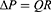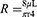Back to Journals » Medical Devices: Evidence and Research » Volume 16
Low-Cost SpO2 Integrated Neonatal CPAP Device for Low Resource Setting
Authors Dawud AA , Abagaro AM
Received 27 January 2023
Accepted for publication 1 June 2023
Published 8 June 2023 Volume 2023:16 Pages 145—156
DOI https://doi.org/10.2147/MDER.S406170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ahmed Ali Dawud, Ahmed Mohammed Abagaro
School of Biomedical Engineering, Jimma Institute of Technology, Jimma University, Jimma, Ethiopia
Correspondence: Ahmed Ali Dawud, Tel +251920846652, Email [email protected]
Introduction: More than 60% of preterm births take place in South Asia and sub-Saharan Africa, making prematurity a primary cause of neonatal mortality. Even though continuous positive airway pressure (CPAP) is a popular treatment for respiratory distress syndrome (RDS) and is safe, practicable, and efficient for use in LMICs, it is crucial to ensure that neonates receive the full benefits of the therapy by monitoring their blood oxygen level.
Methods: A centrifugal fan, power source, control system, and sensors are all included in our design. A centrifugal fan was created to provide air at positive pressure in the range of approximately 4 cmH2O to 20 cmH2O utilizing revolving blades (impeller), a DC motor, and a fixed component. The control unit contains a microcontroller to handle sensor data. The proportional-integral (PI) controller board’s external potentiometer is used to set the pressure level.
Results: To ascertain whether the prototype satisfies the design requirements, it was constructed and put through several iterations and testing. The proposed device’s prototype was tested for accuracy, affordability, and usability. The centrifugal fan speed measurement was accurate to within 94.5%, while the oxygen concentration sensor reading was accurate to within 98.5%.
Conclusion: The design investigates viability of a straightforward, inexpensive, portable SpO2 integrated neonatal CPAP device for use in the delivery room in low-resource countries and to evaluates methods for measuring flows during CPAP treatment by monitoring the level of oxygen in the blood and pressure level delivered by the device using the lowest and safest setting that yields useful results.
Keywords: centrifugal fan, CPAP, hypoxemia, oxygen concentration, prematurity, respiratory distress syndrome
Graphical Abstract:
Introduction
More than 5.9 million children die each year, most of them from illnesses that may be prevented or treated simply, and more than 95% of those fatalities take place in poor nations.1 An estimated 15 million children are born prematurely each year, with more than 60% of these births taking place in south Asia and sub-Saharan Africa.2,3 Prematurity is a primary cause of neonatal mortality. By 2030, the UN plans to lower newborn mortality to 12 deaths per 1000 live births.4 The majority of the world’s low- and middle-income nations (LMICs) are found in Africa, where the regional newborn mortality rate is 27 deaths per 1000 live births.5–8 Most preterm babies breathe when they are born, but since their lungs and respiratory control systems are still maturing, they often require further breathing support.9 To observe a significant improvement, strategies to accomplish this aim must be adapted to LMICs. To prevent lung harm, the focus of respiratory support has recently changed from invasive to noninvasive ventilation. Even if the device compresses the needed volume of air, performing efficient noninvasive ventilation is challenging due to mask leakage and airway blockage.
The main dangerous side effect of pneumonia is hypoxemia or a lack of oxygen in the blood. According to estimates, there will be 120 million occurrences of pneumonia in children under 5 years old in 2020, of which 14 million will progress to serious illness and 1.3 million will result in mortality.10 In nations where resources are few, hypoxemia is one of the best predictors of mortality. A total of 1.86 million instances of hypoxemic pneumonia occur each year, accounting for at least 13.3% of pediatric pneumonia cases and substantially increasing the mortality risk.11,12 Additional newborn disorders, such as birth asphyxia, infection, and low birth weight, all of which can result in hypoxemia, account for 23% of the 5.9 million child fatalities each year.13 These variables underscore the significance of total oxygen monitoring within health systems during CPAP treatment in low-income and middle-income countries and add to the significant burden of hypoxemia. It is important in almost all acute severe illnesses, but frequently overlooked, and blood oxygen concentration monitoring is still a luxury that many seriously ill children admitted to hospitals in impoverished nations cannot afford. This is especially true for newborn cases in small-scale institutions where, even if some installation for covering position oxygen is present, supplies are usually unstable and the advantages of therapy may be diminished by poorly maintained, absence of fully automated bias to cover oxygen level.
It is still unknown how often respiratory distress syndrome (RDS) occurs in newborns. This issue, which has started to get substantial attention in the medical community in the last 15–20 years,14–17 has been the topic of numerous epidemiological studies. Intellectual disability or cerebral palsy, cardiovascular issues, and a higher newborn death rate are all effects of RDS. For people with RDS, continuous positive airway pressure is now the most common type of therapy. However, the assessment of the therapeutic efficacy of this therapy has been restricted to the subjective assessment of physicians following CPAP therapy. Further research on performance, as well as CPAP optimization and resuscitation system fusion, has the potential to reduce the requirement for mechanical breathing and improve outcomes.
Finding methods or strategies to minimize the requirement for mechanical breathing, including both drug therapy and respiratory support systems, have been a primary focus of neonatal research. Recent randomized control studies have shown that CPAP combined with oxygen concentration monitoring is a reliable substitute for treating respiratory distress syndrome and verify that the newborns benefit fully from the treatment.18–20 However, mask leaks and airway blockage make it challenging to perform adequate noninvasive ventilation.21 A CPAP machine’s function is to deliver positive pressure through a noninvasive interface to the neonate’s lungs. As a result, variations must be assessed, and pressure should be kept constant and categorized for as long as the device is functional. In response to evaluating the efficacy and efficiency of the treatment to address concerns regarding the performance of CPAP systems, flow measuring techniques, and monitoring blood oxygen saturation, the objective of this project is to design a device with the functionality of treating respiratory dysfunction and monitoring preterm blood oxygen saturation. The device needs to be made of resources that are found in the region where it is being implemented, easily accessible reliable and user-friendly so that anyone can operate the machine.
Materials and Methods
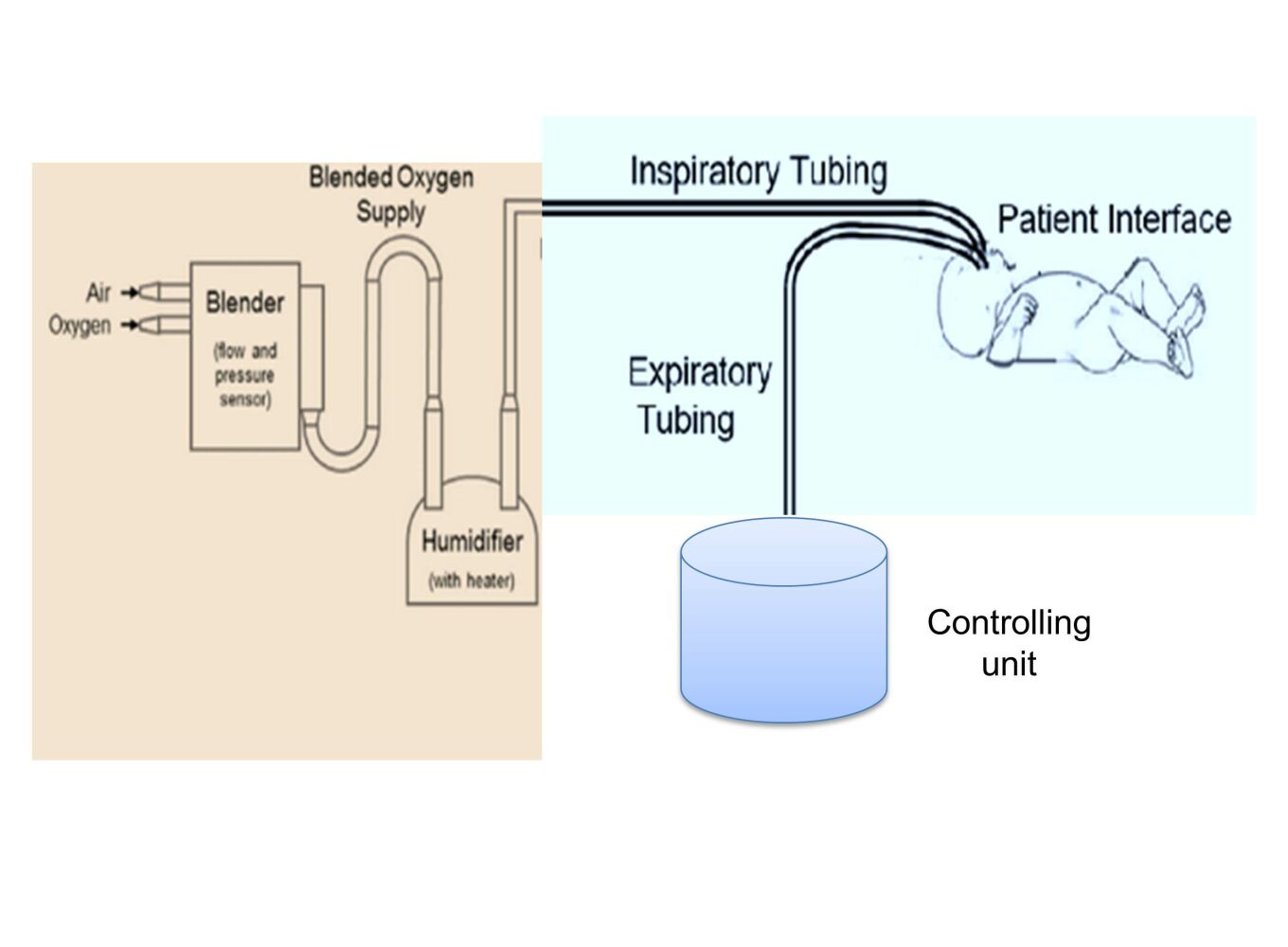
The centrifugal fan, power supply unit, conduit (containing the patient interface), pressure control, and oxygen saturation monitoring are the five essential parts of the proposed design. The respiratory cannula served as the patient interface on the prototype created for this project, which also used silicone tubing as the conduit, a centrifugal fan as the air source, a symmetric voltage power supply, a pressure sensor to regulate the pressure delivered by the device, and SpO2 sensor to measure blood oxygen saturation. The intended newborn respiratory dysfunction therapy and oxygen saturation monitoring device is shown in functional block diagram form in Figure 1. As illustrated in Figure 1, the apparatus additionally included an inbuilt flow meter for monitoring the flow of mixed oxygen and atmospheric pressure through the tube and a pressure gauge for adjusting the pressure of these gases to the required level. In addition, a humidifier was incorporated to replenish the moisture lost by the nasal airways during CPAP therapy and a two-way solenoid valve that alternately produces positive end-expiratory pressure (PEEP) based on the condensate buildup on either side of the expiratory tube.
 |
Figure 1 Block diagram of the proposed SpO2 integrated neonatal CPAP device. |
Air Source
In this project, a centrifugal fan was designed to deliver air at positive pressure in the range of approximately 4 cmH2O to 20 cmH2O utilizing revolving blades known as an impeller. A centrifugal fan is a type of pump that increases the kinetic energy in the fluid by using revolving blades. An impeller linked to a spinning shaft and a stationary casing that houses the impeller make up the two primary parts of the pump. The impeller increases the velocity by utilizing a number of blades placed in a regular pattern around the shaft, and as the velocity decreases, the housing converts the velocity to pressure. System parameters, such as variations in elevation, pipe diameters and lengths, friction factors, and small losses, define the system curve.
It is advised that the operating point occurs at a safe and effective flow rate and pressure for newborns to maintain the device’s mobility. The housing was 83 mm in diameter to provide extra clearance, while the impeller was 75 mm in diameter and contained 9 vanes. Air is sucked into the impeller’s center as it rotates and spins CW is flung outward by the vanes and descends into the base where it is sent out via the exit port. The 3D structure designed on AutoCAD software was printed using the recent 3D technology indicated in Figure 2. Our centrifugal fan design, which has the power to accomplish the extra effort needed to spin faster against the resistance of the air, employed an HDD motor developed for 12 V to 24 V. The output of the designed centrifugal fan using different power source was evaluated (see Supplemental Information Figure SI1).
 |
Figure 2 3D design of the air source: the impeller (A) and stationary component (B). |
Conduit
The inspiratory limb and the expiratory limb make up the two components that comprise the conduit for the developed prototype. The silicone tubes used for inhalation and exhalation are similar and flexible. As a result, air travels from the pump output through the inspiratory limb, nasal cannula, and expiratory limb before arriving at the valve. Each segment of tubing from the pump to the valve will undoubtedly experience a pressure drop because the device’s goal is to supply a continuous, positive pressure to the neonates. Poiseuille’s Law can be used to determine the size of pressure losses and anticipate the pressure that will be applied to the patient.22
Where ΔP is the pressure drop [Pa], Q is the flow rate through the tube [m3/s], and R represents resistance, L length [m], and r radius [m]. The nasal cannula is the patient interface, which lies between the inspiratory and expiratory limbs. Different types of nasal cannulas were analyzed for use in the developed prototype. The RAM cannula was selected for the prototype because it is made of a more flexible, softer material with thinner walled nasal prongs, decreases nasal injury without increasing the need for mechanical ventilation, is more user-friendly than others and is commonly used for CPAP in LMICs.23,24
Pressure Control
A silicon pressure sensor (XGZP6847A) was used in this design, to measure pressure over the designated full-scale pressure range. The silicon piezoresistive pressure sensor used in the XGZP6847A is fully calibrated and temperature compensated for offset, sensitivity, temperature, and nonlinearity so that it satisfies all requirements for repeatability, linearity, stability, and sensibility and can be used right away in medical equipment.25
Blood Oxygen Concentration Monitoring
The percentage of oxygen-binding sites in the blood at the measuring location that is saturated with oxygen is known as blood oxygen saturation. A MAX30100 sensor was used to measure blood oxygen levels. The sensor is an integrated pulse and heart-rate monitor sensor system that detects pulse and heart-rate signals using two LEDs, a photodetector, optimized optics, and low-noise analog signal processing. Deoxygenated blood absorbs red light and passes more infrared light, whereas blood that has been oxygenated absorbs more infrared light and passes more red light.26 The sensor’s primary job is to measure the absorption levels of both light sources and store the results in a buffer that can be accessed through the data interface.
Power Supply
The proposed device needs electricity for its components, but most likely uses less watts. The transformer converts the voltage; it accepts 220 Vac and sends constant, necessary, and reliable voltages to every component of the device. The system has an external power source with a symmetric system of voltage at 5 V, 12 V, and 24 V from this point. For detail information regarding to the design power supply (see Supplemental Information Figures SI2 and SI3).
The materials and their specifications needed to build the prototype are shown in Table 1.
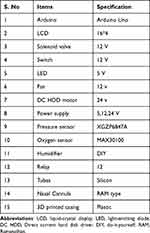 |
Table 1 List of Materials and Specifications Used to Construct the Prototype |
Results
Simulation Result
Before building the prototype and doing actual testing, the design was modeled using Proteus software and an Arduino Uno microcontroller board. Since neither MAX30100 nor XGZP6847A has a library, the inputs for the simulated system were torchlight for MAX30100 and an MPX4115 for XGZP6847A. As a result, these parts are linked to the analog pin on the Arduino board so that they may be simulated, and the output would then be shown on an LCD. A buzzer was also employed to mimic the alarming element. These pressure-flow correlations describe how the blower’s intended function as a pressure source performs.
Since a real neonate cannot be used in the simulation, we make the scientific premise that whenever the neonate expires, there will be an increase in condensate in the expiratory tube and pressure. To simulate a pressure sensor, the pressure from the sensor was manually increased and decreased based on this assumption. By manually manipulating the torchlight as the sensor’s input, a blood oxygen saturation level test simulation was carried out. We manually adjusted the safe range pressure and SpO2 based on the oxygen blending chart given by the WHO to simulate the system. As demonstrated in Figure 3, our device kept working and no warning or red light-emitting diode (LED) blinks were noticed while the pressure and SpO2 measurements were within the safe range established by the physician. The relay did not transfer the expiratory channel to the alternative tube if the pressure measurement was above or below the safe limit because of condensate accumulation, but rather, as illustrated in Figure 4, both the alarm buzzer and red LED were activated to alert the physicians. Our device may continue to function if the SpO2 level is below the safe range, but neonates may not benefit fully from the treatment. In this instance, the red LED and alarm buzzer were both activated to alert the general practitioner, as illustrated in Figure 5. The warning buzzer and red LED were also activated to alert the physician if the pressure and SpO2 measurements were outside the safe range established by the physician, as illustrated in Figure 6.
 |
Figure 3 Both the alarm and LED are off when the pressure and SpO2 are within the safe range. Abbreviations: LED, light-emitting diode; SpO2, blood oxygen concentration. |
 |
Figure 4 Both the alarm and LED are ON when the pressure is below the range. Abbreviations: LED, light-emitting diode; SpO2, blood oxygen concentration. |
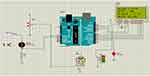 |
Figure 5 Both alarm and LED are ON when SpO2 is below the range. Abbreviations: LED, light-emitting diode; SpO2, blood oxygen concentration. |
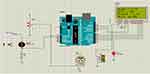 |
Figure 6 Both the alarm and LED are ON when the pressure and SpO2 readings are not in the safe range. Abbreviations: LED, light-emitting diode; SpO2, blood oxygen concentration. |
Prototype Iterations
The final design was developed after several prototype iterations. The final design was accomplished to provide a Spo2 integrated CPAP device that was safe and generally acceptable and could diagnose respiratory problems as well as monitor preterm blood oxygen saturation to determine how well the therapy was working. The centrifugal fan, power supply, controlling unit, and sensors make up this low-cost SpO2 integrated CPAP design. A model of the motor, the impeller, and the housing for the centrifugal fan was made using certain measurements; it is not the final design but rather more of a proof of concept. Then, we improved the design to make the CPAP blower more useful and robust, the housing, which was 83 mm in diameter to allow for greater space, the top and bottom covers, the 22 mm diameter exhaust tube, and the 80 mm diameter impeller, which is 75 mm in diameter and has 9 vanes. To print in extremely high quality and enable the mounting of various-sized tubes on a comparable blower, we ultimately selected to make the tube a distinct piece.
The device is composed of a centrifugal fan built for continuous positive air delivery, a pressure monitoring component (pressure sensor, LCD, and alarm), and a pressure control component called proportional integral (PI) controller board (see Supplemental Information Figures SI4 and SI5), Arduino Uno, and two-way solenoid valve). The final produced prototype also included a nasal cannula (RAM), a relay, and humidifier components. To power the device’s systems, the system requires a varying amount of voltage from an external power source. The whole development process of the low-cost Spo2 integrated CPAP with a pressure control and treatment monitoring system is shown in Figure 7.
Prototype Test Results
Accuracy, safety, affordability, portability, and ease of use were the design criteria used to develop the prototype. Due to a lack of realistic animal or other models, we tested our device by physically inflating the artificial newborn test lung and observed the outcome for ten distinct iterations. This allowed us to conduct the test in the actual world. We agree that clinical testing and assessment in humans should come after tests in animal models. The test findings were compared to the design requirements. As a result, a variety of experimental tests were carried out to determine the correctness of the prototype units. Using a tachometer to monitor speed and analyze the related pressure-flow (P-V) relationship recorded with various power sources, the planned centrifugal fan’s accuracy was evaluated. The centrifugal fan can deliver up to 14 cmH2O with a flow of 105 L/min when powered by a 12 V source, which should be enough for the majority of clinical uses of CPAP treatment. As a result, we decided to use this 12 V power supply option to construct our gadget. The power supply for the centrifugal fan can be raised if a higher pressure is needed. Using a healthy, average individual, the accuracy of oxygen saturation monitors was evaluated. The sensor data were then compared to a gold standard using a pulsometer device. The oxygen level sensor measurement and centrifugal fan speed measurements both have accuracy levels of 98.5% and 94.5%, respectively. The testing procedures employed and the test results are shown in Table 2.
 |
Table 2 Test Methods and Test Results |
Discussion
Preterm babies often have less lubricant in their lungs at birth than full-term infants, which makes it harder for them to maintain lung expansion. This can result in persistent lung conditions, which can be deadly for newborns, especially in developing nations with severely constrained access to healthcare. The other is called neonatal transition, which is marked by a sudden rise in the amount of oxygen available to tissues. As a result, preterm infants with an immature antioxidant system are more vulnerable to oxidative damage. Based on these assumptions, global guidelines advise the use of oxygen in preterm infants and have incorporated SpO2 reference ranges to direct CPAP and oxygen monitoring systems in the delivery room. Continuous positive airway pressure (CPAP) machines are commonly used to assist preterm infants in breathing, giving them a chance at a normal life. There are several devices available right now, such as bubblers.27 However, this device only unfolds, has a fixed pressure manifold, and the pressure needed to treat a newborn is much higher. Additionally, it is quite costly, making it unaffordable for middle- and low-income nations. Additionally, if the newborn is utilizing a CPAP machine, it’s crucial to check that the treatment is working as intended by measuring the blood oxygen level in the newborn.
In this paper, we introduce the feasibility of a simple, low-cost, portable SpO2 integrated neonatal CPAP device for low-resource settings by comparing existing systems used for CPAP treatment using simulated neonatal breathing. To monitor blood oxygen levels, we have also identified and assessed systems and methodologies utilized for measurements of neonatal breathing, with a focus on the in-line versus flow-through position. The sensors were utilized to measure the blood oxygen concentration in newborns as well as the pressure value compressed by the air source. The controller uses these values to control the air source’s pressure, measure the amount of oxygen in the blood, and evaluate the effectiveness of the treatment by driving an HDD DC motor pump to turn the centrifugal fan’s impeller. A proportional integrated board is part of the design, and it regulates how much pressure is applied to the patient. The LCD continuously shows the pressure and oxygen level values in real time.
The proposed method was first simulated in advance of prototype construction. The sensor readings and the feedback mechanism (regulation of the controller) were evaluated using the simulator, and the system was modified correspondingly. The compressing system, the electrical system, and the control mechanism were designed, constructed and tested sequentially, and system unit integration was performed. The body of the final design was constructed using affordable metal sheet materials. The design was made to be simple and user-friendly for physicians to easily adapt to the proposed system with minimum training. The components used for the development of the prototype cost less than 200 USD (excluding design, manufacturing, and other costs), making it potentially economical for low-resource settings. The accuracy of the sensors used was tested against a gold standard and an average accuracy of 94.5% was achieved for the centrifugal fan and 98.5% for the oxygen saturation level. This makes the effectiveness of the ventilation and the Spo2 integrated with a single setting effectively provide respiratory support and monitoring of the treatment.
Our proposed solution is unique due to the following: (1) an HDD motor and a 3D-printed impeller and casing were used in the design of the centrifugal fan; (2) the design integrates a blood oxygen concentration sensor to monitor oxygen saturation in the blood to assess the effectiveness of the treatment; (3) the device has a controlling mechanism to correct levels of oxygen in the blood and reduce pauses in breathing; and (4) the humidifier uses a container of water to produce water vapor. Thus, air is generated from the centrifugal fan, the oxygen source is humidified as it passes through the water vapor, and this air is sent to the infant. Thus, the final prototype generally offers the basic functionality of CPAP and Spo2 devices, is suitable for low-resource settings, is safe for the patient and is user-friendly. Of note, the proposed approach for neonatal CPAP device design empowers the end users in LMICs to fully control the procedure, adapt it to the local conditions and update the components used in response to market availability. In a time when complex devices appear to be needed and only be provided by a very competitive and specialized industry, the simplicity and performance of this designed low-cost Sp02 integrated CPAP device remind us of the need to go back to the basics.
As there was no real-world testing of the device, tests on the animal model should be done before clinical testing and evaluation on humans. In this work, we have demonstrated a potentially effective low-cost SpO2 integrated CPAP device that could, with more evaluation, be effective as a means of treating neonates affected by respiratory distress syndrome and hypoxemia.
Conclusion
The objective of this project is to design a device with the functionality of treating respiratory dysfunction and monitoring preterm blood oxygen saturation.In this work, a device was developed that was low cost, simple, easily accessible, and maintainable and could regulate neonatal respiratory dysfunction and monitor preterm blood oxygen saturation to ensure that the baby received the full benefits from the treatment. The humidifier is very effective and provides clean air for the infant. Our study demonstrates the potential for this low-cost, SpO2-integrated neonatal CPAP device for low-resource settings to become a globally used commercial product for neonates. As there was no real-world testing of the device, tests on the animal model should be done before clinical testing and evaluation on humans.
Acknowledgments
We would like to express our sincere gratitude to Armauer Hansen Research Institute which funded our research. We would like to thank Dr. Sisay Mengistu (a pediatrician at Jimma University Specialized Hospital) for his valuable clinical assistance and advice. Moreover, we would like to thank Jimma University, Office of Research and Community Services, which supported us in conducting the study and continuous follow-up of our research.
Funding
This project work was funded by Armauer Hansen Research Institute, Grand Challenges Ethiopian Award, through Jimma University finance office with grant No. AH0/01011/0028/20.
Disclosure
The authors declare that they have no competing interests.
References
1. World Health Organization. Newborns: Reducing Mortality. World Health Organization; 2019.
2. World Health Organization. Prevention and Control of Noncommunicable Diseases in Kyrgyztan. World Health Organization; 2017.
3. Liao MT, Tsai IJ, Lin FH, et al. Risk factors for in-hospital mortality and acute kidney injury in neonatal-pediatric patients receiving extracorporeal membrane oxygenation. J Formos Med Assoc. 2021;120:1758–1767. doi:10.1016/j.jfma.2021.03.004
4. Hug L, Alexander M, You D, Alkema L. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Heal. 2019;7:e710–e720. doi:10.1016/S2214-109X(19)30163-9
5. Bitew ZW, Alemu A, Ayele EG, Jember DA, Haile MT, Worku T. Incidence density rate of neonatal mortality and predictors in sub-saharan Africa: a systematic review and meta-analysis. Int J Pediatr. 2020;2020:1–14. doi:10.1155/2020/3894026
6. Fox H, Bitter T, Sauzet O, Rudolph V, Oldenburg O. Automatic positive airway pressure for obstructive sleep apnea in heart failure with reduced ejection fraction. Clin Res Cardiol. 2021;110:983–992. doi:10.1007/s00392-020-01701-1
7. Masaba BB, Mmusi-Phetoe RM. Neonatal survival in sub-Sahara: a review of Kenya and South Africa. J Multidiscip Healthc. 2020;Volume 13:709–716. doi:10.2147/JMDH.S260058
8. Alebel A, Wagnew F, Petrucka P, et al. Neonatal mortality in the neonatal intensive care unit of Debre Markos referral hospital, Northwest Ethiopia: a prospective cohort study. BMC Pediatr. 2020;20. doi:10.1186/s12887-020-1963-z
9. Dekker J, van Kaam AH, Roehr CC, et al. Stimulating and maintaining spontaneous breathing during transition of preterm infants. Pediatr Res. 2021;90:722–730. doi:10.1038/s41390-019-0468-7
10. Sharma N, Sethi AS, Sethi RS. Vitamin D level as a predictor of pneumonia and asthma in children less than 5 years of age. Int J Contemp Pediatr. 2020;7:1593. doi:10.18203/2349-3291.ijcp20202623
11. Soheili M, Moradi G, Baradaran HR, Soheili M, Mokhtari MM, Moradi Y. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Neonatal Med. 2021. doi:10.1080/14767058.2021.1888923
12. Rahman AE, Hossain AT, Nair H, et al. Prevalence of hypoxaemia in children with pneumonia in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Glob Heal. 2022;10:e348–e359. doi:10.1016/S2214-109X(21)00586-6
13. Gianino MM, Lenzi J, Bonaudo M, et al. Patterns of amenable child mortality over time in 34 member countries of the Organisation for Economic Co-operation and Development (OECD): evidence from a 15-year time trend analysis (2001–2015). BMJ Open. 2019;9:e027909. doi:10.1136/bmjopen-2018-027909
14. Qari SA, Alsufyani AA, Muathin SH. Prevalence of Respiratory Distress Syndrome in Neonates. Egypt J Hosp Med. 2018;70:257–264. doi:10.12816/0043086
15. Baloch K, Mugheri D, Soomro AM, et al. Assessment of neonatal respiratory distress incidences with causes, mortality and morbidity in a tertiary care hospital. J Pharm Res Int. 2020:6–10. doi:10.9734/jpri/2020/v32i2730849
16. Mirzamoradi M, Hasani Nejhad F, Jamali R, et al. Evaluation of the effect of antenatal betamethasone on neonatal respiratory morbidity in early-term elective cesarean. J Matern Neonatal Med. 2020;33(15):2533–2540.
17. Yadav SK, Giri A. Safety of early rescue surfactant replacement therapy for preterm neonates with respiratory distress syndrome at neonatal intensive care unit of a tertiary hospital. J Nepal Paediatr Soc. 2019;39:162–167. doi:10.3126/jnps.v39i3.27321
18. Huerga SF. Continuous Positive Airway Pressure (CPAP). Res Care Non Invas Mech Vent Supp. 2021;1–607. doi:10.1007/978-3-642-00418-6_1394
19. Shapiro AL. Effect of the CPAP-SAVER Intervention on Adherence. Clin Nurs Res. 2021;30:110–119. doi:10.1177/1054773819865875
20. Labarca G, Saavedra D, Dreyse J, Jorquera J, Barbe F. Efficacy of CPAP for improvements in sleepiness, cognition, mood, and quality of life in elderly patients with OSA: systematic review and meta-analysis of randomized controlled trials. Chest. 2020;158:751–764. doi:10.1016/j.chest.2020.03.049
21. Subramaniam P, Ho JJ, Davis PG. Prophylactic or very early initiation of continuous positive airway pressure (CPAP) for preterm infants. Cochrane Database Syst Rev. 2021;10. doi:10.1002/14651858.CD001243.pub4
22. Pincock C. The derivation of Poiseuille’s law: heuristic and explanatory considerations. Synthese. 2021;199:11667–11687. doi:10.1007/s11229-021-03306-1
23. Maram S, Murki S, Nayyar S, et al. RAM cannula with Cannulaide versus Hudson prongs for delivery of nasal continuous positive airway pressure in preterm infants: an RCT. Sci Rep. 2021;11. doi:10.1038/s41598-021-02988-4
24. Gokce IK, Kaya H, Ozdemir R. A randomized trial comparing the short binasal prong to the RAM cannula for noninvasive ventilation support of preterm infants with respiratory distress syndrome. J Matern Neonatal Med. 2021;34:1868–1874. doi:10.1080/14767058.2019.1651268
25. CFSensor Co. Ltd. XGZP6847 pressure sensor module. CFSensors; 2019. Available from: https://www.sgbotic.com/products/datasheets/sensors/02976-datasheet.pdf.
26. Sacan VG, Ertas KB. Performance assessment of MAX30100 SpO2/heartrate sensor. In:
27. Falk M, Donaldsson S, Drevhammar T. Infant CPAP for low-income countries: an experimental comparison of standard bubble CPAP and the Pumani system. PLoS One. 2018. doi:10.1371/journal.pone.0196683
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.