Back to Journals » Journal of Pain Research » Volume 13
Low-Intensity Continuous Ultrasound for the Symptomatic Treatment of Upper Shoulder and Neck Pain: A Randomized, Double-Blind Placebo-Controlled Clinical Trial
Authors Petterson S, Plancher K, Klyve D , Draper D, Ortiz R
Received 28 January 2020
Accepted for publication 5 May 2020
Published 2 June 2020 Volume 2020:13 Pages 1277—1287
DOI https://doi.org/10.2147/JPR.S247463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Stephanie Petterson, 1 Kevin Plancher, 1– 4 Dominic Klyve, 5 David Draper, 6 Ralph Ortiz 7
1Orthopaedic Foundation, Stamford, CT, USA; 2Department of Orthopaedics, Albert Einstein College of Medicine, New York, NY, USA; 3Department of Orthopaedics, Weill Cornell Medical College, New York, NY, USA; 4Plancher Orthopaedics & Sports Medicine, New York, NY, USA; 5Department of Mathematics, Central Washington University, Ellensburg, WI, USA; 6Department of Exercise Sciences, Brigham Young University, Provo, UT, USA; 7Medical Pain Consultants, Dryden, NY, USA
Correspondence: Ralph Ortiz
Medical Pain Consultants, Dryden, NY, USA
Tel +1 607 844-9979
Email [email protected]
Purpose: Low-intensity continuous ultrasound (LICUS) is an emerging high-dosimetry ultrasound-based therapy for accelerated tissue healing and the treatment of myofascial pain. In this study, LICUS treatment is clinically evaluated for chronic upper neck and shoulder pain in a randomized, multi-site, double-blind, placebo-controlled study.
Patients and Methods: CONSORT guidelines were followed in conducting and reporting the clinical trial. Thirty-three participants with upper trapezius myofascial pain were randomized for treatment with active (n=25) or placebo (n=8) devices. Investigators and subjects were blinded to treatment groups. Participants self-reported pain daily, rating from 0– 10 on the numeric rating scale. If pain rating was more significant than or equal to 3, the LICUS (3MHz, 0.132W/cm 2, 1.3W, 4 hours) was self-applied for total energy dosimetry of 18,720 Joules per treatment. During the 4-week study, daily pain rating was recorded. If LICUS treatment was delivered, pain before, during, and after treatment were recorded as well as the global rate of change (GROC). Independent t-tests were used to assess change from baseline and differences between treatment groups. ClinicalTrials.gov: NCT02135094.
Results: There was a 100% completion rate for participants enrolled in the study and no significant differences between the groups regarding demographic variables or baseline outcome measures. Participants treated with active therapy observed a significant mean pain reduction from baseline of 2.61 points for active (p< 0.001), compared to 1.58 points decrease from baseline for placebo (p=0.087), resulting in a 1.03 points significant decrease in the active group over placebo (p=0.003). The total GROC was significantly higher in the active group at 2.84 points compared to the placebo group at 0.46 points (p< 0.001).
Conclusion: Low-intensity continuous ultrasound treatment significantly reduced pain in patients with upper trapezius myofascial pain of the neck and shoulder. LICUS treatment showed a clinically meaningful improvement in the GROC scores for patients. The results from this clinical trial indicate that the LICUS treatment of 18,720 Joules can effectively be used to treat clinical pain related to upper trapezius myofascial pain. Further research could investigate varying dosimetry to improve efficacy and/or reduce the dose.
Keywords: sustained acoustic medicine, myofascial trigger points, non-steroidal anti-inflammatory drugs, NSAIDs, non-invasive therapy, soft tissue healing, chronic musculoskeletal pain
Corrigendum for this paper has been published
Background
Neck and back pain is the most common musculoskeletal condition, affecting over 84% of the adult population.1,2 Back pain costs the United States healthcare system over $100 billion annually,3–6 and there are several etiologies of back pain including anatomic, nerve root, muscle, myofascial structure, bone, joint, intervertebral disc, and organ abnormalities or injury.6 Myofascial trigger points, a common occurrence in those with back pain, have been described as discrete hypersensitive areas presenting in taut bands of muscle.7–10 Myofascial trigger points are classified as active or latent. Active myofascial trigger points produce local and referred pain when compressed, among other clinical symptoms, such as limiting full muscle lengthening and muscle weakening.7,11 Latent myofascial trigger points are painful only when palpated and do not present with as great of mechanical response as active myofascial trigger points.8,12
Myofascial trigger points are often treated non-invasively with ischemic compression, laser therapy, and ultrasound treatment.13 Ischemic compression is applied with enough manual pressure to produce skin blanching in the treatment area and can result in a reduction in pain score.14 Laser therapy studies have shown conflicting results in the treatment of myofascial trigger points, which could be attributed to the range of parameters used in the treatment and limited depth of penetration.15 Low-intensity ultrasound produces mechanobiological effects stimulating cellular and tissue mechanisms treating multiple conditions, including myofascial trigger points,16–19 back pain,20,21 tendinopathy,22,23 and joint arthritis pain.24–26 The inconsistency of ultrasound treatment effectiveness cited in the literature and metanalysis can be attributed to a lack of optimization of ultrasound duty cycle, frequency, intensity, and power as well as patient compliance.27–29
Over the last decade, research suggests daily increased energy deposition via low-intensity ultrasound can improve patients’ quality of life. Low-intensity continuous ultrasound (LICUS) devices enable patients to self-apply non-invasive therapeutic ultrasound for up to four hours per day, increasing total energy deposition to almost 20,000 Joules compared to an ON/OFF 10 to 20-minute therapist applied ultrasound treatment, which typically involves 700 to 2,000 Joules of energy deposition once a week.30,31 Research into low-intensity continuous ultrasound for musculoskeletal injuries and disorders focuses on daily applied LICUS. Self-applied wearable LICUS devices enable longer treatment durations, increasing energy deposition,22 and accelerate healing.22,31,32 LICUS produces ultrasound without pulses and increases muscle temperature,33,34 creating a potential for increased blood flow,35 increased connective tissue extensibility,36,37 altered nerve conduction velocity,38 and with less probability of forming adverse standing waves leading to potential tissue damage. Recent literature reviews on LICUS have found the treatment is effective in decreasing pain and improving function in musculoskeletal injuries.29
Additionally, ultrasound can have mechanical and biologically driven effects such as myoregeneration,32 improve biomechanics,31 anti-inflammatory,39 and thermal effects.33,34 The objective of this study was to determine the effectiveness of daily 4-hour LICUS at alleviating upper trapezius active myofascial pain and muscle tenderness over a 4-week treatment period. We hypothesized that 4-hours of LICUS treatment of upper neck and back trigger point pain would provide additional pain reduction over only 1-hour of LICUS treatment by Lewis et al (2013).19 The study and methods followed the Consolidated Standards of Reporting Trials (CONSORT)40
Methods
A multi-site, randomized, double-blind placebo-controlled trial design was used to direct this study. The 4-week study design and 4-hour LICUS treatment protocol was chosen to build off of the results of Lewis et al (2013),19 and determine potential increased pain reduction due to the longer (+3 hour) LICUS treatment. For the primary outcome measure, change in pain on the numeric rating scale 0–10 (NRS) after LICUS treatment, participants were recruited on a 3:1 basis to active and placebo, based on statistical power analysis from Lewis et al (2013) 1 hour of LICUS treatment for trapezius muscle spasm pain. Using the mean LICUS pain reduction for the first 2 days of the study (active mean 21.25% ± 9%, placebo mean 4% ± 9%), and mean pain reduction for the entire 10 days of the study (active mean 16% ± 7%, placebo 8% ± 7%) from Lewis et al (2013); A sample size of 24:8 active to placebo provided over 95% power and 80% power for the primary outcome measure NRS pain reduction. We also anticipated a stronger active treatment effect size for pain reduction in our study since LICUS was to be applied for 4-hours per treatment versus only 1-hour per treatment in Lewis et al (2013).19 Total participants enrolled and randomly assigned to an active (n = 25) or placebo (n = 8) treatment group was slightly above target (+1 active). Randomization occurred by participants drawing numbers out of a hat that matched the serial numbers of the unknown device set-up (either active or placebo). The active ultrasound group sample size was powered based on a previous study on LICUS for upper back pain using self-reported pain scales. Adequate placebo was used for comparison.The participants, investigators, and biostatistician were blinded to treatment group assignments.
Participants
Thirty-three participants (12 males and 21 females, age = 33.3 ± 13.3 y, height = 169.6 ± 11.4 cm, mass = 74.4 ± 15.0 kg) were recruited from the patient population at the enrolment sites. All patients enrolled completed the study (Table 1). Participants were recruited and screened per the diagnostic criteria. All participants provided written informed consent prior to enrolling in the study, which was approved by the Institutional Review Board for Human Subjects for Brigham Young University and the study was conducted in accordance with the Declaration of Helsinki.41 Participants were included if they were between the ages of 18–65 y and were diagnosed with upper trapezius trigger points by a health care practitioner. The health care practitioner (physician or athletic trainer) diagnosed the participant with pain caused by trapezius myofascial trigger point if they presented with a palpable taut band, with pain greater or equal to 3.0 out of 10 on a numeric rating scale (NRS) at least 3 times per week and subjectively reported stiffness and/or restricted range of motion in the upper trapezius. Participants were excluded if they were not willing to follow the protocol and follow-up procedures, had a known neuropathy, were type I or type II diabetic, had surgery in the target area within the past 6 months or had other contraindicated conditions to therapeutic ultrasound. If applicable, participants were asked to discontinue the use of pain medications and topical analgesic creams or gels and discontinue massage therapy or spinal manipulation treatments. This was confirmed by asking participants if they had used any treatment other than the LICUS in their daily diaries. The study started in June 2014 and was completed by September 2015 (Clinical trial: NCT02135094).
 |
Table 1 Patient Demographics |
Procedures
Enrolled participants provided demographic and baseline information during their initial visits to the enrollment site. Outcomes measures included: 1) pain rated on a 10-point numeric rating scale (NRS), 2) participant’s overall feeling rated on a 15-point global rate of change (GROC) scale. Baseline measurements were taken on Day 1, and then participants were assigned an active or placebo LICUS device based on their group assignment.
A daily diary was given to the participants, which contained the NRS pain scale and GROC scale questions. Participants were instructed to fill out the daily dairies after treatment, and the diaries were checked for compliance at the 2-week follow-up visits. Participants were instructed only to apply the device if the daily pain rating was greater than or equal to 3/10. Participants made a total of 3 visits to the enrollment site, once at baseline and then at 2-week intervals for 4 weeks. At the week-2 and week-4 visits, the same procedures were used as the initial visit. The study was completed after the 4-week visit, and no further follow-up was conducted (Figure 1).
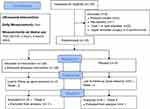 |
Figure 1 The study schematic. Patients were enrolled and evaluated for baseline pain scores on day one of the studies. Two- and four-week follow-ups were included to evaluate compliance. |
LICUS Device Treatment Protocols and Placement
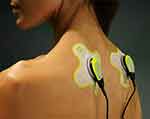
Following device use protocol from the manufacturer’s training, the investigator aided the participant in applying the device the first time. If participants presented with a unilateral trigger point, they placed one transducer over the trigger point of the trapezius muscle on that side. If participants had bilateral trigger points, they were instructed to place one transducer over each trigger point (total of two transducers). Participants placed the transducer over the most painful trigger point if more than one unilateral trigger point was reported. The bilateral application of the LICUS device over the upper trapezius is demonstrated in Figure 2. Participants were instructed to wear the device for 4 hours each time they applied it.
Numeric Rating Scale
The primary outcome measure of the study was pain reduction from LICUS treatment. Participants recorded their NRS score daily for the 4-week clinical trial. The NRS is a validated and consistent measurement of pain for several musculoskeletal conditions, including chronic myofascial trapezius pain.42–44 If the participant’s daily NRS score was greater or equal to a 3/10, the participant was instructed to apply the assigned LICUS device, active or placebo. The participants were asked to record the NRS during the treatment at 30m, 120m, and immediately after the LICUS treatment. A reduction in 1 point on the NRS has been reported as a significant minimal clinically important difference for chronic musculoskeletal pain.44
Global Rating of Change Scale
The secondary outcome measure of the study was GROC improvement when LICUS was applied. On days participants wore the assigned device; they were instructed to record their GROC score after the treatment. The GROC scale assesses a participant’s overall improvement or deterioration during a treatment intervention period.45–48, The “global” aspect of the scale allows participants to consider what is important to them when completing the measurement. Participants responded on a 15-point GROC scale to the following question,48–50 “Consider how your body feels overall right now compared to yesterday and circle the number that describes how you feel.” The 15-point scale was labelled with −7 being “a very great deal worse,” 0 being “no change,” and +7 being “a very great deal better.” GROC was not assessed at baseline because it was only measured after treatments were administered to determine the participants’ global feeling compared to the previous treatment.
GROC scales have high test-retest reliability (ICC = 0.90) in patients with low back pain.49 GROC scales have also been shown to have good face validity.46,49 We used a 15-point scale,50 but the minimal detectable change and minimal clinically important difference using an 11-point scale are 0.45 points and 2 points,19,49,50 respectively. The minimal detectable change and minimal clinically important difference have not been reported for a 15-point scale.
LICUS Device
A LICUS device (SAM®, ZetrOZ Systems, LLC., Trumbull, CT) was used for this study. The device delivers pre-set low-intensity continuous ultrasound at 3 MHz frequency and 0.132 W/cm2 spatial average temporal average intensity (ISATA). The device has single or dual transducer modes and has been FDA approved for home use to wear for up to 4 hours of daily use and deliver 18,720 Joules per treatment (dual transducer).
The LICUS device was self-applied by the participant and operated for the full 4-hour/18,720 Joule treatment. It is easy to use the device with 2 buttons - an on/off button in the middle of the device and a time button on the side of the device. All participants were instructed to press the time button up to 4 hours. The placebo devices supplied for the study had the power wire to the transducer cut by the manufacturer, resulting in no ultrasound energy being produced, but the power to the on/off and time setting lights on the device would remain to function as normal. No alternative instructions were given to the placebo or active treatment group since the device functioned the same for both groups, and the ultrasound intensity was too low to produce a significant sensation.
Data Analysis
Baseline demographic and outcome variable data were compared between treatment groups using independent t-tests to assess adequate randomization.51–53 Chi-squared was used to assess the sex proportion difference between groups. Independent t-tests were used to assess NRS pain scores and GROC change from baseline and difference between active and placebo groups.53
Results
Enrollment and Participants Demographics
Thirty-eight (38) participants were screened, and thirty-three (33) subsequently enrolled in the study (Figure 1). Participants were randomized into active (n = 25) or placebo (n = 8) groups. There were no differences in demographics between groups (Table 1). Overall, averages were (± standard deviation): height 169.6 cm (±11.4 cm), weight 74.1 kg (±15.0 kg), age 33.3 years (±13.3 years). All participants completed the 4-week study with 100% compliance and no adverse events.
Device Use
On average, the active group used the device 2.77 ± 0.476 times per week, while the placebo group used the device 3.2 ± 0.752 times per week. The difference between the groups was not significant (p=0.8, independent t-test)
Pain Change from Baseline
Pain before the intervention was assessed at baseline (day 1), then daily before each treatment during the intervention period of 4 weeks. Post-treatment pain scores were used to evaluate change from baseline. The active group showed a significant decrease in pain from baseline as early as the 1st week and persisted through the 4-week study (p<0.001, independent t-tests of week average compared to baseline) (Table 2). The placebo group did not show a significant difference; however, a trend toward a decrease in pain was noticed at week 3 and week 4 compared to baseline (p=0.070 and p=0.087, respectively). While this could indicate a placebo effect, the difference between groups for change from baseline was significant for all 4 weeks assessed (p<0.001 for weeks 1–3, p=0.003 for week 4), showing a more significant change from baseline for the active LICUS group compared to placebo.
 |
Table 2 Primary Outcomes. The Pain Reported at Baseline and Weekly Averages. Change in Pain from Baseline and Comparison Between Groups Evaluated Using Independent t-tests |
Pain Change During the Treatment Session
The pain level was assessed daily and averaged to group pre-treatment scores (Table 3). When LICUS devices were applied (NRS score ≥ 3), the NRS pain score was reported at 30 minutes into the treatment, 2 hours into treatment, and 4 hours (post-treatment). The active group showed a significant decrease in pain as early as 30 minutes (−0.416 points, p<0.001), and this pain reduction became greater through the 4-hour treatment compared to pre-treatment pain (−2.16 difference, p<0.001). The placebo group did not show a difference at 30 minutes into treatment compared to pre-treatment but did show a significant decrease in pain after 2 hours and post-treatment. Pain reduction in the placebo group was less than 1.0 minimal clinically important difference for NRS pain scores.44 The pain reduction for the active group was significantly greater compared to pain reduction observed in the placebo group at 30 minutes (−0.179 points, p=0.008), 2 hours (−0.747 points, p<0.001), and 4 hours (−1.28 points, p<0.001). Improved pain relief was noted at all follow-up time points beginning at the 1st week.
The Global Rate of Change Assessment
Overall, GROC was significantly greater in the active group at 2.84 ± 2.21 points compared to the placebo group, 0.46 ± 2.08 points (p<0.001). This significant difference is apparent for all weeks during the 4-week study, indicating the active LICUS group considered their overall health improved significantly more than the placebo group (Table 4).
 |
Table 4 The Global Rate of Change Score Reported After (4 hours) of Treatment with SAM. The Comparison Between Groups was Evaluated Using Independent t-tests |
Discussion
Low-Intensity Continuous Ultrasound (LICUS) is believed to inactivate myofascial trigger points by increasing tissue temperature,34 improving local blood flow,54–56 and increasing tissue extensibility.36,37 Previous studies have shown a temperature increase of 4 °C up to 3 cm deep in living human muscle tissue.33 A limitation of our study is that we did not directly measure trigger point molecular features or markers but instead determined the clinical effectiveness and patient satisfaction of LICUS at decreasing the participants’ pain and improving GROC. A significant decrease in pain from baseline was observed in the active LICUS group at 4 weeks (−2.61 points, p<0.001). It should be noted that a trend in change from baseline in the placebo group was observed at 4 weeks and can probably be attributed to the placebo effect (−1.58 points, p=0.087). Regardless of the placebo effect, the change in pain reduction of the active LICUS group was significantly greater than the placebo group every week of the study, including the final week (week 4) of treatment.
Low-intensity ultrasound has been approved for bone healing, considering its mechanotransductive properties leading to bone regeneration. Ultrasound devices that deliver pulsed low-intensity ultrasound (0.03W/cm2,1.5MHz, 20% duty cycle for 20 min, 700 Joules per treatment) for bone healing provide little thermal effect.30 Additionally, non-thermal, low-energy, pulsed low-intensity ultrasound treatments show non-significant clinical effects soft-tissue tendinopathy,57 shoulder pathology,17,58 and myofascial trigger point pain.17,59 On the other end of the energy delivery spectrum, LICUS (0.132W/cm2, 3MHz, 100% duty cycle for 4 hrs, 18,720 Joules per treatment) provides mechanotransducive stimulation leading to tissue regeneration, the continuous nature of ultrasound signal provides increased localized temperature33,34 of the tissue enhancing blood35 and nutrient flow, oxygenation as well as reduction of inflammatory cytokines.22,31 The LICUS treatment creates a 4°C heating increase in approximately 80 minutes when factoring for physiological cooling with participants at rest for multiple hours.33 Additionally, this 4°C heating change using the LICUS is maintained over the remaining treatment time.33 Increase tissue temperature has been shown to increase localized blood flow, tissue oxygenation, and reduced level of inflammatory cytokines, as well as the effects are maintained over for 20 mins post-treatment.33,55,60 We did not measure the acute effects of the treatment of myofascial trigger points such as allodynia, range of motion, or thermal effect, but future research could examine these effects.
LICUS treatment of myofascial trigger points and upper neck and shoulder pain was explored by Lewis et al (2013).19 The placebo-controlled study applied LICUS from one ultrasound transducer for one hour at 1,584 Joules of energy delivered per treatment. Lewis et al (2013) observed a significant pain reduction over placebo on the first two days of treatment (active mean 21.25% ± 9%, placebo mean 4% ± 9%, p<0.05), and mean pain reduction for the entire 10 days of the study (active mean 16% ± 7%, placebo 8%± 7%) which was only significant for male participants p=0.02.19 In this research study, participants self-applied LICUS for 4 hours per treatment delivering 18,720 Joules (12 times more energy than Lewis et al (2013). Participants in our study had significant pain reduction over placebo during all weeks of the study, with the greatest pain reduction occurring in week 4 (NRS −2.61±1.63 points, p<0.001).
Myofascial trigger points are classified as active and latent. Active trigger points are generally always tender to cause pain for the patient, and directly respond to mechanical compression stimulus activating a top neuronal imbalance and segmental muscle dysfunction in the trigger points reference zone. Latent trigger points are clinically quiescent with respect to spontaneous pain, and are painful only when mechanically activated. Latent trigger points occur because of an ischemic condition creating a reduction, a decrease in tissue pH, and stimulation of local nociceptors.8,9,12 Clinically both trigger points are associated with significant pain and loss of function for patients, and treatments that induce central modulation of pain, stimulating serotonin release, and enkephalin regulation, may play a role in decreasing myofascial trigger point pain.10,61 Ultrasound’s ability to alter segmental neural activity is unknown, and its mechanotransductive effects may have a positive impact on neural activity associated with the local tissue healing and regeneration from LICUS treatment.
Low-intensity continuous ultrasound treatment has the potential to reduce healthcare costs for both patients and the healthcare system for the treatment of chronic pain conditions. Opioids and NSAIDs can cost patients over $250 per month for similar pain relief to what was found using a LICUS device.4,5 However, opioids and NSAIDs can have severe side effects on the neural, gastrointestinal, and cardiovascular system, requiring additional medication. Dry Needling is a nonpharmacological treatment for trigger point pain that has also shown similar pain relief to pharmaceuticals (2.1 to 2.6 point reduction).62 The cost of dry needling is approximately $100 per treatment session, and causes minor bleeding or bruising in 5–8% of treatments. Dry needling is generally safe; however, it does pierce the skin and can pose a small risk to infection.63 To help manage chronic pain, 3–6 sessions with 10–20 needles may be required. However, there are currently no clear dosing guidelines for this emerging treatment option.64 Long-duration continuous ultrasound does not have any reported adverse reactions being highly local and non-invasive therapy; there was 100% patient compliance and no reportable side effects across all treatments self-administered by the participants. Additionally, prescription costs are only a fraction (around 14%) of the total expenditure for chronic pain conditions.4 The remainder of the cost is associated with imaging, and outpatient visits, with some research suggesting 18.8 outpatient visits on average per patient. The result is a total cost of over $31,000 per year, which puts a large financial burden on the healthcare system.4
In comparison, the device used here costs $6,800 in this study and $180/month for one-time use coupling patches if treating daily. The annual cost would be $8,960 if patients treated daily. However, this study found that device use was around three times per week, reducing the total cost to approximately $7,800 for the first year and $1,000 for the years following. The number of outpatient visits should also decrease substantially, as there are no adverse reactions to report or monitor. LICUS could reduce the overall economic burden on both patients and the healthcare system while providing safe and effective pain relief for eliminating conditions such as upper back, neck, and shoulder pain.
The current study focused on the application of LICUS as a standalone treatment for upper trapezius myofascial pain relative to the placebo group. Future studies could compare active LICUS to pharmaceutical options, as well as dry needling and trigger point injections. This study focused primarily on patient-rated clinical outcomes and did not investigate acute tissue changes. While the mechanism of action, including increased blood flow, nutrient transfer, mechanical strength,20,32 myoregeneration,32 and thermal effects,33 probably remain true here and impact clinical outcomes, they were not specifically investigated within the scope of this study.
Future research should continue to identify which pathologies LICUS is most effective for and what are its physiological effects. In addition, many myofascial pain conditions are chronic; therefore, future studies should examine the effectiveness and safety over a longer period of use. This evidence is encouraging the development and use of non-drug and non-surgical options to accelerate biological healing and reduce pain naturally.
Conclusion
LICUS delivery of 18,720 Joules per treatment was successful at treating related pain symptoms of upper trapezius myofascial trigger points relative to the placebo group. The reduction in pain seen in the active ultrasound group is clinically significant, indicating the wearable ultrasound device is a possible home-use treatment option with several advantages over prescription pain medication, invasive options, and opioids. LICUS provides an attractive home-use treatment option for patients suffering from upper trapezius myofascial pain.
Data Sharing Statement
The data that support the findings of this study are openly available at ClinicalTrials.gov at https://clinicaltrials.gov/ct2/show/NCT02135094, identifier NCT02135094.
Acknowledgments
This research was conducted in Provo, Utah, USA. Dr. Plancher is a Clinical Professor in the Department of Orthopaedic Surgery at Albert Einstein College of Medicine and an Adjunct Clinical Assistant Professor in the Department of Orthopaedic Surgery at Weill Cornell Medical College, Cornell University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–2196. doi:10.1016/S0140-6736(12)61729-2
2. Chiarotto A, Clijsen R, Fernandez-de-Las-Penas C, Barbero M. Prevalence of myofascial trigger points in spinal disorders: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2016;97(2):316–337. doi:10.1016/j.apmr.2015.09.021
3. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi:10.2106/JBJS.E.01273
4. Park PW, Dryer RD, Hegeman-Dingle R, et al. Cost burden of chronic pain patients in a large integrated delivery system in the United States. Pain Pract. 2016;16(8):1001–1011. doi:10.1111/papr.12357
5. Rasu RS, Vouthy K, Crowl AN, et al. Cost of pain medication to treat adult patients with nonmalignant chronic pain in the United States. J Manag Care Spec Pharm. 2014;20(9):921–928. doi:10.18553/jmcp.2014.20.9.921
6. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi:10.1016/j.jpain.2012.03.009
7. Simons DG, Mense S. Diagnose und Therapie myofaszialer Triggerpunkte. Der Schmerz. 2003;17(6):419–424. doi:10.1007/s00482-003-0253-7
8. Shah JP, Thaker N, Heimur J, Aredo JV, Sikdar S, Gerber L. Myofascial trigger points then and now: a historical and scientific perspective. PMR. 2015;7(7):746–761. doi:10.1016/j.pmrj.2015.01.024
9. Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi:10.1016/j.apmr.2007.10.018
10. Niddam DM, Chan RC, Lee SH, Yeh TC, Hsieh JC. Central modulation of pain evoked from myofascial trigger point. Clin J Pain. 2007;23(5):440–448. doi:10.1097/AJP.0b013e318058accb
11. Bron C, Dommerholt JD. Etiology of myofascial trigger points. Curr Pain Headache Rep. 2012;16(5):439–444. doi:10.1007/s11916-012-0289-4
12. Gerwin RD. Diagnosis of myofascial pain syndrome. Phys Med Rehabil Clin N Am. 2014;25(2):341–355. doi:10.1016/j.pmr.2014.01.011
13. Kannan P. Management of myofascial pain of upper trapezius: a three group comparison study. Glob J Health Sci. 2012;4(5):46–52. doi:10.5539/gjhs.v4n5p46
14. Gatterman MI, McDowell BL. Management of muscle injury and myofascial pain syndromes. Whiplash Mosby. 2012;27.
15. Al-Shenqiti AM, Oldham JA. The use of low reactive level laser therapy in the treatment of myofascial trigger points: an updated critical review. Lasers Med Sci. Conference: S25.
16. Yildirim MA, Ones K, Goksenoglu G. Effectiveness of ultrasound therapy on myofascial pain syndrome of the upper trapezius: randomized, single-blind, placebo-controlled study. Arch Rheumatol. 2018;33(4):418–423. doi:10.5606/ArchRheumatol.2018.6538
17. Ilter L, Dilek B, Batmaz I, et al. Efficacy of pulsed and continuous therapeutic ultrasound in myofascial pain syndrome: a randomized controlled study. Am J Phys Med Rehabil. 2015;94(7):547–554. doi:10.1097/PHM.0000000000000210
18. Ay S, Dogan SK, Evcik D, Baser OC. Comparison the efficacy of phonophoresis and ultrasound therapy in myofascial pain syndrome. Rheumatol Int. 2011;31(9):1203–1208. doi:10.1007/s00296-010-1419-0
19. Lewis GK
20. Ebadi S, Ansari NN, Naghdi S, et al. The effect of continuous ultrasound on chronic non-specific low back pain: a single blind placebo-controlled randomized trial. BMC Musculoskelet Disord. 2012;13(1):192. doi:10.1186/1471-2474-13-192
21. Durmus D, Durmaz Y, Canturk F. Effects of therapeutic ultrasound and electrical stimulation program on pain, trunk muscle strength, disability, walking performance, quality of life, and depression in patients with low back pain: a randomized-controlled trial. Rheumatol Int. 2010;30(7):901–910. doi:10.1007/s00296-009-1072-7
22. Best TM, Moore B, Jarit P, Moorman CT, Lewis GK. Sustained acoustic medicine: wearable, long duration ultrasonic therapy for the treatment of tendinopathy. Phys Sportsmed. 2015;43(4):366–374. doi:10.1080/00913847.2015.1095617
23. Lewis G, Hernandez L, Lewis GK
24. Langer MD, Lewis GK
25. Draper DO, Klyve D, Ortiz R, Best TM. Effect of low-intensity long-duration ultrasound on the symptomatic relief of knee osteoarthritis: a randomized, placebo-controlled double-blind study. J Orthop Surg Res. 2018;13(1):257. doi:10.1186/s13018-018-0965-0
26. Langer MD, Levine V, Taggart R, Lewis GK, Hernandez L, Ortiz R. Pilot clinical studies of long duration, low intensity therapeutic ultrasound for osteoarthritis. Proc IEEE Annu Northeast Bioeng Conf. 2014;2014:1–2.
27. Noori SA, Rasheed A, Aiyer R, et al. Therapeutic ultrasound for pain management in chronic low back pain and chronic neck pain: a systematic review. Pain Med. 2019. doi:10.1093/pm/pny287
28. Wu Y, Zhu S, Lv Z, et al. Effects of therapeutic ultrasound for knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil. 2019;33(12):1863–1875. doi:10.1177/0269215519866494
29. Daniels S, Santiago G, Cuchna J, Van Lunen B. The effects of low-intensity therapeutic ultrasound on measurable outcomes: a critically appraised topic. J Sport Rehabil. 2018;27(4):390–395. doi:10.1123/jsr.2016-0099
30. Higgins A, Glover M, Yang Y, Bayliss S, Meads C, Lord J. EXOGEN ultrasound bone healing system for long bone fractures with non-union or delayed healing: a NICE medical technology guidance. Appl Health Econ Health Policy. 2014;12(5):477–484. doi:10.1007/s40258-014-0117-6
31. Best TM, Wilk KE, Moorman CT, Draper DO. Low intensity ultrasound for promoting soft tissue healing: a systematic review of the literature and medical technology. Intern Med Rev (Wash D C). 2016;2(11).
32. Karnes JL, Burton HW. Continuous therapeutic ultrasound accelerates repair of contraction-induced skeletal muscle damage in rats. Arch Phys Med Rehabil. 2002;83(1):1–4. doi:10.1053/apmr.2002.26254
33. Rigby JH, Taggart RM, Stratton KL, Lewis GK
34. Draper DO, Castel JC, Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. 1995;22(4):142–150. doi:10.2519/jospt.1995.22.4.142
35. Hauck M, Noronha Martins C, Borges Moraes M, et al. Comparison of the effects of 1MHz and 3MHz therapeutic ultrasound on endothelium-dependent vasodilation of humans: a randomised clinical trial. Physiotherapy. 2019;105(1):120–125. doi:10.1016/j.physio.2017.08.010
36. Reed BV, Ashikaga T, Fleming BC, Zimny NJ. Effects of ultrasound and stretch on knee ligament extensibility. J Orthop Sports Phys Ther. 2000;30(6):341–347. doi:10.2519/jospt.2000.30.6.341
37. Acevedo B, Millis DL, Levine D, Guevara JL. Effect of therapeutic ultrasound on calcaneal tendon heating and extensibility in dogs. Front Vet Sci. 2019;6:185. doi:10.3389/fvets.2019.00185
38. Kramer JF. Effect of therapeutic ultrasound intensity on subcutaneous tissue temperature and ulnar nerve conduction velocity. Am J Phys Med. 1985;64(1):1–9.
39. da Silva Junior EM, Mesquita-Ferrari RA, Franca CM, Andreo L, Bussadori SK, Fernandes KPS. Modulating effect of low intensity pulsed ultrasound on the phenotype of inflammatory cells. Biomed Pharmacother. 2017;96:1147–1153. doi:10.1016/j.biopha.2017.11.108
40. Schulz KF, Altman DG, Moher D, Fergusson D. CONSORT 2010 changes and testing blindness in RCTs. Lancet. 2010;375(9721):1144–1146. doi:10.1016/S0140-6736(10)60413-8
41. World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
42. Kim DH, Yoon KB, Park S, et al. Comparison of NSAID patch given as monotherapy and NSAID patch in combination with transcutaneous electric nerve stimulation, a heating pad, or topical capsaicin in the treatment of patients with myofascial pain syndrome of the upper trapezius: a pilot study. Pain Med. 2014;15(12):2128–2138. doi:10.1111/pme.12611
43. Gomes C, Dibai-Filho AV, Politti F, Gonzalez TO, Biasotto-Gonzalez DA. Combined use of diadynamic currents and manual therapy on myofascial trigger points in patients with shoulder impingement syndrome: a randomized controlled trial. J Manipulative Physiol Ther. 2018;41(6):475–482. doi:10.1016/j.jmpt.2017.10.017
44. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–291. doi:10.1016/j.ejpain.2003.09.004
45. Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17(3):163–170. doi:10.1179/jmt.2009.17.3.163
46. Bade M, Cobo-Estevez M, Neeley D, Pandya J, Gunderson T, Cook C. Effects of manual therapy and exercise targeting the hips in patients with low-back pain-a randomized controlled trial. J Eval Clin Pract. 2017;23(4):734–740. doi:10.1111/jep.12705
47. Ibrahim AA, Akindele MO, Bello B, Kaka B. Translation, cross-cultural adaptation, and psychometric properties of the hausa versions of the numerical pain rating scale and global rating of change scale in a low-literate population with chronic low back pain. Spine (Phila Pa 1976). 2020;45(8):E439–E447. doi:10.1097/BRS.0000000000003306
48. Masaracchio M, Kirker K, Collins CK, Hanney W, Liu X. An intervention-based clinical reasoning framework to guide the management of thoracic pain in a dancer: a case report. Int J Sports Phys Ther. 2016;11(7):1135–1149.
49. Bobos P, Ziebart C, Furtado R, Lu Z, MacDermid JC. Psychometric properties of the global rating of change scales in patients with low back pain, upper and lower extremity disorders. A systematic review with meta-analysis. J Orthop. 2020;21:40–48. doi:10.1016/j.jor.2020.01.047
50. Harper B, Steinbeck L, Aron A. Fascial manipulation vs. standard physical therapy practice for low back pain diagnoses: a pragmatic study. J Bodyw Mov Ther. 2019;23(1):115–121. doi:10.1016/j.jbmt.2018.10.007
51. Chazard E, Ficheur G, Beuscart JB, Preda C. How to compare the length of stay of two samples of inpatients? A simulation study to compare type I and type II errors of 12 statistical tests. Value Health. 2017;20(7):992–998. doi:10.1016/j.jval.2017.02.009
52. Lydersen S. Statistical review: frequently given comments. Ann Rheum Dis. 2015;74(2):323–325. doi:10.1136/annrheumdis-2014-206186
53. Livingston EH, Cassidy L. Statistical power and estimation of the number of required subjects for a study based on the t-test: a surgeon’s primer. J Surg Res. 2005;126(2):149–159. doi:10.1016/j.jss.2004.12.013
54. Langer MD, Byrne HK, Henry T, Lewis G, Mattern C. The effect of low intensity wearable ultrasound on blood lactate and muscle performance after high intensity resistance exercise. J Exerc Physiol. 2017;20:14.
55. Morishita K, Karasuno H, Yokoi Y, et al. Effects of therapeutic ultrasound on intramuscular blood circulation and oxygen dynamics. J Jpn Phys Ther Assoc. 2014;17(1):1–7. doi:10.1298/jjpta.Vol17_001
56. Fabrizio PA, Schmidt JA, Clemente FR, Lankiewicz LA, Levine ZA. Acute effects of therapeutic ultrasound delivered at varying parameters on the blood flow velocity in a muscular distribution artery. J Orthop Sports Phys Ther. 1996;24(5):294–302. doi:10.2519/jospt.1996.24.5.294
57. Warden SJ, Metcalf BR, Kiss ZS, et al. Low-intensity pulsed ultrasound for chronic patellar tendinopathy: a randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford). 2008;47(4):467–471. doi:10.1093/rheumatology/kem384
58. Ahmed HA, Ali OI, ElLaithy MH. Continuous versus pulsed ultrasound on myofascial pain syndrome: randomized single blind controlled trial. International. J Ther Rehabil Res. 2017;17(6):2.
59. Van Der Heijden GJ, Leffers P, Wolters PJ, et al. No effect of bipolar interferential electrotherapy and pulsed ultrasound for soft tissue shoulder disorders: a randomised controlled trial. Ann Rheum Dis. 1999;58(9):530–540. doi:10.1136/ard.58.9.530
60. Draper DO. Facts and misfits in ultrasound therapy: steps to improve your treatment outcomes. Eur J Phys Rehabil Med. 2014;50(2):209–216.
61. Buchmann J, Neustadt B, Buchmann-Barthel K, et al. Objective measurement of tissue tension in myofascial trigger point areas before and during the administration of anesthesia with complete blocking of neuromuscular transmission. Clin J Pain. 2014;30(3):191–198. doi:10.1097/AJP.0b013e3182971866
62. Gerber LH, Shah J, Rosenberger W, et al. Dry needling alters trigger points in the upper trapezius muscle and reduces pain in subjects with chronic myofascial pain. PMR. 2015;7(7):711–718. doi:10.1016/j.pmrj.2015.01.020
63. Brady S, McEvoy J, Dommerholt J, Doody C. Adverse events following trigger point dry needling: a prospective survey of chartered physiotherapists. J Man Manip Ther. 2014;22(3):134–140. doi:10.1179/2042618613Y.0000000044
64. Fernandez-Carnero J, Gilarranz-de-Frutos L, Leon-Hernandez JV, et al. Effectiveness of different deep dry needling dosages in the treatment of patients with cervical myofascial pain: a pilot RCT. Am J Phys Med Rehabil. 2017;96(10):726–733. doi:10.1097/PHM.0000000000000733
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.