Back to Journals » Patient Preference and Adherence » Volume 17
Low Adherence is Associated with Chronic Active Disease in Ulcerative Colitis: A Retrospective Study from a Single Referral Center
Authors Viola A , Demarzo MG, Abbruzzese A, Muscianisi M, Chiappetta MF, Costantino G, Ksissa O, Alibrandi A , Fries W
Received 26 September 2022
Accepted for publication 18 February 2023
Published 23 March 2023 Volume 2023:17 Pages 807—816
DOI https://doi.org/10.2147/PPA.S390349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Anna Viola,1 Maria Giulia Demarzo,1,2 Alfredo Abbruzzese,1,3 Marco Muscianisi,1 Michele Francesco Chiappetta,1,3 Giuseppe Costantino,1 Omar Ksissa,1,3 Angela Alibrandi,4 Walter Fries1
1Department of Clinical and Experimental Medicine, IBD-Unit, University of Messina, Messina, Italy; 2Department of Internal Medicine, Ospedale Policlinico San Martino-IRCCS per l’Oncologia, Gastroenterology Unit, University of Genoa, Genoa, Italy; 3Department of Health Promotion, Mother & Child Care, Internal Medicine & Medical Specialties, PROMISE, Gastroenterology & Hepatology Unit, University of Palermo, Palermo, Italy; 4Department of Economics; Unit of Statistical and Mathematical Sciences, University of Messina, Messina, Italy
Correspondence: Anna Viola, Clinical Unit of Gastroenterology and Chronic Bowel Disorders, Department of Clinical and Experimental Medicine, Via Consolare Valeria 1, Messina, 9815, Italy, Tel +39 3338377928, Email [email protected]
Purpose: New therapeutic approaches for ulcerative colitis (UC) are now available, but there is still no robust evidence for predictors of poor outcomes. We aimed to evaluate the factors associated with a chronic active UC disease course.
Patients and Methods: Data of all UC outpatients followed for at least 3 years after diagnosis between 2005 and 2018 were retrospectively collected. The primary aim was to identify risk factors for chronic active disease 3 years after diagnosis. Moreover, the following variables were investigated: proximal disease extension or disease regression, proctocolectomy, early use of biologics (BIO) or immunomodulators (IMM), hospitalization, colorectal cancer, and adherence. We defined adherence as both, taking the prescribed therapy and constancy in scheduled follow-up visits.
Results: A total of 345 UC patients followed for a median period of 82 months were included. Patients with extensive colitis at diagnosis had a higher rate of chronic active disease 3 years after diagnosis (p< 0.012) together with a higher rate of surgery (p< 0.001) at maximum follow-up. Patients with pancolitis showed significant disease regression over time (51%) without differences in treatment. The only factor associated with chronic active disease was non-adherence (p < 0.03; OR 0.49, 95% CI: 0.26– 0.95). Adherent patients developed chronic active disease (p< 0.025) less frequently but did receive more frequent IMM (p< 0.045) or BIO (p< 0.009) therapy.
Conclusion: Patients diagnosed with pancolitis were more likely to have chronic active disease and to undergo colectomy. The only predictor for developing chronically active UC regardless of disease extension was the lack of adherence to therapy within the first 3 years after diagnosis, underlining the importance of tight control of UC patients and the need to timely identify potential risk factors for non-adherence.
Keywords: outcome, adherence, surgery, hospitalization, disease course
Introduction
Ulcerative Colitis (UC) is a chronic, potentially progressive and disabling disease that may extend to the entire colon. The clinical course of UC including the risk for surgery is still difficult to predict being characterized by periods of remission and exacerbation which have variable duration. A discrete percentage of patients will develop chronic active disease leading to very poor quality of life (QoL) and a high rate of surgery.1,2
In a recent paediatric study,3 the clusters adopted by Henriksen et al4 were investigated during the first 5 years after diagnosis showing that more than 30% of the patients will develop a chronic active or intermittent disease course despite appropriate treatment.
A timely identification of patients with a more disabling disease course over time would be helpful in order to recognize who to treat with a more aggressive therapy aimed to prevent complications and irreversible tissue damage, while, in those with indolent disease, overtreatment may be avoided, and consequently, potential side effects. However, despite numerous studies having been conducted, we are not yet able to stratify the majority of patients according to the risk for developing a more aggressive disease over time.5
Unfortunately, beyond the four items of the risk matrix model proposed by Monstad et al6 with important negative predictors such as age <40 years at diagnosis, pancolitis, signs of systemic inflammation and the early need for systemic steroids, with a colectomy rate that reaches 40% at 10 years after diagnosis, in the case of the presence of all four predictors, little progress has been made in predicting a more aggressive disease course.
Several years ago, an attempt to define the overall disease severity was carried out by using the Delphi method and various factors were included, from mucosal lesions to impact on daily life, C-reactive protein (CRP), former biologic (BIO) use and recent hospitalizations.7 The predictive value in the short term of this aforementioned disease severity index (DSI), integrated with other scores concerning the perceived stress, depression, anxiety, and QoL, was demonstrated most recently in a prospective study lasting 12 months.8
Social conditions as well as psychological factors such as distress, depression, or anxiety, besides their negative influence on disease activity9,10 do also impact on adherence to therapy.11–13 Therapy adherence represents one of the key factors in all chronic diseases determining the achievement of treatment goals, i.e. the induction of remission and the prevention of disease progression and complications.
Low adherence in UC is associated with adverse outcomes such as an increased relapse rate at 6 and 12 months.14 Moreover, more hospitalizations and emergency department admissions15 were observed in non-adherent patients such as an increased risk of colorectal cancer according to an inconstant use of 5-aminosalicylate.15 All prospective studies addressing adherence were limited to a 6- to 12-month observation period,14,16 and none of these considered non-adherence as possible risk factor for chronically active disease.
The aim of our study was to assess predictive factors including non-adherence associated with a more disabling UC course in a cohort of patients followed at our center, from diagnosis over a period of at least 36 months and beyond.
Materials and Methods
Patients
In this single-center retrospective study, patients were included according to the following criteria:
Inclusion Criteria
Patients with a confirmed clinical, endoscopic, and histologic diagnosis of UC diagnosed after the year 2005, ≥18 years of age, attending our center at diagnosis or within 6 months after diagnosis and followed for at least 3 years thereafter. The year 2005 coincides with the introduction of the first BIO agent, infliximab, for UC.
Exclusion Criteria
Patients followed before the year 2005, incomplete uploaded data, patients with discontinuous follow-up, patients followed at our unit for less than 3 years or taken in charge later than 6 months from diagnosis. Patients with IBD-U were also excluded.
Objectives
The primary aim was to identify risk factors for chronic active disease (pattern 3 according to the IBSEN study definition4 3 years after diagnosis including the following variables: proximal disease extension or disease regression assessed by endoscopy with respect to onset, proctocolectomy, early use of BIO or IMM, hospitalizations, and adherence. Adherence was defined as taking not only the prescribed medications for UC assessed at each visit but also attending regular visits in our outpatients’ clinic. Since virtually no patient showed a perfect adherence to topical therapies, this parameter was not included in adherence evaluation. Patients were categorized in adherent or not adherent.
Data Collection
Data were extracted from a local database and from clinical charts of patients by the principal (AV) and sub-investigators (G.DM, M.M.). For each patient, the following data were collected at baseline: gender, age at diagnosis, smoking status (never, ex, or active smoker), family history for IBD, and the presence of extraintestinal manifestations (EIMs). Regarding disease extent, patients were classified in accordance with the Montreal classification.17 Concomitant diseases were assessed for each patient and expressed as the Charlson comorbidity index (CCI) together with concomitant treatments.18 Anemia at diagnosis, i.e. a hemoglobin level below 13 g/dL for male patients or below 12 g/dL for female patients19 was recorded. Initial treatment strategies, i.e. use of systemic steroids at diagnosis and early introduction of immunomodulators (IMM) or BIO, were assessed.
All parameters were evaluated at 3 years from diagnosis with the exception of colorectal cancer (CRC), hospitalizations, and proctocolectomy, assessed also at maximum follow-up.
The study was approved by the local Ethics Committee (Comitato Etico Interaziendale-Messina) with protocol n. 137–20. Patient’s data were collected and handled anonymously according to the European and national laws on data protection and in compliance with the Declaration of Helsinki. Patient consent was not required due to the retrospective nature of the study.
Statistical Analysis
Statistical analysis was carried out using SSPS version 22.0 software for Windows. Categorical variables were summarized using absolute frequencies and percentages and compared with Chi-square test. Descriptive statistics included the calculation of mean values with standard deviation (SD) or median with their range, for all continuous variables. Risk factors for outcomes were assessed with stepwise logistic regression and multivariate analysis. We estimated the probability of receiving a BIO treatment according to disease extent using Kaplan–Meier curves, comparisons were performed with Log rank test. P-values <0.05 were considered statistically significant.
Results
Patients and Descriptive Data
From all UC patients followed at our center (n=1031), 345 patients met the inclusion criteria. The numbers of patients who met the inclusion/exclusion criteria are summarized in Supplementary Figure 1.
Patient baseline characteristics are given in Table 1. Patient mean age at diagnosis was 37 ± 17 years, 44% were males, 8% were current smokers, and 44 patients (12%) had a family history of IBD. Median follow-up was 82 months (36–108 months). Disease at diagnosis was located mostly at the left colon (42%) followed by pancolitis (41%). EIMs were present at diagnosis in 56 patients (16%), with joint disease the most frequent form at 8%. Anemia was present in 115 patients (34%).
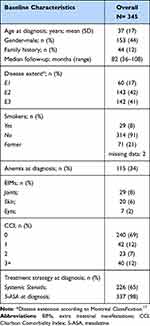 |
Table 1 Baseline Patient’s Characteristics |
Concerning comorbidities, 171 patients (50%) had at least one comorbidity at diagnosis. We observed cardiovascular diseases in 56 (16%) patients, renal disease in 20 (6%) patients, respiratory tract disorders in 12 (4%) patients, liver disease in 14 (4%) patients, metabolic disorders in 37 (11%) patients and neurologic/psychiatric disorders in 18 (5%) patients. Seventy-three patients had comorbidities classified as “others”. Regarding concomitant treatments, 172 (50%) patients were treated with non-IBD drugs at baseline and more specifically were antihypertensives in 56 (16%) patients, anticoagulants or antiplatelet agents in 30 (9%) patients, antidiabetics in 15 (4%) patients, proton pump inhibitors (PPIs) in 64 (19%) patients, drugs for thyroid diseases in 17 (5%) patients, drugs for metabolic diseases in 24 (7%) patients, drugs for neurological/psychiatric diseases in 32 (9%) patients.
Treatment strategies at diagnosis included systemic corticosteroids in 65% of the patients, and almost all patients were also treated with mesalazine (5-ASA) (98%).
Outcomes at 36 Months
Outcomes at 3 years are shown in Table 2A. Patients were divided into two groups according to disease extent at diagnosis: Group 1 (E1+E2) and Group 2 (E3). Thirteen patients (4%) with proctitis and 10 (3%) with left-sided colitis at diagnosis had a proximal disease extension after 3 years for a total of 23 patients (11%) in Group 1. The same group had 81 patients (40%) with endoscopic regression 3 years after diagnosis compared to Group 2 (E3) with 73 patients (51%) that showed a reduction of disease extension compared to diagnosis (p=0.034). Overall, in 168 patients (48%) endoscopic disease extension remained the same.
 |
Table 2 (A) Outcomes at 36 Months After Diagnosis According to Disease Extension at Diagnosis. (B) Outcomes at Maximum Follow-Up According to Disease Extension at Diagnosis |
Chronic active disease was observed in 24% of the patients in Group 1 and in 37% in patients in Group 2 (p=0.009). In the first 3 years from diagnosis, 9 patients (3%) underwent colectomy; there was no statistical difference between the two groups (3 patients of Group 1 and 6 patients of Group 2; p=0.12).
Concerning treatment, 88 patients (25%) received BIO and 107 (31%) IMM within 3 years from diagnosis; no differences were found between the two groups (see Table 2). We assessed the probability to receive BIO or IMM treatment within 3 years from diagnosis between Group 1 and Group 2: no statistically significant differences in the Kaplan–Meier curve were observed (log rank 0.43 and 0.75, respectively) (data not shown). We repeated the same analysis to assess the probability to receive BIO or IMM at 10 years with similar results (data not shown).
Adherence was evaluated after 3 years; 121 patients (66%) and 79 patients (59%), respectively, in Groups 1 and 2, were adherent to the prescribed treatment and to follow-up (not statistically significant).
Outcomes in Further Follow-Up
At maximum follow-up, 25 patients (7%) underwent proctocolectomy (Table 2B). As mentioned above, 9 of these patients underwent colectomy within 3 years from diagnosis and the other 16 at a later timepoint. According to the group stratification, patients of Group 2 had a higher rate of colectomy at maximum follow-up (p<0.001).
Sixty-nine (20%) patients were hospitalized due to IBD and 16 (6%) patients had a diagnosis of cancer (7 of them were diagnosed with CRC). No statistically significant differences were observed between the two groups for either outcome.
Risk Factors for Chronic Active Disease
Factors potentially associated with chronically active disease included in the multivariate analysis were the following: gender, age at diagnosis, family history, smoking, proctitis, anemia at diagnosis, steroids at diagnosis, early use of IMM, early use of BIO, and adherence. At stepwise logistic regression, the only risk factor associated with chronic active disease was non-adherence (p < 0.03; OR 0.49, 95% CI: 0.26–0.95) (Table 3). Finally, we did not identify any risk factors for disease extension in follow-up, proctocolectomy, and early use of BIO or IMM.
 |
Table 3 Factors Associated with Chronically Active Disease |
Outcomes According to Adherence
We performed the same analysis shown in Table 2, regarding the status of “adherent” or “non-adherent” exploring the same outcomes. As reported in Table 4A, adherent patients developed chronic active disease (p=0.025) less frequently and were more frequently treated with BIO therapies and IMMs in the first 3 years from diagnosis (p = 0.018 and p = 0.040, respectively). No differences were found for the other variables. At maximum follow-up (Table 4B), no difference was found between adherent and non-adherent patients for surgery, hospitalizations, and diagnosis of CRC.
 |
Table 4 (A) Outcomes at 36 Months After Diagnosis According to Adherence. (B) Outcomes at Maximal Follow-Up According to Adherence |
Discussion
Adherence
Our study aimed to assess risk factors for chronic active disease 36 months after diagnosis in an adult cohort of patients with UC. Our main findings were that adherent patients developed less frequently chronic active disease and that non-adherence to therapy and follow-up visits was the only variable significantly associated with this more aggressive disease pattern. Adherence represents a major problem in every chronic disease needing continuous therapy. Most studies on adherence in UC focus on 5-ASA maintenance treatment, but non-adherence was reported also with IMM and even BIO therapies.20–24 In the present study, overall adherence according to regular drug utilization and follow-up visits reached 58%, a figure in line with former, dedicated studies.25,26 The strength of our analysis is the length of follow-up, while almost all studies investigating prospectively non-adherence were limited to shorter periods not exceeding 6 to 12 months14,16,20 or were cross-sectional.25
Investigating adherence, most studies adopted the Morisky adherence scale27 or calculated adherence from prescriptions16 or measured 5-ASA metabolites in urines,25 whilst in the present setting we used a wider term for adherence, i.e. we evaluated the willingness to comply with scheduled visits combined with a regular interview on medication adherence. Interestingly, our study demonstrated a higher use of IMM and BIO in adherent patients, which may be an expression of early identification of patients that needed therapy escalation and, thus, may be compared to a form of “tight control” as proposed in the CALM study in Crohn’s disease.28 There was no difference between the two groups for other variables usually associated with worse outcomes such as proximal disease extension, hospitalization, or surgery.
Non-adherence may result from factors related to therapies such as safety concerns or a high pill load, to disease such as being in remission or having a recent diagnosis, to the patient itself,29 or the relationship between patient and its physician.13 Other factors which negatively impact adherence but also QoL of patients with UC are represented by coexisting psychological/psychiatric disorders.10 A systematic review showed that in young IBD patients the presence of psychological morbidity decreased treatment adherence by 12%.30 Anxiety, depression, stress, stigmatization leading to isolation,31–33 are all problems investigated in UC patients. Addressing mental health problems in patients with IBD can improve their adherence to treatment and the somatic disease course and, consequently, reduce morbidity.34
In the present study, non-adherence was identified as being associated with a worse disease course in UC, but an investigation on the causes for non-adherence unfortunately was not included. In our cohort, the rate of psychological morbidity was low (about 5%), but these data were probably underestimated due to the retrospective nature of the study and to the fact that comorbidities and concomitant treatments were registered only at diagnosis for patients with an established psychiatric diagnosis.
Disease Regression
Another important result of our study was disease regression found in 45% of the whole study population, statistically more important in patients with pancolitis at diagnosis. Although numerically higher, in adherent patients, the difference failed to achieve statistical significance. While most studies focus on progression of disease extension,35 less data are available on disease regression over time. Predictors of disease regression were not assessed, and apparently subgroup analysis between E1/E2 and E3 did not reveal a higher use of more aggressive therapy. A statistically significant difference for treatments was found when analyzing adherent versus non-adherent patients. This result is in line with the finding that early introduction of thiopurines may reduce the risk of surgery and proximal disease extension,36 whereas the use of anti-TNF agents in preventing surgery is still matter of debate.37
Proximal Disease Extension
Concerning disease progression, in our cohort 11% progressed to a more extensive form within 3 years. Using the Montreal classification, E3 includes subtotal colitis which may potentially progress to pancolitis and this may have led to an underestimation of the rate of proximal disease extension. Proximal disease extension is deemed to be present in 15% of the patients at 5 years reaching 30% at 10 years and 50% at 30 years.35 In the present study, adherence was associated with halving of disease progression to 5% although this figure failed to reach statistical significance.
A recent retrospective Asian paper in 518 UC patients showed that a disease extension during follow-up occurred with a cumulative rate of 19.6% at 3 years after diagnosis, which is a higher percentage compared to ours (11%). Their only risk factor for disease progression was the disease extent at diagnosis (odds ratio (OR), 1.74, 95% confidence interval (CI) 1.18–2.57, p = 0.01).38
Chronic Active Disease
Chronic active disease represents a subtype of UC leading to extremely poor QoL, functional deficits of the colon, and, in up to 27%, to proctocolectomy at 3 years.2 In the present study, this pattern was present in 37% of the patients with pancolitis and in 24% of the patients with more limited forms. In the present study, we showed that chronic active disease was significantly more frequent 3 years after diagnosis in non-adherent patients.
Colectomy
Our study confirmed that extensive colitis per se at diagnosis had a higher rate of colectomy within the first 3 years from diagnosis. Our rate of colectomy at maximum follow-up reached 15% in patients with extensive colitis, whereas only 2% of the patients with less extensive forms were operated on. The overall surgery rate was 7% in the present study. Our data are comparable to the rate of the largest prospective study on UC long-term prognosis, the IBSEN study, including patients with 20 years of follow-up.6 Although the cumulative colectomy rate for the total cohort [n = 519] was 13.0% [95% CI (11.4–14.6)] at 20 years, almost half of the colectomies were performed within the first 2 years from diagnosis (about 5%) quite similar to our 3% in 36 months of our whole cohort. However, the IBSEN cohort was established between 1990 and 1993, long before the introduction of BIO therapies and was composed of patients mainly on mesalazine and steroids. A quite higher surgery rate was reported in chronic active disease with a colectomy rate at 3 years of 27%.2
Colorectal Cancer
In our population, the rate of CRC was 2% during a median follow-up of 7 years. Our rate was similar or slightly higher compared to other larger studies. In a large two-nation cohort study, CRC was diagnosed in 1.2% of UC patients from Denmark and 1.5% of the patients from Sweden while the IBSEN study showed a cumulative incidence of developing CRC of 1.6% in UC patients after 20 years of follow-up from diagnosis.39,40 In our cohort, patients with extensive colitis had a higher rate of cancer diagnosis compared to Group 1, but this difference was not statistically significant. Analysis on adherence showed no significant differences in cancer development in non-adherent versus adherent patients.
The main strengths of our study are the elevated number of patients (all of them from the era of BIO drugs) and the prolonged observation period. Moreover, we evaluated cumulative adherence after 3 years of follow-up and not only in the short term. Nonetheless, there are several limitations. The retrospective nature of the study that affected data collection including the lack of biochemical markers at diagnosis (ie, CRP) and of numerically scoring of clinical and endoscopic activity during follow-up. Moreover, data on psychiatric comorbidities and data on concomitant treatments were assessed only at baseline and, thus, may be incomplete and underreported. Although the study was conducted at a referral center, the potential bias of following more “severe” patients was overcome by including all patients meeting the inclusion criteria at diagnosis or shortly thereafter.
Conclusion
Our study is a picture of almost 10 years of the course of ulcerative colitis in the era of biologic treatments. Adherence to therapy and follow-up visits led to less chronic active disease and was the only protective factor at multivariate analysis for a lower risk of developing chronic active UC.
Despite the introduction of new of treatments, we confirmed the finding that patients diagnosed with pancolitis were more likely to undergo colectomy or to develop a chronic active disease, but the present study adds a piece to the whole picture underlining the importance of adherence that may permit a timely intervention with more powerful treatments. Psychologic/psychiatric problems interfering potentially with adherence are frequently not investigated during visits but should be addressed, if possible, together with trained psychologist/psychiatrist during follow-up visits.
All clinical information is easy to obtain in daily practice, but the path to identifying other predictors related to poor outcomes is still long and ongoing.
Acknowledgments
The study was supported by an unconditional grant from Ferring S.p.A., Italy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Latella G, Papi C. Crucial steps in the natural history of inflammatory bowel disease. World J Gastroenterol. 2012;18:3790–3799. doi:10.3748/wjg.v18.i29.3790
2. Angelison L, Almer S, Eriksson A, et al.; Swedish Organization for the Study of Inflammatory Bowel diseases (SOIBD). Long-term outcome of infliximab treatment in chronic active ulcerative colitis: a Swedish multicentre study of 250 patients. Aliment Pharmacol Ther. 2017;45:519–532. doi:10.1111/apt.13893
3. Aloi M, Bramuzzo M, Norsa L, et al.; SIGENP IBD Working Group. Disease activity patterns in the first 5 years after diagnosis in children with ulcerative colitis: a population-based study. J Crohns Colitis. 2021;15:367–374. doi:10.1093/ecco-jcc/jjaa203
4. Henriksen M, Jahnsen J, Lygren I, et al.; IBSEN Study Group. Ulcerative colitis and clinical course: results of a 5-year population-based follow-up study (the IBSEN study). Inflamm Bowel Dis. 2006;12:543–550. doi:10.1097/01.MIB.0000225339.91484.fc
5. Reinisch W, Reinink AR, Higgins PD. Factors associated with poor outcomes in adult with newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:635–642. doi:10.1016/j.cgh.2014.03.037
6. Monstad IL, Solberg IC, Cvancarova M, et al. Outcome of ulcerative colitis 20 years after diagnosis in a prospective population-based inception cohort from South-Eastern Norway, the IBSEN study. J Crohns Colitis. 2021;15:969–979. doi:10.1093/ecco-jcc/jjaa232
7. Siegel CA, Whitman CB, Spiegel BMR, et al. Development of an index to define overall disease severity in IBD. Gut. 2018;67:244–254. doi:10.1136/gutjnl-2016-312648
8. Swaminathan A, Fan D, Borichevsky GM, et al. The disease severity index for inflammatory bowel disease is associated with psychological symptoms and quality of life, and predicts a more complicated disease course. Aliment Pharmacol Ther. 2022;56:664–674. doi:10.1111/apt.17058
9. Turnbull GK, Vallis TM. Quality of life in inflammatory bowel disease: the interaction of disease activity with psychosocial function. Am J Gastroenterol. 1995;90:1450–1454.
10. Taft TH, Ballou S, Bedell A, et al. Psychological considerations and interventions in inflammatory bowel disease patient care. Gastroenterol Clin North Am. 2017;46:847–858. doi:10.1016/j.gtc.2017.08.007
11. Gray WN, Denson LA, Baldassano RN, et al. Treatment adherence in adolescents with inflammatory bowel disease: the collective impact of barriers to adherence and anxiety/depressive symptoms. J Pediatr Psychol. 2012;37:282–291. doi:10.1093/jpepsy/jsr092
12. Greene BR, Blanchard EB, Wan CK. Long-term monitoring of psychosocial stress and symptomatology in inflammatory bowel disease. Behav Res Ther. 1994;32:217–226. doi:10.1016/0005-7967(94)90114-7
13. Dasharathy SS, Long MD, Lackner JM, et al. Psychological factors associated with adherence to oral treatment in ulcerative colitis. Inflamm Bowel Dis. 2023;29:97–102. doi:10.1093/ibd/izac051
14. Kane S, Huo D, Aikens J, et al. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114:39–43. doi:10.1016/S0002-9343(02)01383-9
15. van Staa TP, Card T, Logan RF, et al. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573–1578. doi:10.1136/gut.2005.070896
16. Mitra D, Hodgkins P, Yen L, et al. Association between oral 5-ASA adherence and health care utilization and costs among patients with active ulcerative colitis. BMC Gastroenterol. 2012;12:132. doi:10.1186/1471-230X-12-132
17. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi:10.1136/gut.2005.082909
18. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi:10.1016/0021-9681(87)90171-8
19. WHO, UNICEF, UNO. Iron Deficiency Anemia: Assessment, Prevention and Control. Report of a Joint WHO/UNICEF/UNU Consultation. Geneva: World Health Organization; 1998.
20. Kamperidis N, Goodhand JR, Chowdhury FA, et al. Factors associated with nonadherence to thiopurines in adolescent and adult patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2012;54:685–689. doi:10.1097/MPG.0b013e3182475e71
21. D’Incà R, Bertomoro P, Mazzocco K, et al. Risk factors for non-adherence to medication in inflammatory bowel disease patients. Aliment Pharmacol Ther. 2008;27:166–172. doi:10.1111/j.1365-2036.2007.03555.x
22. Campos S, Portela F, Sousa P, et al. Inflammatory bowel disease: adherence to immunomodulators in a biological therapy era. Eur J Gastroenterol Hepatol. 2016;28:1313–1319. doi:10.1097/MEG.0000000000000704
23. Lopez A, Billioud V, Peyrin-Biroulet C, et al. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis. 2013;19:1528–1533. doi:10.1097/MIB.0b013e31828132cb
24. Al Khoury A, Xiao Y, Golovics PA, et al. Assessing adherence to objective disease monitoring and outcomes with Adalimumab in a real-world IBD cohort. Dig Liver Dis. 2021;53:980–986. doi:10.1016/j.dld.2021.02.006
25. Shale MJ, Riley SA. Studies of compliance with delayed-release mesalazine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18:191–198. doi:10.1046/j.1365-2036.2003.01648.x
26. Bernal I, Domènech E, Garcia-Planella E, et al. Medication-taking behavior in a cohort of patients with inflammatory bowel disease. Dig Dis Sci. 2006;51:2165–2169. doi:10.1007/s10620-006-9444-2
27. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi:10.1097/00005650-198601000-00007
28. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled Phase 3 trial. Lancet. 2017;390:2779–2789. doi:10.1016/S0140-6736(17)32641-7
29. Testa A, Castiglione F, Nardone OM, et al. Adherence in ulcerative colitis: an overview. Patient Prefer Adherence. 2017;11:297–303. doi:10.2147/PPA.S127039
30. Brooks AJ, Rowse G, Ryder A, et al. Systematic review: psychological morbidity in young people with inflammatory bowel disease - risk factors and impacts. Aliment Pharmacol Ther. 2016;44:3–15. doi:10.1111/apt.13645
31. Taft TH, Keefer L. A systematic review of disease-related stigmatization in patients living with inflammatory bowel disease. Clin Exp Gastroenterol. 2016;9:49–58. doi:10.2147/CEG.S83533
32. Umar N, King D, Chandan JS, et al. The association between inflammatory bowel disease and mental ill health: a retrospective cohort study using data from UK primary care. Aliment Pharmacol Ther. 2022;56:814–822. doi:10.1111/apt.17110
33. Oyama H, Moroi R, Tarasawa K, et al. Depression is associated with increased disease activity in patients with ulcerative colitis: a propensity score-matched analysis using a nationwide database in Japan. JGH Open. 2022;6:876–885. doi:10.1002/jgh3.12836
34. Arp L, Jansson S, Wewer V, et al. Psychiatric disorders in adult and paediatric patients with inflammatory bowel diseases - a systematic review and meta-analysis. J Crohns Colitis. 2022;16:1933–1945. doi:10.1093/ecco-jcc/jjac095
35. Krugliak cleveland N, Torres J, Rubin DT. What does disease progression look like in ulcerative colitis, and how might it be prevented? Gastroenterology. 2022;162:1396–1408. doi:10.1053/j.gastro.2022.01.023
36. Kariyawasam VC, Mourad FH, Mitrev N, et al. Early thiopurine maintenance is associated with reduced proximal disease progression and colectomy rate in ulcerative colitis. Eur J Gastroenterol Hepatol. 2021;33:1524–1532. doi:10.1097/MEG.0000000000002101
37. Wong DJ, Roth EM, Feuerstein JD, et al. Surgery in the age of biologics. Gastroenterol Rep. 2019;7:77–90. doi:10.1093/gastro/goz004
38. Qiu Y, Chen B, Li Y, et al. Risk factors and long-term outcome of disease extent progression in Asian patients with ulcerative colitis: a retrospective cohort study. BMC Gastroenterol. 2019;19:7. doi:10.1186/s12876-018-0928-2
39. Olén O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123–131. doi:10.1016/S0140-6736(19)32545-0
40. Klepp P, Brackmann S, Cvancarova M, et al. Risk of colorectal cancer in a population-based study 20 years after diagnosis of ulcerative colitis: results from the IBSEN study. BMJ Open Gastroenterol. 2020;7:e000361. doi:10.1136/bmjgast-2019-000361
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
