Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Low Levels of Metrnl are Linked to the Deterioration of Diabetic Kidney Disease
Authors Chen J, Li ZY, Xu F, Wang CQ, Li WW, Lu J , Miao CY
Received 27 November 2023
Accepted for publication 15 February 2024
Published 27 February 2024 Volume 2024:17 Pages 959—967
DOI https://doi.org/10.2147/DMSO.S452055
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Jin Chen,1,2,* Zhi-Yong Li,2,* Fei Xu,2,* Chao-Qun Wang,1 Wen-Wen Li,1 Jin Lu,1 Chao-Yu Miao2
1Department of Endocrinology and Metabolism, Changhai Hospital, Second Military Medical University/Naval Medical University, Shanghai, People’s Republic of China; 2Department of Pharmacology, Second Military Medical University/Naval Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao-Yu Miao, Department of Pharmacology, Second Military Medical University/Naval Medical University, 325 Guo He Road, Shanghai, 200433, People’s Republic of China, Email [email protected] Jin Lu, Department of Endocrinology and Metabolism, Changhai Hospital, Second Military Medical University/Naval Medical University, 168 Chang Hai Road, Shanghai, 200433, People’s Republic of China, Email [email protected]
Objective: Diabetic kidney disease (DKD) is the leading cause of end-stage renal disease. Metrnl is a secreted protein that plays an important role in kidney disease. The aim of this study was to investigate DKD-related factors and the correlation between serum Metrnl levels and the severity of DKD.
Methods: Ninety-six type 2 diabetes mellitus (T2DM) patients and 45 DKD patients were included in the study. A range of parameters were measured simultaneously, including waist-to-hip ratio (WHR), body mass index (BMI), urinary albumin/creatinine ratio (UACR), monocyte–lymphocyte ratio (MLR), albumin/globulin (A/G), liver and kidney function, blood lipid profile, islet function, and others. Subsequently, the related factors and predictive significance of DKD were identified. The correlation between the relevant factors of DKD and serum Metrnl levels with DKD was evaluated.
Results: The duration of the disease (OR: 1.12, 95% CI: 1.01– 1.24, P=0.031), hypertension (OR: 4.86, 95% CI: 1.16– 20.49, P=0.031), fasting blood glucose (OR: 1.23, 95% CI: 1.03– 1.48, P=0.025), WHR (OR: 2.53, 95% CI: 1.03– 6.22, P=0.044), and MLR (OR: 1.91, 95% CI: 1.18– 3.08, P=0.008) are independent risk factors for DKD (P < 0.05). Conversely, A/G (OR: 0.13, 95% CI: 0.02– 0.76, P=0.024) and Metrnl (OR: 0.99, 95% CI: 0.98– 1.00, P=0.001) have been identified as protective factors against DKD. Furthermore, the level of Metrnl was negatively correlated with the severity of DKD (rs=− 0.447, P< 0.001). The area under receiver operating characteristic (ROC) curves for the diagnostic accuracy of Metrnl for DKD is 0.765 (95% CI: 0.686– 0.844).
Conclusion: The duration of the disease, hypertension, fasting blood glucose, WHR, and MLR are major risk factors for DKD. Metrnl and A/G are protective factors for DKD. Serum Metrnl concentrations are inversely correlated with DKD severity.
Keywords: diabetic kidney disease, urinary albumin/creatinine ratio, Metrnl, albumin/globulin
Introduction
Urinary albumin/creatinine ratio (UACR) is a recognized risk factor and a surrogate marker for microvascular disease, such as diabetic kidney disease (DKD).1 Clinically, DKD can be divided into micro- or macro-albumin according to UACR (30–300 mg/g or >300 mg/g).2 With the worldwide increase in diabetes prevalence, the number of DKD patients is expected to increase,3 and it will increase by about 50% in the next 24 years, from 537 million to 783 million.4
DKD is a severe complication of diabetes mellitus, and it is the main cause of end-stage renal disease (ESRD) worldwide.5,6 DKD severely reduces the quality of life of patients with long diabetic duration. Patients with DKD have higher risks of ESRD, cardiovascular disease (CVD), and even death compared to diabetic patients without kidney disease.7 Therefore, identifying the risk and protective factors for DKD is of great importance. The pathogenesis of DKD is extremely complicated, and its underlying molecular mechanism has not yet been thoroughly elucidated. During the occurrence and development of DKD, kidney cells, including glomerular membrane cells, podocyte, endothelial cells, smooth muscle cells, and inflammatory cells are affected by hyperglycemia.8 Many clinical biomarkers can reflect microvascular damage and are associated with the risk of microvascular disease development in T2DM patients. Inflammation has always been paid attention to the progress of DKD. Monocyte–lymphocyte ratio (MLR) is a novel inflammatory marker.9 There are studies reporting a significant correlation between monocytes and serum albumin in the circulation with proteinuria.10 Metrnl is a secreted protein expressed in various tissues of the human body, including the kidney. In 2014, Metrnl was reported as a novel adipokine with high expression in the subcutaneous white adipose tissue, and thus named as subfatin.11 Metrnl is involved in the formation of functional white fat. By generating adipose-specific Metrnl transgenic knockout and overexpression mice, it has been demonstrated that Metrnl plays an important role in improving insulin resistance induced by a high-fat diet or leptin deficiency, and promoting insulin sensitivity.12 Several studies have confirmed the protective effect of Metrnl in diabetes mellitus and coronary artery disease.13–16 However, few studies have examined the relationship between serum Metrnl and diabetic microvascular complications. Therefore, this study aimed to investigate whether serum Metrnl concentration is correlated with DKD.
Materials and Methods
Study Population
A total of 141 patients with T2DM, meeting the ADA diagnostic criteria, were admitted to the Department of Endocrinology at Shanghai Changhai Hospital. To be included in the study, participants had to meet the following diagnostic criteria for T2DM: (1) fasting plasma glucose ≥126 mg/dL (7.0 mmol/L). Fasting is defined as no caloric intake for at least 8 h; (2) 2-h plasma glucose ≥200 mg/dL (11.1 mmol/L) during Oral Glucose Tolerance Test (OGTT). The test should be performed as described by WHO, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water; (3) HbA1c ≥6.5% (48 mmol/mol). The test should be performed in a laboratory using a method that is National Glycohemoglobin Standardization Program (NGSP) certified and standardized to the Diabetes Control and Complications Trial assay; (4) In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥200 mg/dL (11.1 mmol/L). In the absence of unequivocal hyperglycemia, diagnosis requires two abnormal test results from the same sample or in two separate test samples. Participants were divided into two groups based on their UACR results: those with Normoalbuminuria (UACR < 30 mg/g, n = 96) and those with Albuminuria (UACR ≥ 30 mg/g, n = 45). Exclusion criteria included (1) acute complications such as lactic acidosis or diabetic ketoacidosis; (2) type 1 diabetes; (3) secondary diabetes mellitus; (4) severe heart, lung, and liver insufficiency; (5) mental diseases; (6) severe cerebrovascular diseases; (7) neoplasms or diseases of the blood system; (8) acute infection. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Changhai Hospital Ethics Committee. Written consent was obtained from all participants in the study. All the patients were fully informed about the purpose of the study.
Detection Index and Method
Upon admission, basic clinical data were collected, including sex, age, and diabetes history. All participants fasted for at least 8 hours overnight. The following morning, height, weight, waist circumference, and hip circumference were measured to calculate the body mass index (BMI) and waist-to-hip ratio (WHR). Fasting venous blood was collected, and a steamed bread meal test was conducted after at least 10 hours of fasting at night. Blood biochemistry and lipid profile were measured using an automatic biochemical instrument (Hitachi 7020), while fasting insulin (F-ins) and C-peptide (C-P) levels were determined by chemiluminescence (Roche). The ratio of serum albumin concentration to globulin concentration is albumin/globulin. Blood routine was determined by automatic blood cell analyzer (SYSMEX XN9000). The ratio of monocyte count to lymphocyte count is monocyte–lymphocyte ratio. The homeostasis model of insulin resistance index was used to evaluate the insulin resistance (IR) status of the patients [(fasting blood glucose (mmol/L) × fasting insulin (mIU/L))/22.5]. Serum samples were analyzed for Metrnl using a commercial ELISA kit (R&D Systems, USA).
Statistical Analysis
The Kolmogorov–Smirnov test was used initially to test the normality of the distribution of the data, with normally distributed data represented by mean ± standard deviation. The Student’s t-test and the Mann–Whitney U-test were used to analyze the samples with normal and non-normal data distributions, respectively. Categorical variables were analyzed using the Chi-square test. Multivariate logistic regression was used to determine the odds ratio (OR) values and 95% confidence intervals. The Spearman correlation was used to determine the correlation between serum Metrnl levels and UACR. For samples with non-normal distribution, differences between the three groups were assessed by the Kruskal–Wallis test to detect differences in serum Metrnl concentration in the severity of DKD. Receiver operating characteristic (ROC) curves were constructed, and the area under the curve (AUC) was calculated to assess the predictive power of the independent risk factors. All statistical analyses were performed using the SPSS 20.0 statistical software package. Statistical significance was defined as P values < 0.05.
Results
Characteristics of the Study Participants
A total of 141 patients with type 2 diabetes mellitus (T2DM) were included in the study. T2DM patients were divided into two groups: T2DM (n = 96) and DKD (n = 45). As shown in Table 1, the glycosylated hemoglobin (HbA1c) of all these T2DM patients exceeded 9%. The fasting blood glucose (FBG) of patients with DKD was significantly higher than that of patients without DKD (P<0.05). Patients with a longer duration of diabetic disease and high blood pressure are more likely to develop DKD. The WHR and MLR of patients with DKD were higher than those without DKD (all P<0.05). A/G, total bilirubin (TBIL), and serum Metrnl in patients without DKD were significantly higher than those with DKD (all P<0.05). There was no significant difference in other variables between the two groups (all P>0.05).
 |
Table 1 Clinical Characteristics of T2DM and DKD Patients |
Multivariate Logistic Regression Analysis of DKD
Multiple factor analysis was conducted to examine the correlation between each variable and the risk of developing DKD. The analysis revealed that the course of the disease, hypertension, WHR, FBG, and MLR were independent risk factors for DKD, and these variables were significantly correlated with DKD (all P<0.05). Additionally, it was found that A/G and serum Metrnl were protective factors for DKD (all P<0.05) (Table 2 and Figure 1). Forest map demonstrated A/G and serum Metrnl are protective factors more intuitively.
 |
Table 2 Regression Analysis of Risk Factors for DKD |
 |
Figure 1 Forest Map for Predicting DKD. The significance of the green line represents that the OR value is equal to 1. |
ROC Curve Analysis of Metrnl for DKD Prediction
The ROC curve analysis was performed to evaluate the predictive ability of serum Metrnl for DKD (Figure 2). When the optimal critical value was set at 404.92, the sensitivity and specificity were 0.573 and 0.844, respectively. Patients were categorized into three groups according to the UACR: normoalbuminuria (UACR <30mg/g), microalbuminuria (30 ≤ UACR ≤300mg/g), and macroalbuminuria (UACR >300mg/g). The analysis showed that there was a statistically significant difference in Metrnl among the three groups (P<0.05) (Figure 3).
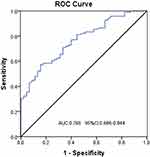 |
Figure 2 ROC Curve of Metrnl for Prediction of Diabetic Nephropathy. |
 |
Figure 3 Serum Metrnl level decreases as with the aggravation of diabetes nephropathy. ** P<0.001. |
Association of Serum Metrnl with UACR
In order to further verify the correlation between serum Metrnl and UACR, Spearman correlation coefficient was used to determine the correlation between serum Metrnl levels and UACR. The results revealed that there was a linear correlation between Metrnl and UACR (rs=−0.447), and the difference was statistically significant (P<0.001) (Figure 4). At the same time, no correlation was found between serum Metrnl levels and MLR (rs=−0.119, P=0.160) (Figure 5).
 |
Figure 4 Association of Metrnl with the Severity of DKD. |
 |
Figure 5 Association of Metrnl with MLR. |
Discussion
Our study analyzed 141 patients with type 2 diabetes. The main finding was that the albuminuria group had significantly lower serum Metrnl concentrations compared to the normoalbuminuria group. Logistic regression analysis demonstrated that the duration of diabetes, hypertension, WHR, MLR, and FBG were independent risk factors for DKD. Serum Metrnl level and A/G were found to be protective factors for DKD. Additionally, our study found that serum Metrnl level was associated with the severity of DKD, suggesting that Metrnl might be a promising new therapeutic target for DKD in patients.
DKD, kidney damage caused by diabetes, is due to metabolic disorder, hemodynamic dysfunction, inflammation, and fibrosis. Approximately 40% of patients with T2DM and 30% of those with type 1 diabetes mellitus (T1DM) develop DKD.3,17 In this study, we found that FBG was an independent risk factor for DKD. The proportion of DKD patients with hypertension was 86.67%, which highlights the need for clinicians to strengthen patient education and pay attention to comprehensive diabetes management.
Bilirubin, an important endogenous antioxidant,18 is negatively correlated with urinary protein and positively correlated with eGFR in patients with T1DM19 and T2DM20–22 as well as in the non-diabetic population.23,24 In 2021, the study investigating the association between serum bilirubin levels and the progression of albuminuria in Taiwanese patients with type 2 diabetes revealed that higher serum bilirubin levels were associated with a lower risk of albuminuria progression.25 Similar results were obtained in our present study that total bilirubin in DKD patients was significantly lower than those in the control group. Fujii et al26 found that bilirubin protected against the progression of DKD in diabetic rats with hereditary hyperbilirubinemia by inhibiting NOX-4.
We also found that the waist-to-hip ratio (WHR) is an independent risk factor for DKD. The possible mechanism is that central or visceral adipose tissue secretes inflammatory factors, such as tumor necrosis factor-α and interleukin-6, leading to glomerular endothelial dysfunction and increased urinary albumin excretion.27,28 The ratio of lymphocytes to monocytes (MLR) is a valuable predictor of DKD.29 Chronic inflammatory reactions caused by abnormal metabolism are closely related to the occurrence and development of diabetes and DKD. Various immune cells and inflammatory cells such as monocytes, lymphocytes, neutrophils, and various cytokines are involved in the occurrence and development of diabetes and DKD.30,31 Therefore, changes in leukocyte subtypes have attracted much attention in the diagnosis and treatment of DKD. In this study, the level of MLR was higher in patients with DKD compared to the control group, suggesting that DKD patients may show a more obvious inflammatory reaction. A/G is an antioxidant marker, and the level of A/G in the group with complications of diabetes decreased significantly compared to healthy subjects,32 suggesting that the A/G ratio is a protective factor for DKD patients. Currently, there is no research on the mechanism of A/G protecting DKD, which needs to be further studied.
In recent years, there have been numerous studies on the protective effect of serum Metrnl in diabetes, coronary heart disease, and other diseases. In this study, we characterized Metrnl as a novel renoprotective factor. Adipokines participate in the regulation of multiple physiological functions, including insulin sensitivity, immunity, and inflammation.33 Our lab has identified Metrnl as an adipokine that is abundantly expressed in rat, mouse, and human subcutaneous white adipose tissue, with relatively lower expression levels found in brown adipose tissue.11
We further demonstrate that transgenic mice overexpressing Metrnl specifically in adipocytes were protected from diet-induced insulin resistance. Metrnl-mediated insulin sensitization occurs through the PPARγ pathway. A number of clinical studies on diabetes have shown that serum Metrnl level in T2DM population is reduced, which is negatively correlated with HOMA-IR.15 Previous studies have demonstrated that serum Metrnl enhances insulin sensitivity and has a negative association with metabolic variables, including fasting insulin, FBG, HbA1C, and lipid levels.14,34 Previous research shows that Metrnl plays a role in regulating insulin resistance and inflammatory responses. Jung et al35 determined that skeletal muscle Metrnl improved insulin sensitivity at the cellular and animal levels. Therefore, Metrnl may be used as a novel therapeutic target for the treatment of T2DM.
Recently, Metrnl was shown to be significantly associated with the pathogenesis of coronary heart disease.13 In this study, we mainly enrolled patients with a long duration of hospitalization, and, therefore, the HOMA-IR was not calculated due to the influence of insulin use. Another study showed that the low level of serum Metrnl in patients with coronary heart disease was negatively correlated with the severity of CAD, and serum Metrnl may be a promising new therapeutic target for CAD.36 Metrnl may play a protective role in great vessel injury through a variety of mechanisms, such as attenuating MI/R injury-induced cardiomyocyte apoptosis by alleviating endoplasmic reticulum stress via activation of AMPK-PAK2 signaling in H9C2 cells.37 Hazem M et al's study showed that low serum Metrnl concentrations were associated with worsening of glucose tolerance, impaired endothelial function, and atherosclerosis.15 Metrnl knockout results in impaired vasodilation function by reducing the eNOS phosphorylation level at Ser1177 and inflammatory activation by enhancing the NF-kB pathway, thus increasing the susceptibility to atherosclerosis and leading to vascular endothelial dysfunction. Exogenous Metrnl can save endothelial dysfunction induced by Metrnl deficiency. Metrnl is a therapeutic target for endothelial dysfunction and atherosclerosis.38
Circulating Metrnl may also be affected by disease severity. We found a negative correlation between circulating Metrnl and albuminuria. Spearman correlation analysis revealed that the serum Metrnl level of DKD patients was negatively correlated with UACR. Metrnl is a potentially important new regulator of kidney metabolism to maintain cellular mitochondrial homeostasis and lipid accumulation in DKD via Metrnl-Sirt3-AMPK/UCP1 signaling in tubular epithelial cells (RTECs).39 The detailed mechanism accounting for serum Metrnl protecting diabetes-induced kidney injury is not entirely clear at present. A comprehensive explanation of why lower blood Metrnl is associated with and the exact function of Metrnl in diabetic kidney disease need to be explored further. The exact relationship needs to be further confirmed with more detailed, well-designed, and controlled clinical studies, especially cohort studies. Although several signaling pathways are regulated by Metrnl, Metrnl is still an orphan ligand. Reboll M et al found Metrnl as a KIT receptor ligand in the context of ischemic tissue repair.40
Our study should be interpreted within the context of its limitations. Firstly, the cross-sectional design of the study does not allow for causal inference. Secondly, the relatively small sample size may have underpowered the results. Thirdly, the DKD patients in our study were all inpatients, which introduces the possibility of selection bias and could confound the results. Additionally, since we only investigated Metrnl levels in Chinese patients, our findings need to be confirmed in other ethnic groups.
Conclusion
In conclusion, Metrnl was mainly expressed in renal tubules, and levels of Metrnl were significantly reduced in DKD patients. Metrnl plays a role in anti-inflammation, in consistent with previous studies reported by our lab. However, the role played by Metrnl in the kidney has not been fully explored. Although some studies have revealed the molecular mechanism of Metrnl in DKD, we need to further study its role.
Ethics Statement
Our study complies with all ethical regulations of the Changhai Hospital Ethics Committee and granted the ethical number: CHEC 2023-233.
Acknowledgments
The authors thank all research staff at the department of pharmacology and endocrinology and metabolism, for their participation in this study.
Funding
This work was supported by the National Natural Science Foundation of China Major Project (82030110 and 82330117) and Youth Initiation Fund of Naval Medical University (2021QN25).
Disclosure
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
1. Bakris G, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care. 2014;37:867–875. doi:10.2337/dc13-1870
2. Molitch M, DeFronzo R, Franz M, et al. Nephropathy in diabetes. Diabetes Care. 2004:S79–83. doi:10.2337/diacare.27.2007.s79
3. Alicic R, Rooney M, Tuttle K. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi:10.2215/cjn.11491116
4. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
5. Martínez-Castelao A, Navarro-González J, Górriz J, de Alvaro F. The concept and the epidemiology of diabetic nephropathy have changed in recent years. J Clin Med. 2015;4:1207–1216. doi:10.3390/jcm4061207
6. Saran R, Robinson B, Abbott K, et al. US renal data system 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidn Dis. 2017;69:A7–A8. doi:10.1053/j.ajkd.2016.12.004
7. Pálsson R, Patel U. Cardiovascular complications of diabetic kidney disease. Advan Chron Kid Dis. 2014;21:273–280. doi:10.1053/j.ackd.2014.03.003
8. Forbes J, Cooper M. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–188. doi:10.1152/physrev.00045.2011
9. Liu J, Liu X, Li Y, et al. The association of neutrophil to lymphocyte ratio, mean platelet volume, and platelet distribution width with diabetic retinopathy and nephropathy: a meta-analysis. Biosci Rep. 2018:38. doi:10.1042/bsr20180172
10. Cristancho C, Hemond C. Serum albumin modifies the effect of peripheral blood monocytes on severity of diabetic nephropathy in an adult population. Can J Diabetes. 2022;46:69–74. doi:10.1016/j.jcjd.2021.06.001
11. Li Z, Zheng S, Wang P, et al. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Ther. 2014;20:344–354. doi:10.1111/cns.12219
12. Li Z, Song J, Zheng S, et al. Adipocyte metrnl antagonizes insulin resistance through PPARγ signaling. Diabetes. 2015;64:4011–4022. doi:10.2337/db15-0274
13. Dadmanesh M, Aghajani H, Fadaei R, Ghorban K. Lower serum levels of Meteorin-like/Subfatin in patients with coronary artery disease and type 2 diabetes mellitus are negatively associated with insulin resistance and inflammatory cytokines. PLoS One. 2018;13:e0204180. doi:10.1371/journal.pone.0204180
14. Lee J, Kang Y, Kim J, et al. Serum Meteorin-like protein levels decreased in patients newly diagnosed with type 2 diabetes. Diabetes Res Clin Pract. 2018;135:7–10. doi:10.1016/j.diabres.2017.10.005
15. El-Ashmawy H, Selim F, Hosny T, Almassry H. Association of low serum Meteorin like (Metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Res Clin Pract. 2019;150:57–63. doi:10.1016/j.diabres.2019.02.026
16. Fadaei R, Dadmanesh M, Moradi N, et al. Serum levels of subfatin in patients with type 2 diabetes mellitus and its association with vascular adhesion molecules. Arch Physiol Biochem. 2020;126:335–340. doi:10.1080/13813455.2018.1538248
17. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394:1145–1158. doi:10.1016/s0140-6736(19)30427-1
18. Stocker R, Yamamoto Y, McDonagh A, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi:10.1126/science.3029864
19. Nishimura T, Tanaka M, Sekioka R, Itoh H. Serum bilirubin concentration is associated with eGFR and urinary albumin excretion in patients with type 1 diabetes mellitus. J diabet complicat. 2015;29:1223–1227. doi:10.1016/j.jdiacomp.2015.07.007
20. Toya K, Babazono T, Hanai K, Uchigata Y. Association of serum bilirubin levels with development and progression of albuminuria, and decline in estimated glomerular filtration rate in patients with type 2 diabetes mellitus. J Diabetes Invest. 2014;5:228–235. doi:10.1111/jdi.12134
21. Okada H, Fukui M, Tanaka M, et al. Low serum bilirubin concentration is a novel risk factor for the development of albuminuria in patients with type 2 diabetes. Metabolism. 2014;63:409–414. doi:10.1016/j.metabol.2013.11.011
22. Mashitani T, Hayashino Y, Okamura S, et al. Correlations between serum bilirubin levels and diabetic nephropathy progression among Japanese type 2 diabetic patients: a prospective cohort study (Diabetes distress and care registry at tenri [DDCRT 5]). Diabetes Care. 2014;37:252–258. doi:10.2337/dc13-0407
23. Targher G, Bosworth C, Kendrick J, et al. Relationship of serum bilirubin concentrations to kidney function and albuminuria in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001-2006. Clin Chem Lab Med. 2009;47:1055–1062. doi:10.1515/cclm.2009.244
24. Kawamoto R, Ninomiya D, Hasegawa Y, et al. Association between serum bilirubin and estimated glomerular filtration rate among elderly persons. PLoS One. 2014;9:e115294. doi:10.1371/journal.pone.0115294
25. Chan W, Tsai S, Li Y, et al. Association between serum bilirubin levels and progression of albuminuria in Taiwanese with type 2 diabetes mellitus. Biomedical Journal. 2021;44:201–208. doi:10.1016/j.bj.2019.12.004
26. Fujii M, Inoguchi T, Sasaki S, et al. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int. 2010;78:905–919. doi:10.1038/ki.2010.265
27. Fischer-Posovszky P, Wabitsch M, Hochberg Z. Endocrinology of adipose tissue - an update. Hormone Metab Res. 2007;39:314–321. doi:10.1055/s-2007-976539
28. Lee J, Lee S, Zhang H, et al. Interaction of IL-6 and TNF-α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS One. 2017;12:e0187189. doi:10.1371/journal.pone.0187189
29. Huang Q, Wu H, Wo M, et al. Monocyte-lymphocyte ratio is a valuable predictor for diabetic nephropathy in patients with type 2 diabetes. Medicine. 2020;99:e20190. doi:10.1097/md.0000000000020190
30. Tesch G. Diabetic nephropathy - is this an immune disorder? Clin Sci. 2017;131:2183–2199. doi:10.1042/cs20160636
31. Lim A, Tesch G. Inflammation in diabetic nephropathy. Mediators Inflammation. 2012;2012:146154. doi:10.1155/2012/146154
32. Adnan Khalaf M, Ghassan Zainal I. Investigation of antioxidant markers in diabetic patients. Arch Razi Inst. 2021;76:1453–1460. doi:10.22092/ari.2021.355755.1717
33. Miao C. Introduction: adipokines and cardiovascular disease. Clin Exp Pharmacol Physiol. 2011;38:860–863. doi:10.1111/j.1440-1681.2011.05598.x
34. DeBoer M, Gurka M, Golden S, et al. Independent associations between metabolic syndrome severity and future coronary heart disease by sex and race. J Am Coll Cardiol. 2017;69:1204–1205. doi:10.1016/j.jacc.2016.10.088
35. Jung T, Lee S, Kim H, et al. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp Mol Med. 2018;50:1–11. doi:10.1038/s12276-018-0147-5
36. Liu Z, Ji H, Yao M, et al. Serum Metrnl is associated with the presence and severity of coronary artery disease. J Cell & Mol Med. 2019;23:271–280. doi:10.1111/jcmm.13915
37. Xu L, Cai Y, Wang Y, Xu C. Meteorin-Like (METRNL) attenuates myocardial ischemia/reperfusion injury-induced cardiomyocytes apoptosis by alleviating endoplasmic reticulum stress via activation of AMPK-PAK2 Signaling in H9C2 cells. Med Sci Monit. 2020;26:e924564. doi:10.12659/msm.924564
38. Zheng S, Li Z, Song J, et al. Endothelial METRNL determines circulating METRNL level and maintains endothelial function against atherosclerosis. Acta pharmaceutica Sinica B. 2023;13:1568–1587. doi:10.1016/j.apsb.2022.12.008
39. Zhou Y, Liu L, Jin B, et al. Metrnl alleviates lipid accumulation by modulating mitochondrial homeostasis in diabetic nephropathy. Diabetes. 2023. doi:10.2337/db22-0680
40. Reboll M, Klede S, Taft M, et al. Meteorin-like promotes heart repair through endothelial KIT receptor tyrosine kinase. Science. 2022;376:1343–1347. doi:10.1126/science.abn3027
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
