Back to Journals » OncoTargets and Therapy » Volume 16
Low-Grade Ductal Carcinoma in situ Within a Fibroadenoma of the Breast: A Rare Case Report and Review of the Literature
Authors Ni XH , An R, Shi QW, Wang CL
Received 31 March 2023
Accepted for publication 3 June 2023
Published 9 June 2023 Volume 2023:16 Pages 399—406
DOI https://doi.org/10.2147/OTT.S413223
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Xi-Hao Ni,1 Ran An,1 Qian-Wen Shi,2 Chang-Liang Wang3
1School of Clinical Medicine, Weifang Medical University, Weifang, Shandong Province, 261041, People’s Republic of China; 2Newborn Department, Weifang People’s Hospital, Weifang, Shandong Province, 261041, People’s Republic of China; 3Department of Breast Surgery, Weifang People’s Hospital, Weifang, Shandong Province, 261041, People’s Republic of China
Correspondence: Chang-Liang Wang, Department of Breast Surgery, Weifang People’s Hospital, No. 151 Guangwen Street, Kuiwen District, Weifang, Shandong Province, 261041, People’s Republic of China, Email [email protected]
Background: Ductal carcinoma in situ within a breast fibroadenoma is a rare malignancy with an incidence of only 0.02– 0.125%. Imaging of low-grade ductal carcinoma in situ within a breast fibroadenoma shows no specific presentation. Therefore, pathology and immunohistochemistry are required for definitive diagnosis. Surgery is currently considered to be an effective treatment. There is no uniform clinical standard for postoperative adjuvant radiotherapy.
Case Summary: A 60-year-old female patient underwent excisional biopsy on October 19, 2022. Pathology and immunohistochemistry confirmed the diagnosis of low-grade ductal carcinoma in situ within the fibroadenoma. Subsequently, breast-conserving surgery and sentinel lymph node biopsy were performed under general anesthesia with tracheal intubation, and no cancer metastasis was observed in the sentinel lymph nodes or incisional margins.
Conclusion: Low-grade ductal carcinoma in situ within a breast fibroadenoma is an extremely rare malignancy, and clinicians should be familiar with its clinicopathological features and treatment methods. Multidisciplinary joint treatment is recommended to maximize the benefits to patients.
Keywords: breast, fibroadenoma, ductal carcinoma in situ, case report, review of the literature
Introduction
Fibroadenoma (FA) is the most common breast tumor found in young women,1 and can occur at any age, especially in young women between the ages of 20 and 40 years. Ductal carcinoma in situ arising within a fibroadenoma is a specific pathological type of tumor that is rarely encountered in the clinic.2 Its incidence ranges from 0.02% to 0.125%, and it is usually discovered incidentally during pathological examination of resected fibroadenoma tissue.3 At present, a few ductal carcinomas in situ within breast fibroadenomas have been reported worldwide. We analyzed a case of low-grade ductal carcinoma in situ within a breast fibroadenoma patient admitted to Weifang People’s Hospital and reviewed the relevant literature. To reduce misdiagnosis and mistreatment, we analyzed the diagnosis and treatment of ductal carcinoma in situ within the breast fibroadenoma.
Case Report
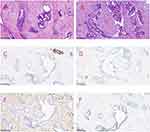
The patient, a 60-year-old female, was admitted to the Breast Surgery Department of Weifang People’s Hospital on October 19, 2022, because a right breast mass was found on examination for 1 year. There was no known family history of breast cancer or other gynecologic cancers. Her physical exam was significant for a one-centimeter palpable, mobile mass in the upper right breast at the twelve o’clock position. There was no axillary or supraclavicular lymphadenopathy. Ultrasound (US) showed that 0.7cm×0.4cm and 0.6cm×0.4cm hyper echoes were detected in the direction of 12 o’clock in the right breast, both of which were adjacent to each other with clear borders and smooth margins and relatively uniform internal echogenicity (Figure 1A and B). Mammography (MMG) showed a right breast nodule with calcified foci in the right breast, which was considered benign calcification. A round-like soft tissue nodule was seen in the upper right quadrant, with a local border that was not smooth. No significant mass was seen in the left breast, and a round-like high-density shadow was seen in the right breast (Figure 2A and B). Dynamic MRI showed a nodular shadow at the edge of the gland in the upper quadrant of the right breast, with a heterogeneous high signal in T2WI and persistent enhancement, which was approximately 0.6 cm × 1.0 cm in extent, with clear borders and irregular morphology, and no obvious burr around it. Multiple foci of punctiform enhancement were also seen in both breasts (Figure 3A and B). The patient underwent excisional biopsy. Pathological examination revealed low-grade ductal carcinoma in situ within a fibroadenoma of the breast with local infiltration of <1 mm in size. Immunohistochemistry showed AR (3+, 90%), ER (3+, 90%), PR (3+, 85%), C-erbB-2 (2+), CK5/6 (+), P63 (+), Calponin (+) (Figure 4A–F). It is worth noting that CK5/6, P63 and Calponin were positively expressed in the myoepithelium but partially absent in myoepithelium, suggesting a local infiltration of carcinoma in situ. Subsequently, breast-conserving surgery and sentinel lymph node biopsy were performed under general anesthesia with tracheal intubation. Pathology showed no cancer metastasis in the sentinel lymph nodes 1, 2, and 3, and the superior, inferior, internal, and external cut margins were clear. The patient recovered well after surgery.
Discussion
Fibroadenoma (FA) is the most common benign tumor of the breast in young women,1 and can occur at any age, especially in young women between the ages of 20 and 40 years. However, ductal carcinoma in situ occurring within a fibroadenoma is rare and has rarely been reported in literature. The reported mean age in various case series is 42.5 years,4,5 which is approximately 20 years later than the peak age of occurrence of fibroadenoma. One study reported that the elderly accounted for 11.8% of patients with fibroadenoma.6 Based on previous case reports and follow-up,7–11 there is no significant difference in the prognosis of ductal carcinoma in situ within fibroadenoma in younger and older women. This is a case of low-grade ductal carcinoma in situ within a fibroadenoma of the breast. The diagnosis, treatment, and adjuvant therapy of this disease are discussed in detail.
Imaging
At present, imaging methods such as US, MMG, and MRI are commonly used in clinical practice to diagnose ductal carcinoma in situ within a fibroadenoma. In sonographic examination of fibroadenomas, irregular shape and contour, extensive hypo-echogenicity, shadowing, echogenic halo and distortion of surrounding tissue are considered to be hints of suspicious malignancy.12 The mammographic features of carcinoma originating within a fibroadenoma include indistinct margins, clustered micro calcifications but it is quite difficult to differentiate the benign fibroadenomas from those harboring malignancy.13 Through dynamic MRI, malignant changes can be differentiated from pure FAs through differences in vascularity.14 FA typically shows on MRI a round, oval-shaped mass with a smooth margin and demonstrates a persistent enhancement until the late phase.14 However, the dynamic MRI commonly shows a rapid early enhancement with variations in delayed enhancement when carcinoma is present.14 In our case, the US showed clear borders with smooth margins and BI-RADS 3. However, MMG showed calcified foci in the right breast, and round-like soft tissue nodules with local unsmooth borders were seen in the right upper quadrant. Dynamic MRI showed a nodular shadow at the edge of the gland in the upper quadrant of the right breast, with a heterogeneous high signal in T2WI and persistent enhancement, which was approximately 0.6 cm × 1.0 cm in extent, with clear borders and irregular morphology. Therefore, we suspected the presence of cancer within this FA and recommended an excisional biopsy.
Clinical Pathology
Ductal carcinoma in situ within breast fibroadenoma is not significantly different from benign fibroadenoma in terms of clinical symptoms, and imaging manifestations are not specific. Therefore, it is easily misdiagnosed as benign fibroadenoma.15–17 Imaging findings can be used as a reference for diagnosis and not as a criterion for diagnosis. The final diagnosis depends on the clinicopathological and immunohistochemical results. Currently, common clinical methods for obtaining pathological information include fine needle aspiration cytology (FNAC), hollow core needle aspiration biopsy (CNB), and mass excision biopsy. However, owing to the heterogeneity of the lesions, FNAC and CNB are not always sufficient to exclude malignancy in benign breast lesions that are at risk of developing cancer.17 Therefore, we recommend open biopsy. In addition, FA can be divided into simple and complex types. Complex fibroadenomas were, according to Dupont et al18 defined as fibroadenomas harboring one or more of the so-called complex features: epithelial calcifications, apocrine metaplasia, sclerosing adenosis, and cysts larger than 3 mm. The risk of developing cancer is 3.10 times greater in patients with complex FA than in non-complex ones.18 Azzopardi et al19 stated that carcinoma involving a fibroadenoma may be related to one of the following: arising in the adjacent breast tissue engulfing and infiltrating a fibroadenoma, in the crevices of a fibroadenoma as well as in the adjacent breast tissue, and carcinoma restricted entirely, or at least dominantly, to a fibroadenoma. Some investigators have defined carcinoma arising within fibroadenomas as having carcinoma cells that are limited to a well-defined fibroadenoma or that only focally extend into the adjacent stroma or ducts.20 According to other researchers,21 the diagnosis of fibroadenoma carcinoma in the breast is based on:1) epithelial heterogeneous hyperplasia or carcinoma in common breast fibroadenoma; 2) cancerous tissue confined to the envelope of fibroadenoma or only small focal infiltration into the surrounding breast tissue; 3) exclusion of infiltration of surrounding breast cancer into fibroadenoma, and coexistence of breast cancer with fibroadenoma cannot be diagnosed as intra-fibroadenoma carcinoma; and 4) support of immunohistochemical marker results. In our case, intraductal carcinoma foci were found within the fibroadenoma and were confined to the fibroadenoma, and epithelial sieve-like hyperplasia originated within the fibroadenoma. Immunohistochemistry showed that CK5/6, P63 and Calponin were positively expressed in the myoepithelium. Therefore, combined with the pathological diagnosis and immunohistochemical findings, we determined that this case was a ductal carcinoma in situ within a fibroadenoma.
Surgery
Surgery is the preferred treatment for ductal carcinoma in situ within a fibroadenoma. The most effective and reasonable surgical procedure is breast-conserving surgery (BCS),5 which includes nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM). In this case, the pathological diagnosis of the excisional biopsy of the mass was low-grade ductal carcinoma in situ within a fibroadenoma, and we performed breast-conserving surgery. Because pathological biopsy suggested low-grade ductal carcinoma in situ within the fibroadenoma with local infiltration, we performed biopsies of the sentinel lymph nodes. During the operation, we removed 3 sentinel lymph nodes, none of which had metastases.
Post-Operative Adjuvant Therapy
Postoperative adjuvant therapy for low-grade ductal carcinoma in situ within a breast fibroadenoma includes radiotherapy and endocrine therapy. Studies have shown that hormone receptor-positive postmenopausal patients taking aromatase inhibitors (such as exemestane and anastrozole) after breast-conserving surgery reduce the incidence of contralateral breast cancer much better than tamoxifen.22 Ductal carcinoma in situ within a fibroadenoma is a heterogeneous disease with substantial variations in local recurrence risks, and hence, the absolute benefits of radiation therapy after surgery in individual patients.23 However, several studies have shown that low-risk patients treated with BCS alone derive less benefit from radiotherapy, whereas high-risk patients benefit more.24 In another study,25 patients treated with BCS without postoperative radiotherapy had 8-year recurrence rates of 0%, 21.5%, and 32.1% in low-, intermediate-, and high-risk patients, respectively. In this retrospective study, the Van Nuys prognostic index (VNPI) was used to calculate the recurrence risk based on tumor grade, size, margin width, and age at diagnosis. Total mastectomy is recommended for VNPI 10–12, local excision alone is feasible for VNPI 4–6, while extensive local excision combined with whole breast radiotherapy is recommended for VNPI 7–9. Genetic testing can also predict the risk of recurrence. Studies have shown that 21-gene recurrence risk assessment is now commonly used, and there is no difference between women with lower recurrence scores receiving or not receiving postoperative radiotherapy.26 In our case, the pathological diagnosis was low-grade ductal carcinoma in situ within a fibroadenoma, and a calculated score of 4 indicated a low risk of recurrence based on VNPI. The genetic test recurrence risk score was lacking because the patient did not undergo genetic testing. In addition, radiotherapy is not without risks, and high costs and reduced quality of life are borne by the patient. Specifically, lung cancer and heart disease are known to be potential long-term complications of radiation therapy, especially in long-term smokers.27 Therefore, radiotherapy is not recommended for low-risk patients. We performed breast-conserving surgery only in patients without postoperative radiotherapy. However, the value of the clinical application of VNPI remains controversial and is for reference only. Hence, the specific criteria for postoperative radiotherapy for low-grade ductal carcinoma in situ should be further explored and studied.
Conclusion
Low-grade ductal carcinoma in situ within a breast fibroadenoma is a rare malignancy with little specificity in its clinical and imaging manifestations. Therefore, patients with this disease are prone to misdiagnosis and missed diagnosis. We can only improve the accuracy of diagnosis by adequately collecting histopathological specimens and combining them with immunohistochemical results. Breast-conserving surgery (BCS) is a more effective treatment method. Adjuvant radiotherapy after surgery remains controversial, and specific treatment criteria need to be further studied. Consequently, clinicians should be familiar with their clinicopathological features and treatment methods to prolong patient survival and improve the cure rate. In addition, we recommend a multidisciplinary approach in which breast surgeons, oncologists, pathologists, radiologists, ultrasonographers, and plastic surgeons work together to improve the health and quality of life of the patient.
Abbreviations
FA, Fibroadenoma; US, Ultrasound; MMG, Mammography; FNAC, Fine needle aspiration cytology; CNB, Core needle aspiration biopsy; BCS, Breast-conserving surgery; NSM, Nipple-sparing mastectomy; SSM, Skin-sparing mastectomy; VNPI, Van Nuys prognostic index.
Ethical Approval
Institutional approval was not required to publish the case details.
Consent for Publication
Written informed consent was obtained from the patient for publication of this paper and any accompanying images.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to OncoTargets and Therapy, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by Weifang Health and Family Planning Commission (wfwsjs_2018_029).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Carty NJ, Carter C, Rubin C, Ravichandran D, Royle GT, Taylor I. Management of fibroadenoma of the breast. Ann R Coll Surg Engl. 1995;77(2):127–130. PMID: 7793802; PMCID: PMC2502143.
2. Fukuda M, Nagao K, Nishimura R, et al. Carcinoma arising in fibroadenoma of the breast--A case report and review of the literature. Jpn J Surg. 1989;19(5):593–596. PMID: 2593394. doi:10.1007/BF02471669
3. Deschênes L, Jacob S, Fabia J, Christen A. Beware of breast fibroadenomas in middle-aged women. Can J Surg. 1985;28(4):372–374. PMID: 2990650.
4. Pick PW, Iossifides IA. Occurrence of breast carcinoma within a fibroadenoma. A review. Arch Pathol Lab Med. 1984;108(7):590–594. PMID: 6329129.
5. Diaz NM, Palmer JO, McDivitt RW. Carcinoma arising within fibroadenomas of the breast. A clinicopathologic study of 105 patients. Am J Clin Pathol. 1991;95(5):614–622. PMID: 1850948. doi:10.1093/ajcp/95.5.614
6. Levi F, Randimbison L, Te VC, La Vecchia C. Incidence of breast cancer in women with fibroadenoma. Int J Cancer. 1994;57(5):681–683. PMID: 8194875. doi:10.1002/ijc.2910570512
7. Shojaku H, Hori R, Yoshida T, et al. Low-grade ductal carcinoma in situ (DCIS) arising in a fibroadenoma of the breast during 5 years follow-up: a case report. Medicine. 2021;100(10):e24023. PMID: 33725814; PMCID: PMC7969289. doi:10.1097/MD.0000000000024023
8. Marumoto A, Steinemann S, Furumoto N, Woodruff S. An uncommon pairing of common tumors: case report of ductal carcinoma in situ within fibroadenoma. Hawaii J Med Public Health. 2019;78(2):39–43. PMID: 30766763; PMCID: PMC6369888.
9. Ooe A, Takahara S, Sumiyoshi K, Yamamoto H, Shiba E, Kawai J. Preoperative diagnosis of ductal carcinoma in situ arising within a mammary fibroadenoma: a case report. Jpn J Clin Oncol. 2011;41(7):918–923. PMID: 21693482. doi:10.1093/jjco/hyr075
10. Hammood ZD, Mohammed SH, Abdulla BA, et al. Ductal carcinoma in situ arising from fibroadenoma; a rare case with review of literature. Ann Med Surg. 2022;75:103449. PMID: 35386780; PMCID: PMC8977904. doi:10.1016/j.amsu.2022.103449
11. Tiwari A, Singh BMK, Varshney S, Yadav ML. Incidental detection of carcinoma in-situ in fibroadenoma of the breast in a young woman: a rare finding. Cureus. 2018;10(12):e3797. PMID: 30868011; PMCID: PMC6402743. doi:10.7759/cureus.3797
12. Skaane P, Engedal K. Analysis of sonographic features in the differentiation of fibroadenoma and invasive ductal carcinoma. AJR Am J Roentgenol. 1998;170(1):109–114. PMID: 9423610. doi:10.2214/ajr.170.1.9423610
13. Borecky N, Rickard M. Preoperative diagnosis of carcinoma within fibroadenoma on screening mammograms. J Med Imaging Radiat Oncol. 2008;52(1):64–67. PMID: 18373829. doi:10.1111/j.1440-1673.2007.01913.x
14. Kato F, Omatsu T, Matsumura W, et al. Dynamic MR findings of ductal carcinoma in situ within a fibroadenoma. Magn Reson Med Sci. 2011;10(2):129–132. PMID: 21720115. doi:10.2463/mrms.10.129
15. Goldman RL, Friedman NB. Carcioma of the breast arising in fibroadenomas, with emphasis on lobular carcinoma. A clinicopathologic study. Cancer. 1969;23(3):544–550. PMID: 5766498. doi:10.1002/1097-0142(196903)23:3<544::aid-cncr2820230305>3.0.co;2-f
16. More IA, Sandison AT. Triple carcinoma of the breast, one arising within a fibro-adenoma. J Pathol. 1973;109(3):263–265. PMID: 4352593. doi:10.1002/path.1711090313
17. Kuijper A, Preisler-Adams SS, Rahusen FD, Gille JJ, van der Wall E, van Diest PJ. Multiple fibroadenomas harbouring carcinoma in situ in a woman with a family history of breast/ovarian cancer. J Clin Pathol. 2002;55(10):795–797. PMID: 12354814; PMCID: PMC1769772. doi:10.1136/jcp.55.10.795
18. Dupont WD, Page DL, Parl FF, et al. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331(1):10–15. PMID: 8202095. doi:10.1056/NEJM199407073310103
19. Azzopardi JG, Ahmed A, Millis RR. Problems in breast pathology. Major Probl Pathol. 1979;11:
20. Kurosumi M, Itokazu R, Mamiya Y, et al. Invasive ductal carcinoma with a predominant intraductal component arising in a fibroadenoma of the breast. Pathol Int. 1994;44(12):874–877. PMID: 7866572. doi:10.1111/j.1440-1827.1994.tb01687.x
21. Weihua H, Yang H, Haixia L. Clinicopathological observation of four cases of intramammary fibroadenoma carcinoma. J Clin Exp Pathol. 2013;29(11):1242–1244. doi:10.13315/j.cnki.cjcep.2013.11.032
22. Margolese RG, Cecchini RS, Julian TB, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, Phase 3 clinical trial. Lancet. 2016;387(10021):849–856. PMID: 26686957; PMCID: PMC4792688. doi:10.1016/S0140-6736(15)01168-X
23. Correa C, McGale P, Taylor C, et al.; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010(41):162–177. PMID: 20956824; PMCID: PMC5161078. doi:10.1093/jncimonographs/lgq039
24. Rakovitch E, Nofech-Mozes S, Hanna W, et al. Multigene expression assay and benefit of radiotherapy after breast conservation in ductal carcinoma in situ. J Natl Cancer Inst. 2017;109(4):djw256. PMID: 30053207; PMCID: PMC6233855. doi:10.1093/jnci/djw256
25. Gilleard O, Goodman A, Cooper M, Davies M, Dunn J. The significance of the Van Nuys prognostic index in the management of ductal carcinoma in situ. World J Surg Oncol. 2008;6(1):61. PMID: 18564426; PMCID: PMC2459183. doi:10.1186/1477-7819-6-61
26. Rakovitch E, Sutradhar R, Nofech-Mozes S, et al. 21-gene assay and breast cancer mortality in ductal carcinoma in situ. J Natl Cancer Inst. 2021;113(5):572–579. PMID: 33369631. doi:10.1093/jnci/djaa179
27. Taylor C, Correa C, Duane FK, et al.; Early Breast Cancer Trialists’ Collaborative Group. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35(15):1641–1649. PMID: 28319436; PMCID: PMC5548226. doi:10.1200/JCO.2016.72.0722
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.