Back to Journals » OncoTargets and Therapy » Volume 15
Long-Term Treatment-Free Survival After Multimodal Therapy in a Patient with Stage IV Lung Adenocarcinoma
Authors Takaoka H, Terai H , Emoto K, Shigematsu L, Ito F, Saito A, Okada M, Ohgino K, Ikemura S, Yasuda H, Nakachi I , Kawada I, Fukunaga K, Soejima K
Received 10 June 2022
Accepted for publication 6 September 2022
Published 14 September 2022 Volume 2022:15 Pages 981—989
DOI https://doi.org/10.2147/OTT.S375959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Hatsuyo Takaoka,1 Hideki Terai,1 Katsura Emoto,2 Lisa Shigematsu,1 Fumimaro Ito,1 Ayaka Saito,1 Masahiko Okada,1 Keiko Ohgino,1 Shinnosuke Ikemura,3 Hiroyuki Yasuda,1 Ichiro Nakachi,4 Ichiro Kawada,1 Koichi Fukunaga,1 Kenzo Soejima3
1Division of Pulmonary Medicine, Department of Medicine, Keio University School of Medicine, Tokyo, Japan; 2Department of Pathology, Keio University School of Medicine, Tokyo, Japan; 3Department of Respiratory Medicine, Yamanashi University School of Medicine, Yamanashi, Japan; 4Pulmonary Division, Department of Internal Medicine, Saiseikai Utsunomiya Hospital, Utsunomiya, Japan
Correspondence: Hideki Terai, Division of Pulmonary Medicine, Department of Medicine, Keio University School of Medicine, 35 Shinanomachi, Tokyo, 160-8582, Japan, Tel +81-3-3353-3474, Fax +81-3-3353-3475, Email [email protected]
Abstract: We report the first case of a patient with non-small cell lung cancer (NSCLC) with malignant pleural effusion (MPE) who achieved disease- and treatment-free survival for nearly 10 years. A 50-year-old man was diagnosed with NSCLC with MPE and underwent chemotherapy and salvage thoracic surgery. The patient received chemotherapy with cisplatin, pemetrexed, and bevacizumab, and a partial response was achieved. After informed consent was obtained from the patient, right middle lobectomy was performed to achieve local tumor control. Postoperative adjuvant chemotherapy with pemetrexed and bevacizumab was discontinued after almost 1 year of chemotherapy due to side effects such as diarrhea and muscle weakness. The patient has survived without recurrence of lung cancer for more than 11 years after being diagnosed and nearly 10 years after discontinuing chemotherapy.
Keywords: malignant pleural effusion, bevacizumab, pemetrexed, salvage surgery, cure
Introduction
Lung cancer remains the leading cause of cancer-related death worldwide.1 Even with the best available therapies, patients with stage IV lung cancer have a poor prognosis and are generally believed to be incurable.2 According to the site and number of distant metastases, stage IV lung cancer is classified into M1a, M1b or M1c, based on the TNM classification of the Union of International Cancer Control (UICC), 8th edition.3 Additionally, the median overall survival between M1a, M1b and M1c is significantly different (M1a: 11.5 months, M1b: 11.4 months, and M1c: 6.3 months).4,5
Most clinical guidelines for lung cancer recommend systemic therapy; surgical resection or radical radiation therapy aimed at cure are not recommended in patients with lung cancer with distant metastasis.6,7 Stage IV lung cancers with a specific characteristic referred to as “oligometastasis” (often classified as stage IV with M1b) have attracted attention recently because multimodal therapies, including local radical therapy, have improved survival and almost achieved cure in clinical trials.8,9 However, patients with malignant pleural effusion (MPE) whose cancers are classified as stage IV with M1a are not considered oligometastatic by consensus guidelines9,10 since cancer cells are disseminated into the intrathoracic space and cannot be removed completely by local therapy.11
Here, we report the case of a patient with non-small cell lung cancer (NSCLC) with MPE treated with multimodal therapy and survived without a recurrence for more than 11 years after diagnosis and 10 years after discontinuing cancer treatment.
Case Presentation
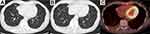
A 50-year-old Japanese man developed dry cough beginning in February 2010. A chest X-ray during a medical checkup in June 2010 showed a 20-mm nodule in the right lung, and a chest computed tomography (CT) scan in July 2010 showed a 26 mm nodule in the medial segment of the middle lobe, a right pleural effusion, and scattered nodules in the interlobar pleura (Figure 1A and B). Lung cancer was suspected, and the patient was referred to the Department of Respiratory Medicine at our hospital for further examination in July 2010.
A whole body 18F–FDG (18F‐fluorodeoxy‐glucose) positron emission tomography (PET)‐CT demonstrated high FDG uptake in the lung nodule on the medial segment. The maximum standardized uptake value (SUVmax) was 6.1 (Figure 1C). Gadolinium-enhanced brain magnetic resonance imaging and bone scintigraphy showed no other metastases.
Biopsy of the lung nodule from the medial segment obtained by bronchoscopy and cytology of the pleural fluid obtained by thoracic puncture confirmed lung adenocarcinoma (Figure 2). The tumor was classified as clinical T1cN0M1a, which is stage IVA according to the TNM classification of the UICC, 8th edition. The patient’s previous medical history was unremarkable except for being a past light smoker (1 pack-years). The Eastern Cooperative Oncology Group performance status (ECOG-PS) at the time of admission was 0. Lung tissue samples showed no mutations in the epidermal growth factor receptor (EGFR) by the peptide nucleic acid-locked nucleic acid PCR clamp.12 The carcinoembryonic antigen (CEA) level was 18.1 ng/mL (normal, 0–5 ng/mL). Beginning in August 2010, the patient received cisplatin (75 mg/sq), pemetrexed (PEM, 500 mg/sq), and bevacizumab (BEV, 1000mg/body) as first-line chemotherapy. After 4 cycles of chemotherapy, the tumor size decreased to 1 cm in diameter, and pleural effusion had disappeared; the 61.5% reduction in tumor size indicated that partial response was achieved according to the Response Evaluation Criteria in Solid Tumors version 1.1 (Figures 3A and B). PET-CT revealed that FDG uptake was negative in the tumor (Figure 3C). The tumor marker CEA levels decreased to 2.4 ng/mL, which was within the normal range (Figure 4).
 |
Figure 2 Cytological examination of pleural effusion. The pleural effusion was positive for adenocarcinoma cells (scale bar: 100 μm). |
 |
Figure 4 Time course of treatment and laboratory findings. Abbreviations: CDDP, cisplatin; PEM, pemetrexed; BEV, bevacizumab; CEA, carcinoembryonic antigen. |
The treatment strategy was debated at the multidisciplinary lung cancer board conference since the patient’s strong will for additional local treatment of the residual tumor on CT imaging. Finally, surgery was selected with the patient’s consent to remove the residual tumor and achieve local tumor control. Surgical findings in the thoracic cavity included a tumor on the mediastinal area of S5 with a mild pleural depression and white nodules that were mainly observed on the interlobar surfaces of the lower, middle, and upper lobes and appeared to be scars of healed pleural dissemination; no similar white nodules were found on the diaphragmatic or mural pleura. The white nodule on the interlobar surface of S8 was partially removed and submitted for frozen section examination, which showed no evidence of malignancy. Rapid pathological diagnosis of the enlarged hilar lymph nodes in the middle lobe using pleural lavage cytology was also negative for malignancy. Finally, surgical resection of the right middle lobe and mediastinal lymph node dissection were performed.
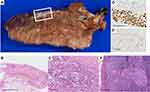
Macroscopic examination of the specimen from the right middle lobectomy revealed that the tumor nodule became a fibrotic lesion adjacent to the pleura (Figure 5A). Upon histological examination, solid tumor cell clusters were observed among lung tissue with collapsed alveoli and fibroelastosis (Figures 5B and C). Immunohistochemical staining of the tumor cells was positive for TTF-1, and approximately 5% were positive for Ki-67. Hence, the tumor cells were diagnosed as residual adenocarcinoma (Figures 5D and E). The tumor bed measured 20×5 mm and included the viable tumor (5%) and stroma (95%) without necrotic area. No viable tumor cells were observed in the pleura. Additionally, low-volume metastasis in the #7 lymph node was confirmed by histological examination (Figure 5F). These results show that the effect of chemotherapy on the primary site was equivalent to a major pathologic response according to the neoadjuvant therapy criteria.13
Pathological staging revealed that the tumor was ypT1aN2M0, stage IIIA. After surgery, the patient received 6 cycles of PEM and BEV as adjuvant chemotherapy and 12 additional cycles of BEV until May 2012. Chemotherapy was discontinued in June 2012 due to fatigue and muscle weakness, although it was unclear whether these symptoms were caused by the drugs.
As of February 2022, the patient has been alive for more than 11 years without disease recurrence after being diagnosed with stage IV NSCLC with MPE. Furthermore, the patient has not received any anti-cancer treatments in over 10 years.
Discussion and Conclusion
Stage IV lung cancer has a high mortality rate. Its 5-year overall survival rate is below 10%, although expected survival times have increased with recent advances in treatment methodology.2,14,15
Several studies have reported factors associated with the long-term survival of patients with advanced stage NSCLC,16–22 including a good ECOG-PS, positive EGFR mutation status, absence of extra-thoracic lesions, and elevated tumor-infiltrating lymphocyte levels (Table 1). Generally, local therapies aimed at radical cure are not indicated for stage IV lung cancer patients.2 However, Aoki et al reported that 80% of patients diagnosed with stage IV lung cancer but achieved complete remission received local radical therapy such as surgery or radiation during treatment, though this complete remission does not directly mean improving their survival.18 This suggests that radical local therapy as a part of comprehensive multimodal treatment could benefit selected patients with stage IV lung cancer to achieve complete remission. In the present case, there was a small size of residual alive tumors in a surgical specimen of primary tumor and lymph node metastasis. Meanwhile, multiple clinical trials have reported that additional local radical therapy can improve prognosis in patients with stage IV lung cancer with oligometastasis and good ECOG-PS, although results from large-scale prospective clinical trials are not yet available.9
 |
Table 1 Cases of Advanced Non-Small Cell Lung Cancer with Long-Term Survival from the Literature |
As previously mentioned, local radical therapy is not generally indicated for patients with lung cancer with MPE, as they are not recognized as having an oligometastatic disease. However, a potential benefit of local treatment, such as surgery, has been reported in patients with MPE and/or malignant pleural nodules in previous studies23–33 (Table 2). For example, Aoki et al reported a few cases. Aoki et al also reported a few similar long-term survivors without cancer treatment in patients with stage IV lung cancer.18 Additionally, several studies reported the effectiveness of hyperthermic intrathoracic chemotherapy (HITHOC) that it can control the symptoms and prolong the survival of patients with MPE.34 Surgery combined with HITHOC has been recently reported to be an effective surgical intervention for selected pM1a patients to prolong life expectancy. A systematic review reported by Migliore et al35 showed favorable outcomes for N0–1 NSCLC patients with MPE undergoing primary tumor resection plus HITHOC with a median survival time of 18 months and 2-year overall survival of 28.5%. The status of lymph node metastasis in surgical specimens has repeatedly been reported as a significant prognostic factor, and it has been demonstrated that patients with positive N2/3 lymph node metastasis have significantly worse outcomes than those with or without N1 lymph node metastasis.23,24 However, there is a large variation among the 5-year survival rates and mean survival times reported in these studies, and it is therefore difficult to determine which cases with MPE could undergo surgery.
 |
Table 2 Summary of Studies Reporting Surgically Treated Patients with Malignant Pleural Disease |
In the present case, we used radical surgery when the patient achieved induced oligometastatic status after systemic chemotherapy. Induced oligometastatic status is defined as the presence of multiple metastatic lesions at the time of diagnosis that are reduced by effective treatment as they are detected only as a limited disease. It has not been determined whether additional local therapy would improve patients’ prognosis following the achievement of induced oligometastatic status. After surgery, a histological examination revealed residual cancer cells in the resected mediastinal lymph node #7, which was defined as the N2 lymph node.
We believe that salvage surgical resection was potentially beneficial to achieving treatment-free survival in this patient, as Davis et al reported22 in their large retrospective study that in addition to a younger age and lower T status, surgery was a good prognostic factor for long-term survival in 44,387 patients with stage IV lung cancer. Although the necessity of adjuvant chemotherapy was controversial in this case, based on previous reports, the risk of recurrence seemed high due to the presence of lymph node metastases in the surgical specimen.23,24,26,28,29,32,33 Given that the preoperative chemotherapy effectiveness is limited in adenocarcinomas,36 we selected pemetrexed and bevacizumab for adjuvant chemotherapy, as administration of these drugs before surgery resulted in a remarkable reduction of the tumor size on pathological evaluation. This is a rare case with stage IV NSCLC with MPE and N2 lymph node metastasis, who survived for more than 11 years after surgery without relapse. It is difficult to conclude whether surgery is a good option in patients with stage IV lung cancer who achieve a similar induced oligometastatic status. Little is known about the significant factors that contribute to improved prognosis and survival rates that approach those of remission in stage IV lung cancer patients. To elucidate the mechanisms to achieve treatment-free survival in patients with stage IV lung cancer, it is necessary to document similar cases. The number of cases with induced oligometastatic status is expected to increase with the recent approval of promising new drugs (eg, drugs that target driver genes and immune checkpoint inhibitors), and evidence from prospective trials will be required to further evaluate these cases.37
Abbreviations
UICC, Union of International Cancer Control; NSCLC, non-small cell lung cancer; MPE, malignant pleural effusion; CT, computed tomography; FDG-PET, 18Fluorodeoxyglucose positron emission tomography; ECOG-PS, the eastern cooperative oncology group performance status; CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor; HITHOC, hyperthermic intrathoracic chemotherapy.
Ethics Approval and Informed Consent
This study was approved by the ethical review boards of Keio University School of Medicine (approval no. 20110171).
Consent for Publication
Written informed consent was obtained from the patient to publish this case report and any accompanying images.
Acknowledgments
We would like to thank Editage for English language editing.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by the Japan Society for the Promotion of Science to H.T. 22K08289.
Disclosure
Dr Kenzo Soejima reports grants and honoraria from AstraZeneca and Taiho Pharmaceutical; honoraria from Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb Japan, MSD Oncology, Eli Lilly Japan, Novartis Pharma, and Takeda Pharmaceutical, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Hanna N, Johnson D, Temin S, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(30):3484–3515. doi:10.1200/JCO.2017.74.6065
3. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi:10.1016/j.jtho.2015.09.009
4. Kay FU, Kandathil A, Batra K, Saboo SS, Abbara S, Rajiah P. Revisions to the tumor, node, metastasis staging of lung cancer (8). World J Radiol. 2017;9(6):269–279. doi:10.4329/wjr.v9.i6.269
5. Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2015;10(11):1515–1522. doi:10.1097/JTO.0000000000000673
6. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi:10.1093/annonc/mdy275
7. Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254–266. doi:10.6004/jnccn.2021.0013
8. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10. doi:10.1200/JCO.1995.13.1.8
9. Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for research and treatment of cancer consensus recommendation. Lancet Oncol. 2020;21(1):e18–e28. doi:10.1016/S1470-2045(19)30718-1
10. Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–166. doi:10.1016/j.radonc.2020.04.003
11. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18(2):402–419. doi:10.1183/09031936.01.00225601
12. Nagai Y, Miyazawa H, Tanaka T, et al. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005;65(16):7276–7282. doi:10.1158/0008-5472.Can-05-0331
13. Travis WD, Dacic S, Wistuba I, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709–740. doi:10.1016/j.jtho.2020.01.005
14. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. doi:10.1056/NEJMoa1916623
15. Takano N, Ariyasu R, Koyama J, et al. Improvement in the survival of patients with stage IV non-small-cell lung cancer: experience in a single institutional 1995–2017. Lung Cancer. 2019;131:69–77. doi:10.1016/j.lungcan.2019.03.008
16. Kaira K, Takahashi T, Murakami H, et al. Long-term survivors of more than 5 years in advanced non-small cell lung cancer. Lung Cancer. 2010;67(1):120–123. doi:10.1016/j.lungcan.2009.03.014
17. Huang CY, Chen BH, Chou WC, Yang CT, Chang JW. Factors associated with the prognosis and long-term survival of patients with metastatic lung adenocarcinoma: a retrospective analysis. J Thorac Dis. 2018;10(4):2070–2078. doi:10.21037/jtd.2018.03.143
18. Aoki T, Akiba T, Nishiyama J, et al. Analysis of key clinical features for achieving complete remission in stage III and IV non-small cell lung cancer patients. Respir Res. 2019;20(1):263. doi:10.1186/s12931-019-1235-3
19. Hirashima T, Suzuki H, Okamoto N, et al. Important factors for achieving survival of five years or more in non-small cell lung cancer patients with distant metastasis. Oncol Lett. 2014;8(1):327–334. doi:10.3892/ol.2014.2107
20. Giroux Leprieur E, Lavole A, Ruppert AM, et al. Factors associated with long-term survival of patients with advanced non-small cell lung cancer. Respirology. 2012;17(1):134–142. doi:10.1111/j.1440-1843.2011.02070.x
21. Okamoto T, Maruyama R, Shoji F, et al. Long-term survivors in stage IV non-small cell lung cancer. Lung Cancer. 2005;47(1):85–91. doi:10.1016/j.lungcan.2004.06.006
22. Davis JS, Prophet E, Peng HL, et al. Potential influence on clinical trials of long-term survivors of stage IV non-small cell lung cancer. JNCI Cancer Spectr. 2019;3(2):pkz010. doi:10.1093/jncics/pkz010
23. Okamoto T, Iwata T, Mizobuchi T, et al. Pulmonary resection for lung cancer with malignant pleural disease first detected at thoracotomy. Eur J Cardiothorac Surg. 2012;41(1):25–30. doi:10.1016/j.ejcts.2011.04.010
24. Iida T, Shiba M, Yoshino I, et al. Surgical intervention for non-small-cell lung cancer patients with pleural carcinomatosis: results from the Japanese Lung Cancer Registry in 2004. J Thorac Oncol. 2015;10(7):1076–1082. doi:10.1097/JTO.0000000000000554
25. Fiorelli A, Santini M. In lung cancer patients where a malignant pleural effusion is found at operation could resection ever still be justified? Interact Cardiovasc Thorac Surg. 2013;17(2):407–412. doi:10.1093/icvts/ivt153
26. Ren Y, Dai C, Shen J, et al. The prognosis after contraindicated surgery of NSCLC patients with malignant pleural effusion (M1a) may be better than expected. Oncotarget. 2016;7(18):26856–26865. doi:10.18632/oncotarget.8566
27. Fukuse T, Hirata T, Tanaka F, Wada H. The prognostic significance of malignant pleural effusion at the time of thoracotomy in patients with non-small cell lung cancer. Lung Cancer. 2001;34(1):75–81. doi:10.1016/s0169-5002(01)00228-8
28. Ichinose Y, Tsuchiya R, Koike T, et al. Prognosis of resected non-small cell lung cancer patients with carcinomatous pleuritis of minimal disease. Lung Cancer. 2001;32(1):55–60. doi:10.1016/s0169-5002(00)00206-3
29. Kodama K, Doi O, Higashiyama M, Yokouchi H, Tatsuta M. Long-term results of postoperative intrathoracic chemo-thermotherapy for lung cancer with pleural dissemination. Cancer. 1993;72(2):426–431. doi:10.1002/1097-0142(19930715)72:2<426::aid-cncr2820720218>3.0.co;2-s
30. Mordant P, Arame A, Foucault C, Dujon A, Le Pimpec Barthes F, Riquet M. Surgery for metastatic pleural extension of non-small-cell lung cancer. Eur J Cardiothorac Surg. 2011;40(6):1444–1449. doi:10.1016/j.ejcts.2011.02.076
31. Ohta Y, Tanaka Y, Hara T, et al. Clinicopathological and biological assessment of lung cancers with pleural dissemination. Ann Thorac Surg. 2000;69(4):1025–1029. doi:10.1016/s0003-4975(99)01579-9
32. Shiba M, Kakizawa K, Kohno H, et al. Prognostic implication of Ki-67 immunostaining in treating subclinical pleural cancer found at thoracotomy in lung cancer patients. Ann Thorac Surg. 2001;71(6):1765–1771. doi:10.1016/s0003-4975(01)02589-9
33. Ichinose Y, Tsuchiya R, Koike T, et al. The prognosis of patients with non-small cell lung cancer found to have carcinomatous pleuritis at thoracotomy. Surg Today. 2000;30(12):1062–1066. doi:10.1007/s005950070002
34. Li H, Liu T, Sun Z, Wang Z, Liu X, Yang F. New horizons in non-small-cell lung cancer patients with ipsilateral pleural dissemination (M1a): review of the literature. Ann Transl Med. 2021;9(11):959. doi:10.21037/atm-20-6188
35. Migliore M, Nardini M. Does cytoreduction surgery and hyperthermic intrathoracic chemotherapy prolong survival in patients with N0-N1 nonsmall cell lung cancer and malignant pleural effusion? Eur Respir Rev. 2019;28(153):190018. doi:10.1183/16000617.0018-2019
36. Qu Y, Emoto K, Eguchi T, et al. Pathologic assessment after neoadjuvant chemotherapy for NSCLC: importance and implications of distinguishing adenocarcinoma from squamous cell carcinoma. J Thorac Oncol. 2019;14(3):482–493. doi:10.1016/j.jtho.2018.11.017
37. Brighenti M, Petrelli F, Barni S, et al. Radical treatment of oligometastatic non-small cell lung cancer: ready for prime time? Eur J Cancer. 2017;79:149–151. doi:10.1016/j.ejca.2017.04.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.