Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 12
Low-Level Radiofrequency Exposure Does Not Induce Changes in MSC Biology: An in vitro Study for the Prevention of NIR-Related Damage
Authors Alessio N , Santoro E, Squillaro T , Aprile D , Briccola M, Giubbini P , Marchesani R, Muoio MR, Lamberti M
Received 5 February 2019
Accepted for publication 10 July 2019
Published 18 December 2019 Volume 2019:12 Pages 49—59
DOI https://doi.org/10.2147/SCCAA.S204166
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Nicola Alessio,1,* Elisa Santoro,2,* Tiziana Squillaro,3 Domenico Aprile,1 Massimo Briccola,4 Paolo Giubbini,4 Raffaella Marchesani,4 Maria Rosaria Muoio,2 Monica Lamberti2
1Department of Experimental Medicine, Biotechnology and Molecular Biology Section, University of Campania “Luigi Vanvitelli”, Naples, Italy; 2Department of Experimental Medicine, Occupational Medicine Section, University of Campania "Luigi Vanvitelli", Naples, Italy; 3Department of Medical, Surgical, Neurological, Metabolic Sciences, and Aging, 2nd Division of Neurology, Center for Rare Diseases and InterUniversity Center for Research in Neurosciences, University of Campania “Luigi Vanvitelli”, Naples, Italy; 4e-distribuzione SpA, Rome, Italy
*These authors contributed equally to this work
Correspondence: Nicola Alessio
Department of Experimental Medicine, Biotechnology and Molecular Biology Section, University of Campania “Luigi Vanvitelli”, Naples, Napoli I-80138, Italy
Tel +39 081 5667585
Fax +39 081 5667547
Email [email protected]
Monica Lamberti
Department of Experimental Medicine, Occupational Medicine Section, University of Campania “Luigi Vanvitelli”, Naples, Napoli I-80138, Italy
Tel +39 081 5667731
Email [email protected]
Background: The ubiquitous diffusion of radiofrequency (RF) radiation across human living environments has attracted the attention of scientists. Though the adverse health effects of RF exposure remain debatable, it has been reported that the interaction of such radiation with biological macromolecular structures can be deleterious for stem cells, inducing impairment of their main functions involving self-renewal and differentiation.
Purpose: The purpose of this study was to determine whether exposure to RF of 169 megahertz (MHz) that is part of very high radiofrequency (VHF) range 30–300 MHz, could cause damage to stem cells by inducing senescence and loss of regenerative and DNA repair capacity.
Methods: The study was conducted on mesenchymal stromal cells (MSCs) containing a subpopulation of stem cells. The MSCs were exposed to RFs of 169 MHz administered via an open meter 2G “Smart Meter” for different durations of time.
Result: We did not observe modifications in MSC biology as a result of the RF exposure conducted in our experiments.
Conclusion: We concluded that MSCs are insensitive to RF radiation exposure at 169 MHz for various time intervals, including longer durations.
Keywords: 169 MHz, stem cell, senescence, CFU
Introduction
Electromagnetic fields (EMFs) are generated by a wide range of occupational and non-occupational activities that include: research; communications; medical applications; power production, transmission and distribution; broadcasting; and air and maritime navigation. The effects of EMFs spark debate in developed countries because they are generated whenever electricity is used. In most cases, the field strength is such that it would not cause harmful effects.
Furthermore, Radio Frequency (RF) radiation is used in aesthetic medicine in the forms of cosmetic rejuvenation treatment1 and minimally invasive surgery (i.e., radio-ablation)2 in magnetic resonance imaging (MRI)3 and, in the last years, as a treatment of cancer;4 according to some authors, these treatments are not risk-free.5,6
The omnipresence of RF radiation in human living environments has attracted the attention of scientists and researchers who have undertaken epidemiological and in-vitro studies to assess its effects. Radiofrequency energy is non-ionizing radiations emitted at a frequency that is not strong enough to cause ionization of atoms and molecules.7,8 The biological effects related to RF exposure are divided into three levels: 1) interaction with biological macromolecular structures without clinical effects;9 2) reversible morphological and functional modifications in macromolecular structures with instrumentally, clinically or subjectively demonstrable effects that quickly disappear upon cessation of the stimulus;10 and 3) permanent biological damage that does not disappear upon cessation of the stimulus, wherein the biological effects exceed efficacy thresholds of damage repair, adaptation, and compensatory mechanisms.11
In subjects exposed to electromagnetic fields in both urban areas and in occupational contexts, higher reactive oxygen species (ROS) production observed in neutrophils is reported to be responsible for lower levels of polymorphonuclear leukocytes.12
The molecular changes induced by such radiation depend on a multitude of factors including the duration of radiation, tissue permeability, generation of heat, and the intensity and frequency of the waves. Furthermore, cellular response to these changes depends on specific characteristics of the waves such as waveform (sine or square), the number of changes and their biological effects, and the type of cells exposed to radiation.13
As stated above, the side effects induced by RF radiation can be deleterious for stem cells by impacting their key role in homeostatic maintenance of tissue and organs. Stem cells are undifferentiated biological cells that can differentiate into specialized cells. Their main function is self-renewal to produce progeny that replenish dying or damaged cells throughout an organism’s lifetime.14 Among all of the adult stem cells present in an organism, a widely shared view claims that Mesenchymal Stromal Cells (MSCs) are ubiquitous in human connective tissues, coinciding with ubiquitous pericytes and sharing a potential to differentiate into adipocytes, osteocytes, and chondrocytes as assessed by in vitro and in vivo differentiation assays.15,16
These premises serve to make MSCs an appropriate model for our study. The adverse health effects of RF radiation remain debatable. Indeed, same studies reported that RF exposure can induce DNA strand breaks while others showed no significant effect on DNA damage.17 Yao et al18 evaluated as 1800 MegaHertz (MHz) RF radiation cause DNA damage in human lens epithelial cells by the Comet assay. Franzellitti et al19 examined the same RF range, demonstrating that 1800 MHz RF radiation causes directly DNA damage.
On the contrary, some studies reported that in vivo and in vitro applications with different frequency RF radiation did not cause DNA single- and double-strand breaks and did not affect genotoxicity.20
In the last year, the company e-distribuzione S.p.A, the main Italian electricity distributor, has started the massive installation of the Open Meter 2G “Smart Meter” that will replace the first generation meters. These smart meters have new different characteristics. Namely, the communication features were particularly improved. In addition to the traditional main programmable logic controller (PLC) channel, a radio module with transmission around the frequency of 169 MHz was added.
In this context, the aim of this study was to evaluate whether exposure to the range of radiofrequency of 169 MHz that are part of Very High Radiofrequency (VHF), range 30–300 MHz, administered via an Open Meter 2G “Smart Meter”, has the potential to cause DNA damage in MSCs.
Materials and Methods
MSC Cultures
Bone marrow was obtained from healthy donors (18 to 40 years of age) who provided written informed consent in accordance with the Declaration of Helsinki (1964) and Campania region Ethical Committee (nr 379/CE of Dec 16, 2016). We used plastic adherence and other minimal criteria suggested by the International Society for Cellular Therapy to identify MSCs containing a subpopulation of stem cell (Supplementary Files 1 and 2). Cells were separated on a Ficoll density gradient (GE Healthcare, Milan, Italy) and the mononuclear cell fraction was collected and washed in Phosphate Buffer saline (PBS). We seeded 1–2.5⋅105 cells/cm2 in growth medium containing: alpha-Modified Eagle Medium (alpha-MEM) containing 10% Fetal Bovine Serum (FBS) and 3ng/mL of bFGF (PeproTech, Inc., Rocky Hill, NJ, USA). After 72 hrs, non-adherent cells were discarded and adherent cells were cultivated to confluence. Cells were then further propagated for the assays reported below. Where not specified otherwise, all reagents were obtained from Microgem (Napoli, Italy). Exponentially growing cells (passage 3) were used for exposure experiments. All experimental conditions were carried out by seeding 5×103 cells/cm2.
RF Exposure
The cells were transferred to an incubator and cultured in the presence of RF of 169 MHz. The RF was administered by prototype Open Meter 2G “Smart Meter” (e-distribuzione S.p.A., Rome, Italy). Our prototype-“Smart Meter” emit RF (E=0.1J) has a duration of 0.2s every 2s differently the normal-“Smart Meter” used for massive installation emit RF has a duration of 0.2s one for week. For this reason, we chose to analyze the effects of RF at different time points (0, 30, 90, 180 and 1440 mins). The maximum time exposure (1440mins) corresponds to around 100 years of RF emit by normal-“Smart Meter”.
The positive control (PC) of our experimental procedure was obtained with exposure for 2s the cells at 2998.5 MHz via Mevatron machine (Siemens, Italy) operating at 6 MeV and emit RF (E=12J) for 3·10−6s.
The distance between MSCs and RF sources (Smart Meter and Mevatron) is 0.05m.
In situ Senescence-Associated Beta-Galactosidase Assay
Following 169MHz RF exposure time intervals (0, 30, 90, 180 and 1440 mins) cells and relative positive control were fixed in a solution of 2% formaldehyde and 0.2% glutaraldehyde. After that, cells were washed with PBS (Microgem, Naples, Italy) and then incubated at 37°C overnight with a staining solution (citric acid/phosphate buffer (pH 6), K4Fe(CN)6, K3Fe(CN)6, NaCl, MgCl2, X-Gal). The percentage of senescent cells was calculated by the number of blue, β-galactosidase-positive cells out of at least 300 cells in different microscopic fields, as previously reported.21 Where not specified otherwise, all reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Nexin V Assay
After 169MHz RF exposure at different time points (0, 30, 90, 180 and 1440 min) the cells and relative positive control were collected for apoptosis analysis. Apoptotic cells were detected using fluorescein-conjugated annexin V with a Nexin® kit on a Guava® easyCyte™ (MilliporeSigma, Burlington, MA, USA) flow cytometer following the manufacturer’s instructions. The kit used two separate dyes (Annexin V and 7-Aminoactinomycin D (7-ADD)) to identify apoptotic and non-apoptotic cells (necrosis), respectively. Annexin V (red) binds to phosphatidylserine on the external membrane of apoptotic cells, while 7-AAD (blue) permeates and stains DNA of late-stage apoptotic and dead cells. Staining allows the identification of three cell populations: non-apoptotic cells (annexin V– and 7-AAD–); early apoptotic cells (annexin V+ and 7-AAD–); and late-apoptotic or dead cells (annexin V+ and 7-AAD+). Early and late apoptotic cells were grouped together in our experiments.
Cell Proliferation
We used a colorimetric assay (Cell Counting Kit-8 (CCK-8); Dojindo Molecular Technologies, Inc., Rockville, MD, USA) to determine cell viability. The highly water-soluble tetrazolium salt, WST-8, is reduced by dehydrogenase activity in cells to yield a yellow-color formazan dye. The amount of the formazan dye generated by the activity of cellular dehydrogenases is directly proportional to the number of living cells. We evaluated cell viability after 24, 48, and 72hrs in cells exposed for different time points at 169MHz RF (0, 30, 90, 180 and 1440 mins) and in relative positive control. The assay was carried out on a microplate reader at 450 nm (Infinite 200, TECAN, Männedorf, Switzerland).
Cell Cycle Analysis
The cells after 169MHz RF exposure for different times (0, 30, 90, 180 and 1440 mins) and relative positive control exposure were collected and fixed in 70% ethanol at −20°C, overnight (O.N.), three washed with 1X PBS, and finally dissolved in a hypotonic buffer containing propidium iodide (1XPBS), 100μg/mL of RNAse A (Promega, Madison, WI, USA) and 40μg/mL of Propidium iodine (Sigma-Aldrich) and incubated for 30mins at RT in dark. Samples were acquired on a Guava® easyCyte™ flow cytometer (Millipore, Sigma) and analyzed using the standard procedure recommended by the easyCyte™ software.
Adipo-Osteo and Condro Differentiation and Relative Staining
After 0 and 1440mins of 169MHz RF exposure, 1.5×104/cm2 cells were seeded and osteogenic (Dulbecco Modified Eagle Medium (DMEM) (Microgem), 10% FBS (Microgem), 0.05 mM ascorbic acid, 10 mM β-glycerophosphate and 100 nM dexamethasone) or adipogenic (DMEM) (Microgem), 10% Horse Serum (Microgem), 1 mM dexamethasone, 10 µg/mL insulin, 0.5 mM 3-isobutyl-1-methylxanthine, and 200 µM indomethacin) or chondrogenic (DMEM) (Microgem), 10% FBS (Microgem), 100 IU/mL penicillin (Microgem), 100 mg/mL streptomycin (Microgem), 50 nM ascorbate-2-phosphate, 0.1 mM dexamethasone, and 10 ng/mL human transforming growth factor (hTGF)-β1 (PeproTech, Inc., Rocky Hill, NJ, USA) differentiation was performed. Not differentiated cells were cultivated in DMEM medium (Microgem) supplemented with 10% FBS (Microgem). All conditions were performed for 21 days, renewing the medium every 3 days. Osteogenic differentiation was evaluated by alizarin red S staining, adipogenic differentiation by Oil Red O staining and chondrogenic differentiation by Alcian blue staining as previously described.21 Where not specified otherwise, all reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Colony Forming Units (CFU) Assay
After 0 and 1440 mins of 169mHz RF treatment, 1000 MSCs were seeded in every 10 cm culture dish and incubated for 14 days in a growth medium. Subsequently, the medium was discarded and colonies were fixed with 100% methanol for 10 mins at −20°C. Colonies were then stained with 0.01% (w/v) crystal violet (Sigma-Aldrich) in 25% methanol in PBS for 30 mins. For each experimental condition, we counted the number of colonies in each culture dish using a microscope (Leica DMi1, Leica, Wetzlar, Germany).
RNA Extraction and RT-qPCR
Total RNA was extracted from MSCs following 0 or 1440mins of 169MHz RF exposure using EUROGOLD TriFast (Euroclone) according to the manufacturer’s protocol. mRNA expression levels of the genes of interest were analyzed by RT-qPCR as previously reported.22,23 Primer pairs for RT-qPCR reactions were designed using Primer Express® software (Applied Biosystems/Thermo Fisher Scientific, Foster City, CA, USA). We used the appropriate regions of GAPDH cDNA as internal controls and each RT-qPCR reaction was repeated at least 3 times. The information about primers used were reported in Supplementary File 3.
Immunocytochemistry for Detection of ATM and Gamma-H2AX
Briefly, after 0 and 1440min of 169MHz RF exposure, the cells were fixed in 4% formaldehyde (Sigma-Aldrich) solution from 15mins at RT (20–23°C). Then, we permeabilized the cells with 0.3% Triton-X100 (Roche, Basel, Switzerland) on ice for 5 min and then added a 5% FBS solution in PBS and 0.1% Triton-X100 for 1 h at RT (Blocking Solution). Subsequently, the antibodies ATM (1:1000, ab36810, ABCAM, Cambridge, UK) and gamma-H2AX (1:600, #2577, Cell Signaling Technology, Danvers, MA, USA) were detected according to manufacturers’ protocols. The FITC-conjugated secondary antibody, goat anti-rabbit (1:400, Gtx-Rb-003D488) or TRITC-conjugates secondary antibody goat anti-mouse (1:400, Gtx-Mu-003D594) were obtained from ImmunoReagents (Raleigh, NC, USA). All the antibodies were diluted in blocking solution. Nuclear staining was performed using DAPI mounting medium (ab104139, ABCAM) and micrographs were captured under a fluorescence microscope (Leica DM2000,-DMC5400, Leica, Wetzlar, Germany). The percentage of ATM- and gamma-H2AX-cells was calculated by counting at least 300 cells in different microscopic fields.
Western Blotting
Cells after 169MHz RF exposure (0 and 1440mins) were lysed in a buffer containing 0.1% Triton-X100 (Roche) for 30 mins on ice. Twenty micrograms of each lysate was electrophoresed on a polyacrylamide gel and electroblotted onto a nitrocellulose membrane. All of the primary antibodies were used according to manufacturers’ instructions. Immunoreactive signals were detected using a horseradish peroxidase-conjugated secondary antibody: goat anti-mouse (1:4000, sc-2005) or goat anti-rabbit (1:4000, sc-2004) from Santa Cruz Biotechnology, Inc., Dallas, TX, USA with ECL plus reagent (GE Healthcare). The following primary antibodies were used: RB1 (1:2000, #9303) and p27KIP1 (1:1000, #3686) from Cell Signaling Technology; RB2/P130 (1:1000, MN610261) from BD Biosciences (San Jose, CA, USA); p107 (1:200, sc-318), p53 (1:200, DOI-1), p21CIP1 (1:200, C-19) from Santa Cruz Biotechnology; and p16INK4A (1:1000, ab54210) from ABCAM. All antibodies were diluted in T-TBS 0.1% and 3% of Blotting-Grade Blocker (Bio-Rad, Hercules, CA, USA).
Soft Agar
After 0 or 1440 min of 169MHz RF exposure, 2500 MSCs were seeded in 0.5 mL DMEM composed of 0.35% agarose (Sigma-Aldrich) and 20% FBS in 35 mm Petri dishes pre-treated with 0.5% agar (Sigma-Aldrich) in DMEM with 20% FBS. After 21 days of incubation, the cells were centrifuged at 2000 rpm. Then, the pellets were fixed in 100% methanol for 10 mins at −20°C. Colonies were then stained with 0.01% (w/v) crystal violet (Sigma-Aldrich) in 25% methanol for 30 mins. Subsequently, the cells were washed with PBS three times and resuspended in 100% methanol for 30 min. Finally, the tubes were centrifuged again and the collected supernatant was quantified using a microplate reader at 600 nm (Infinite 200, TECAN). We used HEK-293 cells as a positive control.
Statistical Analysis
Statistical significance was evaluated using ANOVA followed by Student’s t and Bonferroni tests. We used mixed-model variance analysis for data with continuous outcomes. All data were analyzed using GraphPad Prism version 5.01 statistical software package (GraphPad Software, Inc., San Diego, CA, USA).
Results
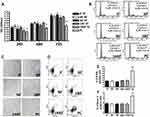
We exposed the MSC cultures to 169 MHz RF waves generated by a prototype-Meter 2G for different durations of time (0, 30, 90, 180 and 1440 min), while relative positive control was obtained exposing the cells at 2998.5 MHz via Mevatron machine (Siemens, Italy). Then, we assessed RF effects on proliferation rate, cell cycle, apoptosis, and senescence. Exposure to different durations of 169MHz RF exposure did not show any modification in MSC proliferation rate (Figure 1A) differently from the positive control where we observed a reduction in the growth rate. These results were in accordance with cell cycle analysis (Figure 1B) where the percentage of cells in S phase was similar among all time intervals considered for the cells exposed to 169MHz. Differently, we observed a reduction of S phase in the relative positive control. We also did not observe statistically significant differences in the percentage of senescent (Figure 1C and E) and apoptotic cells (Figure 1D and F) for the cells exposed to 169MHz. In relative positive control cultures, we observed an increase of apoptotic and senescent cells. We decided to seek further insight into the effects of RF on the biology of MSCs. To this end, we carried out further analysis by treating cells only for the longer duration of 1440 mins. It is well known that the control of such stem cell properties as self-renewal and multipotentiality is of fundamental importance in order to preserve the functionality of a stem cell—that is—to regenerate and repair damaged tissues within an organism.24,25
Therefore, we carried out a CFU assay on these cells to test their clonogenicity, i.e., their ability to expand at the single-cell level, which is an important feature in the self-renewal of stem cells. Neither the RF exposure for 0 min (control) nor for 1440 mins produced modification in the number of clones observed in 100 mm plates seeded with MSCs at low density (Figure 2A). We also evaluated multipotentiality of MSCs according to their tripotential differentiation capacity in adipo-, osteo-, and chondrocytes (Figure 2B). We did not observe modifications in this capacity. Also, the expression of several genes involved in the processes of self-renewal and multipotentiality like OCT4, NANOG, KLF4, and SALL4 is not modified after RD exposure (Figure 2C). Together, these data suggest that MSCs are insensitive to RF exposure at 169 MHz, including for prolonged durations of time.
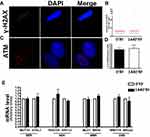
An increased incidence of cancer among people living near mobile phone base stations has been reported in the literature, suggesting that RF radiation may alter the fidelity of DNA.26; in fact, exposure to RF at 1800 MHz in human fibroblasts induces DNA single- and double-strand breaks.27 For these reasons, we decided to evaluate the presence of damaged DNA by H2AX (gamma isoform, γ-H2AX) and ATM immunostaining. H2AX histone is a key regulator of cellular responses to DNA damage and is considered a hallmark of damaged DNA nuclear foci. Ataxia telangiectasia mutated kinase (ATM) is a kinase that regulates DNA repair. Activation of ATM by autophosphorylation at Ser1981 (ph-ATM) occurs in response to exposed DNA. In several experiments, we did not observe any difference between MSCs cultured for 1440mins in the presence of RF waves and control MSCs (0min) for both γ-H2AX (Figure 3A and B) and ph-ATM (Figure 3C and D). We then conducted a more in-depth analysis of whether exposure to RF would change the expression levels of genes involved in different types of DNA repair. We selected a variety of genes involved in the regulation of base and nucleotide excision repair (BER and NER, respectively), mismatch repair (MER), and double-strand break repair (DSBR).28,29 We observed the expression of several genes involved in DNA repair: MMRE11A and BRCA2 genes for DSB; MLH1 and MSH5 genes for MER; RAD23A and ERCC3 genes for NER; and MUTYH and NTHL1 for BER. We did not observe any modification between MSCs cultured with RF exposure and MSC controls (Figure 3E).
After analyzing the biological consequences of RF exposure in MSCs, we decided to understand whether the molecular pathways involved in these phenomena were compromised. Therefore, we analyzed the mRNA expression levels of several genes involved in different pathways known to control cell cycle arrest, differentiation, apoptosis, and senescence. The analysis of the levels of proteins involved in these phenomena did not show any modification between cells treated with RF and controls (Figure 4A and B).
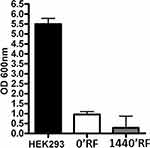
Cells exhibiting small changes in DNA that do not compromise normal cellular function can experience neoplastic transformation after long periods of time. For this reason, we decided to evaluate whether MSCs exposed to radiofrequency waves had accumulated damage that would transform them into a tumorigenic state. Transformed cells can grow independently of a solid surface. This feature is a hallmark of carcinogenesis and was evaluated by the soft agar colony formation assay. Cells cultured for 24 h with RF exposure and their respective controls did not show anchorage-independent growth until 21 days after the exposure (Figure 5).
Discussion
Radiofrequency (RF) refers to the rate of oscillation of electromagnetic radio waves in the range of 3 kHz to 300 GHz, as well as the alternating currents carrying the radio signals. This is the frequency band that is used for communications transmission and broadcasting.30 Today, RF is used in many fields of medicine and the sciences;2–4 despite its universal success, it remains unclear whether the technology is risk-free.5,6 For this reason, an in-depth study of RF exposure is necessary.
Among all cell types present in an organism, stem cells represent an important model in medicine because of their potential to regenerate and repair damaged tissue. Certain therapies such as bone marrow transplantation already make use of stem cells, while other therapies under investigation involve transplanting stem cells into a damaged organism and assessing their ability to grow and differentiate into healthy tissue.15,16
Mesenchymal stromal cells represent a good study model because they are ubiquitous in human connective tissues31 and contain a subpopulation of stem cells capable to differentiate in adipocytes, osteocytes, and chondrocytes. MSCs originating from the mesoderm and residing in various tissues are currently receiving a lot of attention for their therapeutic capacity. They not only contribute to tissue renewal but also support site-specific epithelial and endothelial responses through paracrine effects, secreting growth factors (e.g., fibroblast growth factors, WTN ligands, hepatocyte growth factor, and vascular endothelial growth factor) and Extracellular Membrane proteins; they also exhibit local anti-inflammatory capacity.15,16
As a preliminary study, we decided to investigate whether exposure to RF for different durations of time might be deleterious for MSC biology.
To this end, we exposed MSC cultures to 169 MHz RF generated by a Meter 2G for different time (0, 30, 90, 180 and 1440 mins). Then, we assessed RF radiation effects on proliferation rate, cell cycle, apoptosis, and senescence. The preliminary data did not reveal any biological effects of RF exposure on MSCs for brief durations of time, making us assume that the low doses of RF could be not deleterious for MSCs.
Subsequently, we sought to assess the effects of RF on the biology of MSCs by focusing our attention exclusively on the longest exposure time, treating cells with only the longer exposure of 1440 min.
We concentrated on two important aspects that characterize stem cells: self-renewal and differentiation capacity. We carried out a CFU assay on these cells to test their clonogenicity, i.e., their ability to expand at the single-cell level, which is an important feature of self-renewing stem cells, as well as their differentiation into adipocytes, osteocytes, and chondrocytes using an appropriate medium. We did not observe any differences between control and RF-exposed cells, suggesting that a radiofrequency of 169 MHz may be harmless to MSCs.
Yakymenko et al showed that the exposure of living cells to low-level of RF may cause overproduction of free radicals, in particular of ROS.32 Uncontrolled generation of ROS and their accumulation in cells cause oxidative stress. Recent studies have reported that chronic exposure to conditions that increase oxidative stress induced an increased risk of cancer. Vice versa, elevated levels of cancer have been observed in populations with increased residential exposure to RF radiation.33 An increased incidence of cancer among people living near mobile phone base stations has been reported in the literature.26,34,35 This suggests that RF waves may alter the fidelity of DNA;33 in fact, human fibroblasts exposed to RF at 1800 MHz exhibit DNA single- and double-strand breaks.27 For these reasons, we decided to evaluate the presence of damaged DNA by H2AX (gamma isoform) and ATM immunostaining. In several experiments, we did not observe any difference between MSCs cultured for 24 hrs in presence of RF radiation and control MSCs (Figure 3A–C).
The ability to modulate levels of oxidative DNA damage is also related to the function of genes involved in DNA repair.36 For these reasons, we analyzed whether exposure to RF would induce changes in the expression levels of genes involved in different types of DNA repair mechanisms. We selected genes involved in the regulation of base and nucleotide excision repair (BER and NER, respectively), mismatch repair (MER), and double-strand break repair (DSBR).28,29,37 We did not observe any modification between MSC cultures exposed to 0ʹ or 1440ʹ RF (Figure 3D).
Despite the fact that our study revealed that low doses of RF radiation are harmless to MSCs, several studies claim that the risks induced by RF, specifically, the epidemiological studies of cell phone use, increase the possibility of tumor arise.38 To ascertain whether RF had caused genetic changes to MSCs that would induce neoplastic changes, we carried out a soft agar assay to demonstrate that the exposure of MSCs at 169 MHz for 1640 min was not able to genetically modify MSCs, even when maintained in culture for an additional 21 days.
Our study demonstrated that low-dose RF (169 MHz) administered via an prototype-Open Meter 2G “Smart Mete” is harmless for MSCs in vitro, in accordance with other studies demonstrating that RF can be safe for human life.39,40 Another important aspect to consider is definitely the Dose Rate (dose absorbed per unit of time). In fact, recent studies have shown that low-dose fractionated exposures with a short time interval for a given dose induce stronger cytotoxic effects.41 Our results show that 169MHz RF exposure every 2s of interval time administrated by prototype-Open Meter 2G “Smart Meter” for 1440mins had no effect on the vitality of MSCs. These results are encouraging considering that the Dose Rate of the prototype- Open Meter 2G “Smart Meter”, that emits a RF of 169 MHz for 0.2 s every 2 s, is more high respect to normal-Open Meter 2G “Smart Meter”, that emits a RF of 169 MHz for 0.2s every week.
Nevertheless, it is important to note that studies claiming that RF is dangerous for human life42 mostly involved significantly higher levels of RF. Our in vitro study is innovative in that it demonstrates that there are no changes in MSC biology after exposure to 169 MHz RF.
Conclusion
Compared to controls (not exposed cells), exposed MSCs showed neither loss of differentiation capacity nor increased apoptosis and reactive oxygen species. In conclusion, different exposure times at low RF (169MHz) level administered by prototype-Open Meter 2G “Smart Meter” do not modify the biology of MSCs.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Data Sharing Statement
All data used to support the findings of this study are included within the article.
Acknowledgment
We thank e-distribuzione SpA, Via Ombrone 2, Rome, Italy, for funding support.
Author Contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
Massimo Briccola, Paolo Giubbini, and Raffaella Marchesani were employed by e-distribuzione SpA at the time of the study. The authors report no other conflicts of interest in this work.
References
1. Bhargava S, Cunha PR, Lee J, Kroumpouzos G. Acne scarring management: systematic review and evaluation of the evidence. Am J Clin Dermatol. 2018;19(4):459–477. doi:10.1007/s40257-018-0358-5
2. Sieber DA, Kenkel JM. Noninvasive methods for lower facial rejuvenation. Clin Plast Surg. 2018;45(4):571–584. doi:10.1016/j.cps.2018.06.003
3. Winter L, Ozerdem C, Hoffmann W, et al. Design and evaluation of a hybrid radiofrequency applicator for magnetic resonance imaging and RF induced hyperthermia: electromagnetic field simulations up to 14.0 Tesla and proof-of-concept at 7.0 Tesla. PLoS ONE. 2013;8(4):e61661. doi:10.1371/journal.pone.0061661
4. Garcia-Tejedor A, Guma A, Soler T, et al. Radiofrequency ablation followed by surgical excision versus lumpectomy for early stage breast cancer: a randomized phase II clinical trial. Radiology. 2018;289(2):317–324. doi:10.1148/radiol.2018180235
5. Singh S, Repaka R. Temperature-controlled radiofrequency ablation of different tissues using two-compartment models. Int J Hyperthermia. 2016;33(2):1–13.
6. Panizo JG, Barra S, Mellor G, Heck P, Agarwal S. Premature ventricular complex-induced cardiomyopathy. Arrhythmia Electrophysiol Rev. 2018;7(2):128–134. doi:10.15420/aer.2018.23.2
7. Li DK, Chen H, Ferber JR, Odouli R, Quesenberry C. Exposure to magnetic field non-ionizing radiation and the risk of miscarriage: a prospective cohort study. Sci Rep. 2017;7(1):17541. doi:10.1038/s41598-017-16623-8
8. Houston BJ, Nixon B, King BV, De Iuliis GN, Aitken RJ. The effects of radiofrequency electromagnetic radiation on sperm function. Reproduction. 2016;152(6):R263–R276. doi:10.1530/REP-16-0126
9. Prohofsky EW. RF absorption involving biological macromolecules. Bioelectromagnetics. 2004;25(6):441–451. doi:10.1002/(ISSN)1521-186X
10. Zhong Y, Wang Z, Zhao Y. Impact of radio frequency, microwaving, and high hydrostatic pressure at elevated temperature on the nutritional and antinutritional components in black soybeans. J Food Sci. 2015;80(12):C2732–2739. doi:10.1111/1750-3841.13131
11. Rodier F, Munoz DP, Teachenor R, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124(Pt 1):68–81. doi:10.1242/jcs.071340
12. Pedata P, Garzillo EM, Miranda R, et al. Functional changes in human peripheral neutrophils in workers with different exposure to noxious agents. Int J Environ Health Res. 2012;22(5):458–467. doi:10.1080/09603123.2011.654329
13. Panagopoulos DJ, Chavdoula ED, Margaritis LH. Bioeffects of mobile telephony radiation in relation to its intensity or distance from the antenna. Int J Radiat Biol. 2010;86(5):345–357. doi:10.3109/09553000903567961
14. Van Zant G, Liang Y. Concise review: hematopoietic stem cell aging, life span, and transplantation. Stem Cells Transl Med. 2012;1(9):651–657. doi:10.5966/sctm.2012-0033
15. Squillaro T, Galano G, De Rosa R, Peluso G, Galderisi U. Concise review: the effect of low-dose ionizing radiation on stem cell biology: a contribution to radiation risk. Stem Cells. 2018;36(8):1146–1153. doi:10.1002/stem.2836
16. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25(5):829–848. doi:10.3727/096368915X689622
17. Akdag MZ, Dasdag S, Canturk F, Karabulut D, Caner Y, Adalier N. Does prolonged radiofrequency radiation emitted from Wi-Fi devices induce DNA damage in various tissues of rats? J Chem Neuroanat. 2016;75(Pt B):116–122. doi:10.1016/j.jchemneu.2016.01.003
18. Yao K, Wu W, Wang K, et al. Electromagnetic noise inhibits radiofrequency radiation-induced DNA damage and reactive oxygen species increase in human lens epithelial cells. Mol Vis. 2008;14:964–969.
19. Franzellitti S, Valbonesi P, Ciancaglini N, et al. Transient DNA damage induced by high-frequency electromagnetic fields (GSM 1.8 GHz) in the human trophoblast HTR-8/SVneo cell line evaluated with the alkaline comet assay. Mutat Res. 2010;683(1–2):35–42. doi:10.1016/j.mrfmmm.2009.10.004
20. Falzone N, Huyser C, Franken DR, Leszczynski D. Mobile phone radiation does not induce pro-apoptosis effects in human spermatozoa. Radiat Res. 2010;174(2):169–176. doi:10.1667/RR2091.1
21. Alessio N, Pipino C, Mandatori D, et al. Mesenchymal stromal cells from amniotic fluid are less prone to senescence compared to those obtained from bone marrow: an in vitro study. J Cell Physiol. 2018;233(11):8996–9006. doi:10.1002/jcp.26845
22. Melone MA, Giuliano M, Squillaro T, et al. Genes involved in regulation of stem cell properties: studies on their expression in a small cohort of neuroblastoma patients. Cancer Biol Ther. 2009;8(13):1300–1306. doi:10.4161/cbt.8.13.8890
23. Cirillo A, Di Salle A, Petillo O, et al. High grade glioblastoma is associated with aberrant expression of ZFP57, a protein involved in gene imprinting, and of CPT1A and CPT1C that regulate fatty acid metabolism. Cancer Biol Ther. 2014;15(6):735–741. doi:10.4161/cbt.28408
24. Lompardia SL, Papademetrio DL, Mascaro M, Alvarez EM, Hajos SE. Human leukemic cell lines synthesize hyaluronan to avoid senescence and resist chemotherapy. Glycobiology. 2013;23(12):1463–1476. doi:10.1093/glycob/cwt074
25. Abdel-Rassoul G, El-Fateh OA, Salem MA, et al. Neurobehavioral effects among inhabitants around mobile phone base stations. Neurotoxicology. 2007;28(2):434–440. doi:10.1016/j.neuro.2006.07.012
26. Hardell L, Carlberg M, Hedendahl LK. Radiofrequency radiation from nearby base stations gives high levels in an apartment in Stockholm, Sweden: a case report. Oncol Lett. 2018;15(5):7871–7883. doi:10.3892/ol.2018.8285
27. Diem E, Schwarz C, Adlkofer F, Jahn O, Rudiger H. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res. 2005;583(2):178–183. doi:10.1016/j.mrgentox.2005.03.006
28. Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411(6835):366–374. doi:10.1038/35077232
29. Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27(3):247–254. doi:10.1038/85798
30. Ihnat P, Ihnat Rudinska L, Zonca P. Radiofrequency energy in surgery: state of the art. Surg Today. 2014;44(6):985–991. doi:10.1007/s00595-013-0630-5
31. Sacchetti B, Funari A, Remoli C, et al. No identical “Mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 2016;6(6):897–913. doi:10.1016/j.stemcr.2016.05.011
32. Yakymenko I, Tsybulin O, Sidorik E, Henshel D, Kyrylenko O, Kyrylenko S. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn Biol Med. 2016;35(2):186–202. doi:10.3109/15368378.2015.1043557
33. Zothansiama ZM, Lalramdinpuii M, Jagetia GC. Impact of radiofrequency radiation on DNA damage and antioxidants in peripheral blood lymphocytes of humans residing in the vicinity of mobile phone base stations. Electromagn Biol Med. 2017;36(3):295–305. doi:10.1080/15368378.2017.1350584
34. Group IS. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol. 2010;39(3):675–694. doi:10.1093/ije/dyq079.
35. Baan R, Grosse Y, Lauby-Secretan B, et al. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12(7):624–626. doi:10.1016/S1470-2045(11)70147-4
36. Zanichelli F, Capasso S, Di Bernardo G, et al. Low concentrations of isothiocyanates protect mesenchymal stem cells from oxidative injuries, while high concentrations exacerbate DNA damage. Apoptosis Int J Programmed Cell Death. 2012;17(9):964–974. doi:10.1007/s10495-012-0740-3
37. Ronen A, Glickman BW. Human DNA repair genes. Environ Mol Mutagen. 2001;37(3):241–283.
38. Wall S, Wang ZM, Kendig T, Dobraca D, Lipsett M. Real-world cell phone radiofrequency electromagnetic field exposures. Environ Res. 2018;171, 581–592.
39. Knudsen M, Riishede A, Lucke A, Gelineck J, Keller J, Baad-Hansen T. Computed tomography-guided radiofrequency ablation is a safe and effective treatment of osteoid osteoma located outside the spine. Dan Med J. 2015;62(5).
40. Smith SL, Bowers D, Jennings P, Soomal R. Pulmonary radiofrequency ablation in a district general hospital: is it a safe and effective treatment? Clin Radiol. 2016;71(9):939e931–938. doi:10.1016/j.crad.2016.03.021
41. Terashima S, Hosokawa Y, Tsuruga E, Mariya Y, Nakamura T. Impact of time interval and dose rate on cell survival following low-dose fractionated exposures. J Radiat Res. 2017;58(6):782–790. doi:10.1093/jrr/rrx025
42. Jauchem JR. Effects of low-level radio-frequency (3kHz to 300GHz) energy on human cardiovascular, reproductive, immune, and other systems: a review of the recent literature. Int J Hyg Environ Health. 2008;211(1–2):1–29. doi:10.1016/j.ijheh.2007.05.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.