Back to Journals » OncoTargets and Therapy » Volume 11
Loss of miR-516a-3p mediates upregulation of ABCC5 in prostate cancer and drives its progression
Authors Zhang H, Lian Z, Sun G, Liu R, Xu Y
Received 7 March 2018
Accepted for publication 4 June 2018
Published 6 July 2018 Volume 2018:11 Pages 3853—3867
DOI https://doi.org/10.2147/OTT.S167463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Hongtuan Zhang, Zhenpeng Lian, Guangyu Sun, Ranlu Liu, Yong Xu
Department of Urology, National Key Specialty of Urology, The Second Hospital of Tianjin Medical University, Tianjin Key Institute of Urology, Tianjin Medical University, Tianjin, China
Abstract: To gain a comprehensive understanding of whether ABCC5 can regulate prostate cancer (PCa) progression, we performed microarray data analyses and identified that ABCC5 was drastically increased in primary PCa relative to normal samples, metastatic PCa relative to primary PCa, and castration-resistant PCa relative to hormone naïve PCa, respectively. Multivariate Cox regression analysis suggested that ABCC5 overexpression in PCa was an independent prognostic factor for both poor biochemical recurrence-free and overall survival. We demonstrated that ABCC5 knockdown significantly inhibits PCa cell proliferation, migration, and invasion in vitro and suppresses tumor growth and metastasis in vivo. We also demonstrated that miR-516a-3p was significantly downregulated in PCa. We finally demonstrated that ABCC5 was a direct target of miR-516a-3p. miR-516a-3p overexpression can phenotypically copy ABCC5 knockdown-induced phenotypes, whereas forced expression of ABCC5 can drastically reverse the inhibitory effects of miR-516a-3p. miR-516a-3p may modulate the sensitivity of cancer cells to adriamycin and docetaxel by targeting ABCC5 with important implications in the design of new therapeutic agents. Taken together, our results indicated that loss of miR-516a-3p expression and thus uncontrolled ABCC5 upregulation might drive PCa progression and influence chemosensitivity.
Keywords: ABCC5, miR-516a-3p, prostate cancer, metastasis, chemosensitivity
Introduction
Prostate cancer (PCa) is the most commonly diagnosed noncutaneous malignancy and the second most common cause of male cancer-related deaths.1 It is well known that the etiology of PCa development and progression is complex. In addition to the environmental and genetic factors, lifestyle and diet can also contribute to this disease. Although localized PCa is highly curable, many patients will die of metastatic disease. Androgen-deprivation therapy (ADT) can result in rapid responses in metastatic PCa patients; however, nearly all patients eventually progress to lethal, metastatic castration-resistant PCa (CRPC). Chemotherapy is considered therapeutically efficacious for metastatic CRPC. Drug resistance poses a great challenge in treating chemorefractory PCa patients. Due to poor prognosis of metastatic CRPC, an improved and detailed understanding of the molecular and cellular mechanisms underlying PCa development, progression, and castration resistance is urgently needed, and is necessary to the development of novel effective therapies. Therefore, understanding the molecular mechanisms underlying PCa development and progression would help to improve therapies for the disease. Assessment of the expression and role of metastasis and castration resistance-associated oncoproteins in PCa is required for defining molecular variables associated with tumor aggressiveness, identifying novel PCa biomarkers, and developing more effective therapeutic interventions.
The ATP-binding cassette (ABC) transporters consist of a family of integral membrane proteins capable of unidirectional transport of a wide variety of compounds across cell membranes. Based on sequence homology and domain organization, the ABC family is subdivided into seven subfamilies (ABCA–ABCG). The multidrug resistance (MDR) ABCC family consists of nine related transporters (ABCC1-6, ABCC10-12, or MRP1-9); the MRP5 (ABCC5) protein has two hydrophobic transmembrane domains and two cytoplasmic domains. Next to their natural function, most ABCC transporters have been implicated in drug resistance, but they have a wide range of different substrate specificities. The ABCC5, as one of the critical ABC transporter molecules, is capable of transporting monophosphorylated nucleoside analogs, which can confer resistance to 6-mercaptopurine, 6-thioguanine, and 9-(2-phosphonyl-methoxyethyl) adenine.2 ABCC5 has also been shown to be involved in antimetabolite resistance.3 ABCC5 is also involved in paclitaxel drug resistance.4 The mechanism is based on the efflux of the somewhat polar phosphonate and of the monophosphates of 6-mercaptopurine and 5-fluorouracil out of the cell.5 Because the physiological function of ABC transporters is the efflux of xenobiotics, including chemotherapeutic drugs, the increased expression of MDR genes is the expected cell response to chemotherapy. Recent findings showed that ABCC5 expression is aberrantly upregulated in several human malignancies,6–8 and it promotes invasion and metastasis in these malignancies. These results indicated that ABCC5 can regulate cancer cell invasiveness. The above results also indicated that ABCC5 may act as an important oncogene that is involved in the formation and development of malignancies and might influence the chemoresistance of malignancies. However, the molecular mechanism and the role of ABCC5 in PCa development and progression have not yet been investigated.
Accelerating evidence showed the important role of miRNAs in the regulation of the target gene expression. miRNAs, a class of small noncoding RNAs, have been reported to play important roles in malignancy development and progression.9,10 miRNAs can regulate gene expression at the posttranscriptional level by blocking mRNA translation or degrading mRNAs by binding to the 3′-untranslated regions (3′-UTRs) of specific mRNAs.11 Accumulating studies have shown that miRNAs can be abnormally expressed in malignancies, and deregulated miRNAs were associated with malignancy initiation, promotion, and progression.11,12 In view of the extensive molecular functions of miRNAs and ABCC5 in various malignancies and the potential critical role of ABCC5 in prostate carcinogenesis, it will be very interesting to identify the molecular mechanism and role of ABCC5 in PCa and to find whether ABCC5 gene expression can be regulated by a specific miRNA in PCa.
In the current study, we first conducted microarray data analyses and identified ABCC5 as a metastasis and castration resistance-associated oncogene, and then we found significant correlations between ABCC5 expression and the clinicopathological factors and prognosis of PCa patients. We further explored its effects on the malignant phenotype of PCa cells. We also identified that the miR-516a-3p can regulate the invasiveness of PCa cells and the metastasis of PCa xenografts by regulating ABCC5. Taken together, the above findings have advanced our knowledge of the molecular mechanisms of PCa metastasis, castration resistance, and chemoresistance, provided potential effective molecular biomarkers for the prognosis, and developed effective potential therapeutic targets for the treatment of PCa.
Materials and methods
Clinical specimens
Written informed consent was obtained from all of the 180 patients with PCa.13 We also collected 90 normal prostate samples, 30 metastatic PCa, and 20 CRPC tissues. Written informed consent was also obtained from the above 140 patients. All experiments were approved by the ethics committee of Tianjin Medical University (TMUhMEC2016019). Biochemical recurrence (BCR) was detected if prostate-specific antigen (PSA) value after radical prostatectomy was ≥0.2 ng/mL.
Plasmid construction
A cDNA sequence containing one pre-miR-516a-3p unit was inserted into pcDNA3.1. The ABCC5 shRNA was designed using online shRNA designer tool. Two strands were annealed following the instructions, followed by insertion into pcDNA6.2-GW/EmGFP-miR vector. The ABCC5 cDNA containing the coding sequence was cloned by PCR, and the PCR product was inserted into the pcDNA3.1 vector. The insert was confirmed by DNA sequencing.
Cell line, cell culture, and transfection
Human LNCaP and PC-3 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in the presence of 5% CO2. PCa cells were plated in six-well plates for stable transfection using Effectene (Qiagen) following the manufacturer’s protocol.
RNA extraction and qRT-PCR
Total RNA was extracted using Trizol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the provided instructions. The miR-516a-3p level was investigated by TaqMan miRNA assays (Thermo Fisher Scientific) according to the manufacturer’s protocol. miRNA U6 was used for normalization. ABCC5 mRNA level was studied by SYBR green qPCR assay (Takara, Dalian, China). β-Actin was used as control.
Western blot and immunohistochemistry
Extracts containing 50 μg of proteins were separated in 10% SDS-PAGE gels and electroblotted onto nitrocellulose membranes. The membranes were blocked using Tris-buffered saline/Tween-20 containing 5% nonfat milk followed by overnight incubation at 4°C with primary antibodies (ab24107; Abcam). After three washes, secondary antibodies were added and incubated for 1 hour. Then anti-β-actin antibody was used as a loading control. Immunohistochemistry was performed following the manufacturer’s protocol. ABCC5 protein level was measured by combining the proportion and intensity of positively stained immunoreactive cells.
Colony formation assay
The measured PCa cells were seeded on 35 mm dishes. These cells were fixed and stained. Finally, positive colony formation (>50 cells/colony) was counted.
Cell migration and invasion assay in vitro
Transwell migration and invasion assays were conducted with 8.0 mm pore following the provided protocol (BD Bioscience, San Jose, CA, USA) and were conducted with uncoated and coated Matrigel, respectively. Finally, the migrated and invaded PC-3 and LNCaP cells were fixed, stained, and counted.
Luciferase reporter assay
The miR-516a-3p-binding site in the 3′-UTR of ABCC5 (wild or mutant-type) was inserted downstream of the firefly luciferase gene in a pGL3-promoter vector (Promega, Madison, WI, USA). The assay was conducted according to the provided protocol. Luciferase activity was investigated by the dual luciferase reporter assay system (Promega).
PC-3 cells’ chemosensitivity to adriamycin and docetaxel studies
Adriamycin was initially dissolved in 0.9% sodium chloride and was diluted with fresh medium immediately before each experiment. The concentration of adriamycin varied from 0.01 to 1 μmol/L. Docetaxel is used at a concentration of 10 nM. The PC-3 cell survival was determined using MTT assay. Twenty microliters of MTT was added to each well and incubated at 37°C for 4 hours. Then the medium in each well was removed and 150 μL DMSO was added. The number of PC-3 cells was assessed by measurement of absorbance at 492 nm using Multiskan MS.
PCa xenograft studies
Xenografts were developed with the stable expressing miR-516a-3p, ABCC5 knockdown, or control LNCaP and PC-3 cells, respectively. PC-3 or LNCaP cells were implanted into the dorsal flank of male Athymic nude mice subcutaneously. Tumor volumes were calculated biweekly [tumor volume = (width2 × length)/2]. The xenograft weights were investigated 7 weeks after the inoculation. Then, the DNA extraction of cervical lymph nodes and human Alu sequence PCR amplification was conducted to study the distant metastasis status.14 All procedures and experiments involving animals in this study were approved by the Committee on the Ethics of Animal Experiments of Tianjin Medical University (TMUaMEC2016023) and were performed in compliance with the guidelines of the Animal Welfare Act of the National Institutes of Health (NIH Publications no 8023, revised 1978).
Statistical analysis
Student’s t-test was conducted for continuous factors. Spearman correlation test was performed for examining the correlations between ABCC5 expression and the clinical and pathological factors. Survival curves were conducted by the Kaplan–Meier and studied using the log-rank test. P<0.05 was considered statistically significant. Statistical analysis was conducted by GraphPad Prism 5 and SPSS 17.0 software.
Results
ABCC5 as a potential metastasis and castration resistance-associated oncogene in PCa
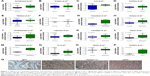
In order to identify whether any difference of ABCC5 expression level exists in primary PCa, metastatic PCa, CRPC, and normal prostate tissues, we analyze the microarray data from The Cancer Genome Atlas and several datasets (GSE55945, GSE35988, GSE68545, GSE6099, GSE6956, GSE6919, GSE3325, GSE6608, GSE3933, GSE68882, GSE21032).15–26 The data suggested that ABCC5 was significantly elevated in primary PCa tissues relative to normal prostate tissues (Figure 1A–F). Similarly, increased ABCC5 mRNA was identified in metastatic PCa tissues compared with primary PCa tissues (Figure 1G–O). Furthermore, ABCC5 was significantly overexpressed in CRPC samples compared with hormone naïve PCa samples (Figure 1P). To validate these results on the protein level, we further conducted immunohistochemistry study to investigate ABCC5 expression in PCa. As shown in Figure 1Q, the ABCC5 positive staining was localized within the cytoplasm. The results indicated that ABCC5 protein expression was increased in PCa tissues compared with benign prostatic hyperplasia (BPH), and the difference was significant. Similarly, increased ABCC5 was identified in aggressive PCa (Gleason ≥7) relative to less aggressive PCa (Gleason <7). We also analyzed ABCC5 gene expression of CRPC and hormone-naïve PCa tissue; CRPC showed higher expression of ABCC5 protein relative to hormone-naïve PCa. Taken together, the above results indicated that ABCC5 as a potential metastasis and castration resistance-associated oncogene increases gradually during the PCa progression, indicating that it has a very critical role in PCa progression.
ABCC5 overexpression is significantly associated with PCa progression and poor prognosis
We performed microarray data analyses and identified that ABCC5 was significantly increased in patients with BCR compared with patients without BCR at 3 and 5 years, respectively (Figure 2A–F).26,27 The Welsh et al and several datasets (GSE3933, GSE68882, GSE10645, GSE68907, and GSE3325) also indicated that ABCC5 was significantly increased in patients with higher grade, higher stage, and higher Gleason score relative to patients with lower grade, lower stage, and lower Gleason score, respectively (Figure 2G–T).24,25,27–30 In order to validate these observations, we further studied the ABCC5 protein expression pattern by immunohistochemical analysis in specimens from patients who underwent radical prostatectomy. Our data showed ABCC5 protein overexpression is significantly associated with lymph node metastasis, clinical stage, preoperative PSA, Gleason score, and BCR (Table S1). We further identified that the high ABCC5 expression patients had a shorter overall or BCR-free survival duration compared with the patients with low ABCC5 expression. We also identified that ABCC5 was the independent prognostic factor for poor BCR-free (Table S2) and overall survival (Table S3). Collectively, our data showed ABCC5 overexpression is significantly associated with PCa progression and poor prognosis.
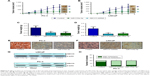
ABCC5 knockdown significantly inhibits PCa cell proliferation, migration, and invasion
To investigate the functional significance of ABCC5 overexpression in PCa, we decreased ABCC5 expression level in PCa cells and tested its impacts on cell proliferation, migration, and invasion. We utilized the stable knockdown strategy targeting ABCC5 in two PCa cell lines, LNCaP and PC-3. The efficiency of ABCC5 knockdown was confirmed by Western blot and RT-PCR (Figure S1). We identified a significant decrease in colony formation upon stable knockdown of ABCC5 relative to control cells (Figure 3A). We also identified that ABCC5 knockdown significantly reduced PC-3 and LNCaP cell migration and invasion (Figure 3B and C). ABCC5 protein was dramatically downregulated in the shRNA transfection group compared with the control group (Figure 3D).
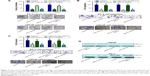
ABCC5 is a direct target gene of miR-516a-3p
Accelerating evidence indicated that some miRNAs can act as cancer and metastasis suppressors by targeting multiple metastasis-associated oncogenes leading to their repression. To investigate if ABCC5 is subject to regulation by miRNAs in PCa, we utilized miRNA target prediction algorithms provided by miRWalk, TargetScan, and miRanda. After integrating the information, we selected miR-516a-3p for further validation due to its antiproliferation and antimetastasis properties in gastric cancer.31 However, to date, its roles in PCa and other cancers are still unclear. Through computational analysis, the binding site for miR-516a-3p at 3′-UTR of ABCC5 was depicted (Figure 4A). We generated firefly luciferase reporter constructs with the 3′-UTR of ABCC5 mRNA and transfected them into PCa cells with miR-516a-3p. We identified that cotransfection with miR-516a-3p in PCa cells significantly reduced the luciferase activity when the construct contained the 3′-UTR of ABCC5 (Figure 4B). Mutation of the binding sites reversed the observed inhibitory effects. Taken together, the above results indicated that ABCC5 was a direct target of miR-516a-3p. We further performed qRT-PCR and Western blot in LNCaP and PC-3 cells to identify whether restoration of miR-516a-3p can change the expression of ABCC5. The expression levels of ABCC5 mRNA and protein were significantly inhibited in miR-516a-3p transfectants when compared with control PCa cells (Figure 4C and D). We also identified that ABCC5 mRNA expression level was inversely associated with the expression level of miR-516a-3p (Figure 4E). Collectively, our results showed that miR-516a-3p may negatively regulate ABCC5 by directly binding to its 3′-UTR.
miR-516a-3p as a potential metastasis and castration resistance-associated miRNA is downregulated in PCa and PCa cell lines
We identified that miR-516a-3p is significantly decreased in primary PCa compared with BPH, metastatic PCa relative to primary PCa, and CRPC relative to hormone naïve PCa, respectively (Figure S2). We also examined miR-516a-3p expression in the different PCa cell lines and prostate epithelial cell line (RWPE-1). We identified that LNCaP cell line expressed higher miR-516a-3p levels in contrast to more aggressive PC-3 cell lines that expressed lower miR-516a-3p levels (Figure S2). The data showed that miR-193a-5p expression was significantly decreased in two PCa cell lines compared with RWPE-1 cell line (Figure S2). Taken together, the above results indicate that the miR-516a-3p as a potential metastasis and castration resistance-associated miRNA may be responsible for PCa progression.
miR-516a-3p regulates cell proliferation, invasion, and migration through directly inhibiting ABCC5
We studied whether miR-516a-3p could affect the effects of ABCC5 on PCa cell proliferation, invasion, and migration. The results indicated that ABCC5 overexpression significantly abrogated the inhibition of PCa cell colony formation induced by miR-516a-3p (Figure 3A). Similarly, ectopic expression of ABCC5 can reverse the inhibition of PCa cell migration and invasion induced by miR-516a-3p (Figure 3B and C). The efficiency of miR-516a-3p overexpression was confirmed by Western blot (Figure 3D). As expected, our results suggested that ABCC5 knockdown resulted in similar results induced by miR-516a-3p expression in PCa cells. Taken together, these results indicated that miR-516a-3p repressed PCa cell proliferation, migration, and invasion through inhibiting ABCC5.
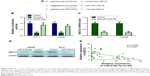
Restoration of miR-516a-3p promotes PC-3 cells’ sensitivity to adriamycin and docetaxel via the suppression of ABCC5
Previous study indicated that ABC proteins can contribute to chemoresistance through the efflux of anticancer drugs from cancer cells. Therefore, ABCC5 knockdown may improve the PCa cells’ sensitivity to chemotherapeutic drugs. To investigate whether miR-516a-3p overexpression can change the PC-3 cells’ sensitivity to chemotherapeutic drugs, the inhibition values for adriamycin and docetaxel in these cells were determined by MTT assay. As shown in Figure S3, the data indicated that the miR-516a-3p overexpression could significantly enhance the chemosensitivity of PC-3 cells to adriamycin (1 μmol/L) and docetaxel (10 nM), compared with the control groups. Forced overexpression of ABCC5 can reverse the promotion of the chemosensitivity of PC-3 cells to both adriamycin and docetaxel induced by miR-516a-3p (Figure S3). We also identified that both miR-516a-3p overexpression and ABBCC5 silencing could result in a comparable promotion of the chemosensitivity of PC-3 cells to adriamycin and docetaxel (Figure S3). Collectively, these data indicated that restoration of miR-516a-3p can promote PC-3 cells’ sensitivity to both adriamycin and docetaxel via the suppression of ABCC5.
ABCC5 plays an essential role in PCa growth and metastasis in vivo
In order to investigate the potential role of ABCC5 on PCa growth in vivo, we employed a murine xenograft model using stable ABCC5 knockdown LNCaP and PC3 cells, and measured the tumor volume, tumor weight, and metastasis to distant organs. Depletion of ABCC5 resulted in significantly reduced tumor volume and weight compared with control cells (Figure 5A–D). ABCC5 knockdown xenograft tumors showed decreased staining for ABCC5 (Figure 5E–G). ABCC5 knockdown of PC-3 also significantly impaired their ability to metastasize from xenograft to cervical lymph nodes compared with control cells (Figure 5H). As expected, restoration of miR-516a-3p displayed attenuated metastasis compared with the control group and resulted in similar results induced by ABCC5 knockdown (Figure 5H). We did not observe any metastases in control or experimental LNCaP xenografts. Collectively, the above results clearly indicated that ABCC5 plays a critical role in PCa growth and metastasis in vivo.
Discussion
The current staging system was unable to accurately find PCa subsets that are prone to progress to aggressive lethal disease, even if Gleason score and PSA level were employed as combined prognostic biomarkers. This issue has contributed to a serious dilemma of overtreatment. However, recent developments in high-throughput technologies have paved the way for the identification of new tumor markers for screening and prognostication. Cancer invasion and metastasis is a complex and multistep process.32 In the current study, we studied the expression and role of ABCC5 in PCa. Our results indicated an essential role for ABCC5 in PCa progression and metastasis in vitro and in vivo. These results indicated that ABCC5 may act as a potential progression molecular biomarker and therapeutic target gene.
PSA acted as the most common molecular biomarker for following PCa patients. However, the course of PCa progression and outcomes of PCa patients can be totally different even in patients with the same PSA. Therefore, it is very urgent to find more sensitive biomarkers for following PCa and improvement of PCa prognosis. ABCC5 is overexpressed in several cancer entities.6–8 To the best of our knowledge, there has been no report on ABCC5 functional analyses in PCa; so, we first analyze the ABCC5 level in PCa in several microarray datasets. Then, our study aimed to investigate the functional significance of ABCC5 in PCa. Here, we found that ABCC5 was upregulated in primary PCa relative to BPH, metastatic PCa relative to primary PCa, and CRPC relative to hormone naïve PCa through microarray analysis, indicating ABCC5 as a metastasis and castration resistance-associated oncogene may play an essential role in PCa progression. We further studied the association of ABCC5 with clinicopathological variables in PCa. Results indicated that ABCC5 overexpression was significantly associated with PCa progression at both mRNA and protein levels and can serve as an independent predictor for the poor BCR-free and overall survival. Results also suggested that ABCC5 overexpression was independently associated with unfavorable outcome of PCa patients. The prognostic value of ABCC5 was statistically significant in multivariate analysis adjusted for significant variables from univariate analysis, which suggested ABCC5 overexpression may be a good molecular marker to predict PCa prognosis. This is the first direct evidence of the association between ABCC5 and clinicopathological factors of PCa and the prognostic role of ABCC5 in PCa. Further large-scale cohort studies may be essential to identify whether ABCC5 is an effective biomarker.
These data suggested that ABCC5 may act as a potential critical metastasis and castration resistance-associated oncogene. However, its underlying molecular mechanism remains unclear. The functional roles of ABCC5 in PCa cells were also studied in the current study. It is well known that proliferation and metastasis are the two most common causes for the malignancy-related death. To investigate the functions of ABCC5, the stable ABCC5 silence/overexpression LNCaP and PC-3 cell lines were constructed and the effects of the knockdown/overexpression of ABCC5 on cell proliferation, migration, and invasion ability were investigated. Our research results suggested that ABCC5 knockdown can significantly inhibit the proliferation of the PCa cell lines in vitro. The results also indicated that PCa cells became less aggressive and invasive after transfected with the ABCC5 knockdown construct, suggesting that the ABCC5 functioned as a metastasis-associated oncogene in PCa. In addition, we demonstrated that silencing of ABCC5 significantly inhibits tumor growth and metastasis in vivo.
Accelerating evidence suggested that miRNAs can act as master regulators of gene expression in a sequence-specific pattern and play critical roles in several biological processes, including cell differentiation, angiogenesis, proliferation, migration, and invasion. We then performed a computational search for the potential miRNAs that can regulate ABCC5. Among these miRNAs, miR-516a-3p, which is a kind of highly conserved miRNA, attracted our attention. In PCa samples, the expression level of miR-516a-3p was found to be correlated inversely with ABCC5 mRNA expression. We further investigated whether miR-516a-3p can suppress the ABCC5 expression level. We treated LNCaP and PC-3 cell lines with miR-516a-3p and measured ABCC5 level. We found that miR-516a-3p can negatively regulate ABCC5 expression in PCa. As expected, ABCC5 knockdown can result in similar results induced by miR-516a-3p expression in PCa cells. Furthermore, ectopic overexpression of ABCC5 can significantly reverse the suppressive effects of miR-516a-3p. Taken together, we identified that miR-516a-3p can significantly suppress PCa cell proliferation, invasion, and migration in vitro, and inhibit tumor growth and metastasis in vivo through the downregulation of ABCC5 by directly binding to its 3′-UTR.
It is well known that ADT is the first line of therapeutic intervention for metastatic PCa. Chemotherapy is a choice for androgen-independent PCa. Unfortunately, many CRPC patients develop drug resistance during chemotherapy, leading to treatment failure in CRPC patients. Therefore, further understanding of the molecular mechanisms underlying drug resistance and progression would be helpful for effective treatment of the disease. Chemotherapy is known to induce the cellular stress response. Previous study reported an interesting mechanism for the regulation of gene expression by miRNAs within stress-related conditions.33 In the cells, a basal level of target mRNA expression and, simultaneously, a certain constant level of miRNAs, which downregulate the target mRNA, are maintained, endowing their expression to be kept at a constant level. A level of target mRNA is dramatically raised following a stress response, saturating the threshold miRNA that results in its insufficiency to disrupt the mRNA and allows its translation. As a result of deleted MDR genes, their expression in tumor cells is not sufficiently increased in response to treatment, and the tumor becomes chemosensitive. We identified that miR-516a-3p restoration can promote PC-3 cells’ sensitivity to adriamycin and docetaxel via the suppression of ABCC5. All the results indicated that miR-516a-3p may modulate the sensitivity of cancer cells to adriamycin and docetaxel by targeting ABCC5 with important implications in the design of new therapeutic agents.
Conclusion
Taken together, this is the first study to indicate that ABCC5 overexpression, most likely mediated by the loss of miR-516a-3p expression, is involved in PCa progression, metastasis, drug resistance, and castration resistance. Our observations demonstrated that ABCC5 plays a critical role in PCa progression and could serve as a viable therapeutic target.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NO: 81502220 and NO: 81772758), Tianjin Research Program of Application Foundation and Advanced Technology (NO: 15JCZDJC35900), and Science Foundation of Tianjin Key Institute of Urology (NO: MNQN201502).
Author contributions
HZ, ZL, GS, RL, and YX conceived and designed the project, performed the experiments, and wrote the manuscript. HZ, RL, and YX contributed to the writing and to the critical reading of the manuscript. HZ, ZL, and GS performed patient collection and clinical data interpretation. HZ, GS, and RL participated and performed the statistical analysis. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel R, Miller K, Jemal A, Statistics C. CA Cancer J Clin. 2015;2015(63):5–29. | ||
Fukuda Y, Schuetz JD. ABC transporters and their role in nucleoside and nucleotide drug resistance. Biochem Pharmacol. 2012;83(8):1073–1083. | ||
Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther. 2005;4(5):855–863. | ||
Park S, Shimizu C, Shimoyama T, et al. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2006;99(1):9–17. | ||
Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10(2):147–156. | ||
Li B, Chen H, Wu N, Zhang WJ, Shang LX. Deregulation of miR-128 in ovarian cancer promotes cisplatin resistance. Int J Gynecol Cancer. 2014;24(8):1381–1388. | ||
Hamamoto J, Yasuda H, Aizawa K, et al. Non-small cell lung cancer PC-9 cells exhibit increased sensitivity to gemcitabine and vinorelbine upon acquiring resistance to EGFR-tyrosine kinase inhibitors. Oncol Lett. 2017;14(3):3559–3565. | ||
Mourskaia AA, Amir E, Dong Z, et al. ABCC5 supports osteoclast formation and promotes breast cancer metastasis to bone. Breast Cancer Res. 2012;14(6):R149. | ||
Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. | ||
Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. | ||
Li H, Yang BB. Stress response of glioblastoma cells mediated by miR-17-5p targeting PTEN and the passenger strand miR-17-3p targeting MDM2. Oncotarget. 2012;3(12):1653–1668. | ||
Zhang H, Li S, Yang X, Qiao B, Zhang Z, Xu Y. miR-539 inhibits prostate cancer progression by directly targeting SPAG5. J Exp Clin Cancer Res. 2016;35:60. | ||
Zhang H, Qi C, Wang A, et al. Prognostication of prostate cancer based on NUCB2 protein assessment: NUCB2 in prostate cancer. J Exp Clin Cancer Res. 2013;32:77. | ||
Kim J, Yu W, Kovalski K, Ossowski L. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell. 1998;94(3):353–362. | ||
Arredouani MS, Lu B, Bhasin M, et al. Identification of the transcription factor single-minded homologue 2 as a potential biomarker and immunotherapy target in prostate cancer. Clin Cancer Res. 2009;15(18):5794–5802. | ||
Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. | ||
Luo JH, Yu YP, Cieply K, et al. Gene expression analysis of prostate cancers. Mol Carcinog. 2002;33(1):25–35. | ||
Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39(1):41–51. | ||
Wallace TA, Prueitt RL, Yi M, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68(3):927–936. | ||
Chandran UR, Ma C, Dhir R, et al. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer. 2007;7:64. | ||
Holzbeierlein J, Lal P, Latulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164(1):217–227. | ||
Varambally S, Yu J, Laxman B, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8(5):393–406. | ||
Yu YP, Landsittel D, Jing L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22(14):2790–2799. | ||
Lapointe J, Li C, Higgins JP, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101(3):811–816. | ||
Latulippe E, Satagopan J, Smith A, et al. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 2002;62(15):4499–4506. | ||
Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. | ||
Nakagawa T, Kollmeyer TM, Morlan BW, et al. A tissue biomarker panel predicting systemic progression after PSA recurrence post-definitive prostate cancer therapy. PLoS One. 2008;3(5):e2318. | ||
Singh D, Febbo PG, Ross K, et al. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell. 2002;1(2):203–209. | ||
Vanaja DK, Cheville JC, Iturria SJ, Young CY. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003;63(14):3877–3882. | ||
Welsh JB, Sapinoso LM, Su AI, et al. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61(16):5974–5978. | ||
Takei Y, Takigahira M, Mihara K, Tarumi Y, Yanagihara K. The metastasis-associated microRNA miR-516a-3p is a novel therapeutic target for inhibiting peritoneal dissemination of human scirrhous gastric cancer. Cancer Res. 2011;71(4):1442–1453. | ||
Spahn M, Kneitz S, Scholz CJ, et al. Expression of microRNA-221 is progressively reduced in aggressive prostate cancer and metastasis and predicts clinical recurrence. Int J Cancer. 2010;127(2):394–403. | ||
Leung AK, Sharp PA. MicroRNA functions in stress responses. Mol Cell. 2010;40(2):205–215. |
Supplementary materials
  | Table S1 Clinicopathologic variables and ABCC5 protein expression in 180 PCa patients |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.