI (Figure 1A).
Discussion
This cross-sectional study comprehensively evaluated arterial ischemia by combining the ABI, TBI, and TcPO2, and revealed that arterial ischemia was an independent risk factor for sudomotor dysfunction. Of note, this ischemia covered lesions from large arteries to microvessels. To the best of our knowledge, this is the first comprehensive study to confirm the relationship between lower limb arterial ischemia and sudomotor function, more importantly, BTA small arteries and microvascular ischemia may also be involved in the impairment of sudomotor function.
DPN1 is characterized by extensive clinical manifestations and long-term asymptomatic, which hinders the timely diagnosis of the disease. Intraepidermal nerve fiber density assessed by skin biopsy demonstrated that small nerve fibers innervating sweat glands were first damaged in DPN.20 Meanwhile, sudomotor dysfunction caused by damaged sweat gland fibers significantly increased the risk of DFU. In this study, the proportion of patients with sudomotor dysfunction assessed by Neuropad was relatively high (75%). Therefore, it is of great clinical significance to analyze the related risk factors of sudomotor dysfunction for its prevention and in turn decreases the incidence of DFU.
Previous studies have shown that PAD diagnosed by ABI was related to sudomotor dysfunction, and more patients with sudomotor dysfunction were found in PAD group compared with non-PAD group.8,9 However, in this research, the low ABI group was not associated with sudomotor dysfunction. The main reason may be related to the too small sample size of the low ABI group, only 7 people. Another potential reason is that ABI is affected by arterial calcification.21 Although an ABI > 1.3 is considered as arterial calcification in the guidelines, it does not mean that there is no calcification in an ABI from 0.9 to 1.3. Joachim et al22 stated that when the ABI < 1.3, 170 patients with type 1 diabetics had a very high proportion of calcification (49%). Moreover, patients with diabetic neuropathy are more prone to arterial calcification, called Mönckeberg’s sclerosis.23
In addition to ABI, TBI and TcPO2 are also commonly used POCDs for lower limb arterial ischemia. TBI is not affected by arterial calcification and can reflect BTA small vessels ischemia less than 3mm,24 which recognizes lower limb ischemia earlier than ABI. The cutoff value of TBI varies from 0.60 to 0.75. TBI<0.75 was selected here, because Dean’s study25 showed that TBI<0.75 had better diagnostic efficacy for DPN patients. TcPO2 reflects microvascular ischemia, and it can also reflect the perfusion of large and small vessels.14,26 However, it should be noted that TcPO2 decreases with the perfusion of large and small vessels only when the reductions in arterial perfusion are being so severe that they reduce tissue oxygen supply. This is due to in the case of mild perfusion reduction, the skin microvessels have the compensatory mechanism of post-ischemia hyperemia27 and a curvilinear relationship exists between TcPO2 values and local perfusion pressures,28 which is sufficient to maintain normal tissue oxygenation. There was no serious large and small arteries ischemia in the present research (ABI ≤ 0.4 or TBI ≤ 0.25), hence TcPO2 mainly reflected the blood supply of microvascular herein.
The combination of these three indicators was adopted in this study, which is helpful for early identification and comprehensive management of lower limb arterial ischemia. After excluding the relevant confounding factors, comprehensive arterial ischemia independently increased the risk of sudomotor disorders. In addition, lower Slop4 and higher incidence of sudomotor dysfunction observed in the low TBI and low TcPO2 groups. Binary logistic regression analysis demonstrated that low TcPO2 was the risk factor of sweating disorders. In the multiple linear regression, Slop4 was positively correlated with TBI and TcPO2. Above results signified that BTA small arteries and microvascular ischemia were also involved in the occurrence of sweating disorders. Therefore, any type of ischemia should not be ignored clinically, even BTA arteriolar and microvascular ischemia, because they could increase the risk of DFU by affecting the sweating function.
It was also found in this study that some patients with sudomotor dysfunction had normal lower limb arterial ischemia indicators (the proportion of sudomotor dysfunction and arterial ischemia was 75% and 47%, respectively), which reminds us that there are other factors involved in the damage of sweat gland fibers. Jeong-Beom et al29 concluded that poor sudomotor function was found in women and older patients. Z.-Q. Feng et al30 confirmed that time in range (TIR), an indicator of blood glucose control, was an independent risk factor for sudomotor dysfunction. A cross-sectional study31 proved that age, diabetes duration, foot deformities, and HbA1c level were associated with sudomotor function in patients with T2DM. Consistent with these studies, the sudomotor dysfunction group was older and had longer diabetes duration than the normal group in the present study, but there was no significant difference in HbA1c between the two groups. The potential reason is that the blood glucose level has been interfered by hypoglycemic drugs. Furthermore, patients with sudomotor dysfunction had poor renal function in this study, which may be due to the chronic accumulation of organic waste induced by the decline of glomerular filtration rate, triggering oxidative stress and over-generation of free radicals, thereby leading to autonomic nerve damage.32
The main strength of this study is that arterial ischemia was evaluated by ABI, TBI and TcPO2, which can more comprehensively elucidate the relationship between arterial ischemia and sudomotor dysfunction. Moreover, easily available POCDs were used to evaluate sudomotor function and lower limb arterial ischemia, which facilitated rapid clinical screening. Finally, the qualitative and quantitative evaluation of the sudomotor function enhanced objectivity in assessment, which was superior to the qualitative evaluation method. In fact, most previous studies only used the traditional visual Neuropad method.
There are some limitations in the present research. First, this was a cross-sectional study, the cause-and-effect between lower limb arterial ischemia and sudomotor function cannot be demonstrated. Second, skin nerve biopsy was not applied in this study to diagnose small fiber neuropathy, which is the gold standard for diagnosing small fiber neuropathy. Thirdly, ultrasound was not used as a means to evaluate the arterial ischemia to compare the diagnostic efficacy with non-invasive point-of-care indicators. Finally, the patients involved were all from a single center, bias of choice and information could not be avoided.
Conclusions
To sum up, this research preliminarily confirmed that lower limb arterial ischemia reflected by composite indicators is an independent risk factor for sudomotor dysfunction, even for BTA small arteries and microvascular ischemia. The present findings provide new insights and a theoretical basis for the intervention of lower limb arterial ischemia. In addition to patients with low ABI, patients with abnormal TBI or TcPO2 should also receive timely early intervention, such as providing foot nursing care, to reduce the incidence of sudomotor disorders and prevent the occurrence of DFU.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors declare that they have no competing interests.
References
1. Pop-Busui R, Boulton A, Feldman EL, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care. 2017;40(1):136–154.
2. Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep. 2019;19(10):86.
3. Vinik AI, Nevoret ML, Casellini C. The New Age of Sudomotor Function Testing: a Sensitive and Specific Biomarker for Diagnosis, Estimation of Severity, Monitoring Progression, and Regression in Response to Intervention. Front Endocrinol (Lausanne). 2015;6:94.
4. Tentolouris N, Marinou K, Kokotis P, Karanti A, Diakoumopoulou E, Katsilambros N. Sudomotor dysfunction is associated with foot ulceration in diabetes. Diabet Med. 2009;26(3):302–305.
5. Panagoulias GS, Eleftheriadou I, Papanas N, et al. Dryness of foot skin assessed by the visual indicator test and risk of diabetic foot ulceration: a prospective observational study. Front Endocrinol (Lausanne). 2020;11:625.
6. Weber F, Ziegler A. Axonal neuropathy in chronic peripheral arterial occlusive disease. Muscle Nerve. 2002;26(4):471–476.
7. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, Group KS. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3):464–469.
8. Argyriou AA, Tsolakis I, Papadoulas S, Polychronopoulos P, Gourzis P, Chroni E. Sympathetic skin response in patients with peripheral arterial occlusive disease. Clin Neurophysiol. 2006;117(2):414–419.
9. Chahal S, Vohra K, Syngle A. Association of sudomotor function with peripheral artery disease in type 2 diabetes. Neurol Sci. 2017;38(1):151–156.
10. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vasc Med. 2017;22(3):Np1–Np43.
11. Meloni M, Izzo V, Giurato L, Gandini R, Uccioli L. Below-The-ankle arterial disease severely impairs the outcomes of diabetic patients with ischemic foot ulcers. Diabetes Res Clin Pract. 2019;152:9–15.
12. Hoyer C, Sandermann J, Petersen LJ. The toe-brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg. 2013;58(1):231–238.
13. Manu CA, Freedman B, Rashid H, Winkley K, Edmonds ME. Peripheral Arterial Disease Located in the Feet of Patients With Diabetes and Foot Ulceration Demands a New Approach to the Assessment of Ischemia. Int J Low Extrem Wounds. 2020;2:1534734620947979.
14. Biro K, Sandor B, Kovacs D, et al. Lower limb ischemia and microrheological alterations in patients with diabetic retinopathy. Clin Hemorheol Microcirc. 2018;69(1–2):23–35.
15. Zografou I, Iliadis F, Sambanis C, Didangelos T. Validation of Neuropad in the Assessment of Peripheral Diabetic Neuropathy in Patients with Diabetes Mellitus Versus the Michigan Neuropathy Screening Instrument, 10g Monofilament Application and Biothesiometer Measurement. Curr Vasc Pharmacol. 2020;18(5):517–522.
16. Curry SJ, Krist AH, Owens DK, et al. Screening for Peripheral Artery Disease and Cardiovascular Disease Risk Assessment With the Ankle-Brachial Index US Preventive Services Task Force Recommendation Statement. JAMA-J Am Med Assoc. 2018;320(2):177–183.
17. Hinchliffe RJ, Forsythe RO, Apelqvist J, et al. Guidelines of the international writing group on the diabetic foot on diagnosis. Diabetes-Metab Res. 2020;36.
18. Fife CE, Smart DR, Sheffield PJ, Hopf HW, Hawkins G, Clarke D. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence. Undersea Hyperb Med. 2009;36(1):43–53.
19. Ponirakis G, Petropoulos IN, Fadavi H, et al. The diagnostic accuracy of Neuropad (R) for assessing large and small fibre diabetic neuropathy. Diabetic Med. 2014;31(12):1673–1680.
20. Ziegler D, Papanas N, Zhivov A, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63(7):2454–2463.
21. Lanzer P, Boehm M, Sorribas V, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35(23):1515–1525.
22. Ix JH, Miller RG, Criqui MH, Orchard TJ. Test characteristics of the ankle-brachial index and ankle-brachial difference for medial arterial calcification on X-ray in type 1 diabetes. J Vasc Surg. 2012;56(3):721–727.
23. Jeffcoate WJ, Rasmussen LM, Hofbauer LC, Game FL. Medial arterial calcification in diabetes and its relationship to neuropathy. Diabetologia. 2009;52(12):2478–2488.
24. Ferraresi R, Mauri G, Losurdo F, et al. BAD transmission and SAD distribution: a new scenario for critical limb ischemia. J Cardiovasc Surg (Torino). 2018;59(5):655–664.
25. Williams DT, Harding KG, Price P. An evaluation of the efficacy of methods used in screening for lower-limb arterial disease in diabetes. Diabetes Care. 2005;28(9):2206–2210.
26. Faglia E, Clerici G, Caminiti M, Quarantiello A, Curci V, Somalvico F. Evaluation of feasibility of ankle pressure and foot oximetry values for the detection of critical limb ischemia in diabetic patients. Vasc Endovascular Surg. 2010;44(3):184–189.
27. Rossi M, Carpi A. Skin microcirculation in peripheral arterial obliterative disease. Biomed Pharmacother. 2004;58(8):427–431.
28. Catella J, Long A, Mazzolai L. What Is Currently the Role of TcPO2 in the Choice of the Amputation Level of Lower Limbs? A Comprehensive Review. J Clin Med. 2021;10(7):89.
29. Lee JB, Kim JH, Murota H. Perspiration Functions in Different Ethnic, Age, and Sex Populations: modification of Sudomotor Function. Curr Probl Dermatol. 2016;51:109–119.
30. Feng ZQ, Guo QY, Wang W, et al. Time in range, especially overnight time in range, is associated with sudomotor dysfunction in patients with type 1 diabetes. Diabetol Metab Syndr. 2021;13(1):119.
31. Shivaprasad C, Amit G, Anish K, Rakesh B, Anupam B, Aiswarya Y. Clinical correlates of sudomotor dysfunction in patients with type 2 diabetes and peripheral neuropathy. Diabetes Res Clin Pract. 2018;139:188–194.
32. de Camargo CRS, Schoueri JHM, Alves BDA, da Veiga GRL, Fonseca FLA, Bocci MR. Uremic neuropathy: an overview of the current literature. Rev Assoc Med Bras. 2019;65(3):469–474.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

