Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Long-term safety of tiotropium/olodaterol Respimat® in patients with moderate-to-very severe COPD and renal impairment in the TONADO® studies
Authors LaForce C, Derom E, Bothner U , Kloer IM, Trampisch M, Buhl R
Received 4 January 2018
Accepted for publication 22 February 2018
Published 1 June 2018 Volume 2018:13 Pages 1819—1831
DOI https://doi.org/10.2147/COPD.S161489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Video abstract presented by Craig LaForce.
Views: 718
Craig LaForce,1 Eric Derom,2 Ulrich Bothner,3 Isabel M Kloer,3 Matthias Trampisch,4 Roland Buhl5
1NC Clinical Research, Raleigh, NC, USA; 2Department of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium; 3Pharmacovigilance, Boehringer Ingelheim International GmbH, Ingelheim, Germany; 4Biostatistics & Data Sciences, Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany; 5Pulmonary Department, Mainz University Hospital, Mainz, Germany
Introduction: The safety, lung function efficacy, and symptomatic benefits of combined tiotropium and olodaterol in patients with COPD were established in the 1-year TONADO® studies (NCT01431274; NCT01431287). As tiotropium is predominantly excreted by the kidneys, the long-term safety profile of tiotropium/olodaterol was investigated in patients with renal impairment in a prespecified safety analysis of the TONADO studies.
Methods: These were 2 replicate, randomized, double-blind, parallel-group, 52-week Phase III studies that assessed tiotropium/olodaterol compared with tiotropium or olodaterol alone (all via Respimat®) in patients with moderate-to-very severe COPD. In this analysis, renal impairment was defined as mild (creatinine clearance [CLcr] 60–89 mL/min), moderate (CLcr 30–59 mL/min) or severe (CLcr 15–29 mL/min). Adverse events (AEs) were pooled from both studies.
Results: Of 3,041 patients included in this analysis, 1,333 (43.8%) had mild, 404 (13.3%) had moderate, and 5 (0.2%) had severe renal impairment; these were distributed equally between treatment groups. Almost one-quarter of all treated patients (23.4%) had a history of cardiac disorder, 45.6% had hypertension, and 13.3% had glucose metabolism disorders, including diabetes. AEs with olodaterol, tiotropium, and tiotropium/olodaterol occurred in 75.1%, 70.8%, and 72.0% of patients with no renal impairment, 75.7%, 74.0%, and 73.3% with mild renal impairment, and 84.3%, 79.5%, and 79.7% with moderate renal impairment, respectively. There was no notable effect of renal impairment on the proportion of patients with an AE, and no differences were observed between tiotropium/olodaterol versus the monocomponents. There was no difference in the incidence of major adverse cardiac events, renal and urinary tract AEs, or potential anticholinergic effects with increasing severity of renal impairment.
Conclusion: Over half the patients enrolled in the TONADO studies had renal impairment, and there was a high level of pre-existing cardiovascular comorbidity. The safety and tolerability of tiotropium/olodaterol is comparable to the monocomponents, irrespective of the level of renal impairment.
Keywords: COPD, renal impairment, comorbidities, tiotropium, olodaterol, safety
Plain language summary
Why was the study done? This analysis of patients participating in two large clinical trials (TONADO®; NCT01431274 and NCT01431287) was performed to assess the safety of combined treatment with 2 bronchodilators (drugs that open the airways) compared with treatment with a single bronchodilator in patients with COPD and impaired kidney function.
What did the researchers do and find? The researchers found that impaired kidney function in patients participating in the TONADO studies was common, and that these patients often had other medical conditions in addition to COPD. No safety concerns were reported in patients with renal impairment who received combined treatment with the 2 bronchodilators, tiotropium (a long-acting muscarinic antagonist) and olodaterol (a long-acting β2-agonist).
What do these results mean? These findings suggest that treatment with tiotropium in combination with olodaterol is as safe as treatment with either drug alone in patients with COPD who have impaired kidney function. This is important because tiotropium is predominantly excreted by the kidneys.
Introduction
COPD is a leading cause of morbidity and mortality, with an estimated global prevalence of 11.7% (2010 figures), equivalent to 384 million cases.1 In 2005, COPD accounted for 5% of all deaths globally, and this is projected to increase by >30% over the next 10 years.2 Long-acting bronchodilators are the mainstay of COPD treatment, and are recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for maintenance therapy of patients with moderate-to-very severe disease.3 Tiotropium (Spiriva®) is an established once-daily long-acting muscarinic antagonist (LAMA) shown to improve lung function, patient-reported outcomes such as dyspnea and quality of life, and reduce exacerbations in patients with COPD.4 Olodaterol (Striverdi® Respimat®) is a recently approved and marketed novel long-acting β2-agonist (LABA) that provides 24-hour bronchodilation and symptomatic benefits in patients with COPD.5,6 In COPD patients who are not adequately controlled despite the use of a single bronchodilator, dual bronchodilation with tiotropium and olodaterol as a fixed-dose combination (FDC) was shown to be safe and efficacious over 52 weeks versus the monocomponents in the 2 Phase III TONADO trials.7
While olodaterol is mainly metabolized by the liver, and its metabolites excreted in the feces, tiotropium is primarily eliminated by the kidneys, and increasing plasma concentrations have been noted in patients with moderate-to-severe renal impairment (creatinine clearance [CLcr] ≤50 mL/min). Although no dose reduction for tiotropium or tiotropium/olodaterol is recommended for patients with renal impairment based on pharmacokinetic data, it is recommended that the use of tiotropium and tiotropium/olodaterol in patients with severe renal impairment be closely monitored.8–10 Observational studies have raised questions regarding whether urinary retention, particularly in elderly male patients with COPD and coexisting lower urinary tract symptoms or benign prostate hyperplasia,11 may increase with systemic availability of inhaled anticholinergics.12,13
Increasing age is a risk factor for COPD,14 and renal impairment is a frequent comorbidity in elderly patients. Therefore, there is a concern that this group may be at greater risk of adverse events (AEs) due to decreased elimination and increased systemic availability of tiotropium. Nevertheless, extensive post-marketing experience has been collected with the use of tiotropium under real-world conditions in patients with comorbidities,15,16 and in a recent pooled analysis including 24,555 patients, renal impairment did not result in an increased incidence of tiotropium-related AEs.17 To date, there have been no known safety issues with renal impairment in patients treated with olodaterol, and there are no special considerations regarding the use of olodaterol in patients with renal impairment, although clinical trial data are lacking for patients with severe renal impairment.
Thus, the objective of this analysis was to describe the patient risk characteristics associated with renal impairment in patients with moderate-to-very severe COPD being treated with tiotropium/olodaterol or the monocomponents in the TONADO studies, and to investigate whether renal impairment is associated with additional safety concerns in this patient population.7
Methods
Study design
The TONADO studies were replicate 52-week, multinational, Phase III, multicenter, parallel-group, randomized, double-blind, active-controlled studies (Study 1237.5: NCT01431274; Study 1237.6: NCT01431287) comparing tiotropium/olodaterol FDC with the monocomponents, all delivered by the Respimat inhaler, in patients with moderate-to-very severe COPD (GOLD 2–4).7
The main inclusion and exclusion criteria of the TONADO studies are described elsewhere;7 briefly, outpatients aged ≥40 years with a history of moderate-to-very severe COPD (GOLD Stage 2–4); post-bronchodilator forced expiratory volume in 1 second (FEV1) <80% of predicted normal; post-bronchodilator FEV1/forced vital capacity <70%; and current or ex-smokers with a smoking history of >10 pack-years were included. Patients with a significant and unstable disease other than COPD were excluded from the study.7 Patients with moderate or severe renal impairment (CLcr ≤50 mL/min) were included, but were monitored closely by the investigator.7 The studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation’s Harmonised Tripartite Guideline for Good Clinical Practice, and local regulations. Approval was granted from institutional review boards (Coordinating Investigator’s [Prof. Roland Buhl] Independent Ethics Committee: Ethik-Kommission bei der Landesärztekammer Rheinland-Pfalz, Deutschhausplatz 3, 55116 Mainz, Germany) and patients provided written informed consent for these studies.
Evaluations and outcome measures
For this analysis, renal impairment categories (normal, mild, moderate, and severe) were defined per US Food and Drug Administration (FDA) guidance by estimated CLcr calculated from serum creatinine (Cockroft–Gault formula) at baseline (FDA Guidance for Industry, Clinical Pharmacology; 2010; Table 1).18
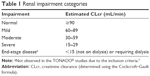  | Table 1 Renal impairment categories |
In a prespecified safety analysis, investigator-reported treatment-emergent AEs (TEAEs) were pooled from both TONADO studies. In TONADO, all AEs and serious AEs (SAEs) occurring from consent through 21 days after the last dose of the study medication were collected. The duration, intensity, relatedness to study drug, treatment required, outcomes, seriousness, and action taken were recorded by the investigator. AEs were classed as mild, moderate, or severe using predefined criteria. An SAE was defined as any AE that resulted in death, was immediately life-threatening, resulted in persistent or significant disability or incapacity, required hospitalization, or resulted in a congenital anomaly/birth defect. All deaths and SAEs were adjudicated by an external independent, blinded adjudication committee of clinicians.
Analysis
AEs were coded as per the Medical Dictionary for Regulatory Activities version 16.1 and assigned to system organ classes (SOCs). A composite endpoint of major adverse cardiovascular events (MACE) was included, representing fatal AEs in cardiac disorder and vascular disorder SOCs, in combination with fatal and nonfatal myocardial infarction, fatal and nonfatal stroke, as well as sudden death, sudden cardiac death, and cardiac death.
Data are reported for patients who received tiotropium/olodaterol 5/5 μg, tiotropium 5 μg, or olodaterol 5 μg, and who had baseline CLcr data available.
Results
Patient population
In TONADO, 5,162 patients were treated and 3,100 received study drugs at the marketed doses (olodaterol 5 μg: n=1,038; tiotropium 5 μg: n=1,033; tiotropium/olodaterol 5/5 μg: n=1,029). Of these, 3,041 patients had baseline CLcr data available. At baseline, 1,333 (43.8%) patients had mild renal impairment (CLcr 60–89 mL/min) and 404 (13.3%) had moderate renal impairment (CLcr 30–59 mL/min), and were distributed equally between treatment groups. Five patients (0.2%) had severe renal impairment at baseline (CLcr 15–29 mL/min); of these, 2 received olodaterol and 3 received tiotropium/olodaterol (Table 2). Because of the small number of patients with severe renal impairment in the TONADO studies, these are not included in the stratified comparative analysis and are described case by case.
  | Table 2 Demographic and baseline patient characteristics by renal impairment category |
Baseline characteristics and demographics
Approximately two-thirds of patients were male (69.6%–77.6%), with no differences observed between treatment groups or renal impairment category (Table 2). Age and smoking history (pack-years) tended to increase with severity of renal impairment, with no differences observed between treatment groups (Table 2). Notably, the proportion of current smokers among all analyzed patients decreased with increasing severity of renal impairment (42.6%, 36.5%, and 22.8% of patients with no, mild, and moderate renal impairment, respectively).
Among all treated patients with normal renal function, 77.1% received pulmonary medication at baseline, compared with 79.9% and 83.2% of those with mild and moderate renal impairment, respectively. This increase was not related to an increase in COPD severity, as determined by GOLD stage, at baseline (Table 2), and no clear differences were observed between treatment groups. The proportion of patients receiving tiotropium at baseline increased with renal impairment severity (31.5%, 36.6%, and 39.6% in patients with no, mild, and moderate renal impairment, respectively), whereas no differences were observed among the proportions of patients receiving a LABA (47.4%, 44.6%, and 46.0%, respectively) or inhaled corticosteroids (47.0%, 47.1%, and 50.7%, respectively). There was some increase in the proportion of patients receiving supportive pulmonary medication (mucolytics) at baseline with increasing severity of renal impairment, irrespective of no increase in COPD severity at baseline (Table 2). Similar proportions of patients with normal (61.0%), mild (53.6%), and moderate (62.1%) renal impairment received cardiovascular medication at baseline. Of the 5 patients with severe renal impairment, 4 (80%) were receiving cardiovascular medication at baseline.
Considerable comorbidity was observed in the TONADO study population; almost one-quarter of treated patients included in this analysis (23.4%) had a history of cardiac disorder, 45.6% had hypertension, and 13.3% had disorders of glucose metabolism, including diagnosed diabetes mellitus. Overall, the proportion of patients with comorbidities did not tend to increase uniformly with the severity of renal impairment (Figure 1).
  | Figure 1 Baseline comorbidity by renal impairment category. |
Exposure to study medication
Exposure to study drug was similar among all patients, irrespective of treatment group or the presence of renal impairment. Among patients with severe renal impairment, median (minimum–maximum) exposure to olodaterol and tiotropium/olodaterol was 368 (range 364–372) and 365 (range 14–367) days, respectively; no patients with severe renal impairment were exposed to tiotropium monotherapy (Table 2).
AEs and SAEs
Safety outcomes by baseline renal impairment and treatment group are summarized in Table 3, Figures 2 and S1. Overall, the proportion of patients with an AE or SAE was high, as would be expected in a typically sick population of patients with COPD.
  | Table 3 Summary of adverse events by baseline renal impairment |
Among all treated patients, 72.6% with normal renal function reported an AE compared with 74.3% with mild and 78.7% with moderate renal impairment (Table 3). AEs with olodaterol, tiotropium, and tiotropium/olodaterol occurred in 75.1%, 70.8%, and 72.0% of patients with no renal impairment; 75.7%, 74.0%, and 73.3% with mild renal impairment; and 84.3%, 79.5%, and 79.7% with moderate renal impairment, respectively (Table 3; Figures 2 and S1). TEAEs occurred in 5.6% of patients with normal renal function, 6.9% of those with mild renal impairment, and 8.9% with moderate renal impairment (Table 3).
SAEs occurred in 15.3% of patients with normal renal function, compared with 16.4% and 23.0% of patients with mild and moderate renal impairment, respectively (Table 3). Among patients receiving olodaterol, tiotropium, and tiotropium/olodaterol, SAEs occurred in 18.2%, 13.6%, and 14.1% of patients with normal renal function; in 13.8%, 18.7%, and 16.7% with mild renal impairment; and in 26.1%, 19.7%, and 23.2% with moderate renal impairment, respectively (Table 3). The proportion of patients with a fatal SAE was low in all treatment groups, with a similar incidence reported among those receiving tiotropium/olodaterol regardless of the level of renal impairment (normal 1.5%; mild 2.0%; moderate 2.2%; Table 3). SAEs requiring hospitalization occurred in 14.1%, 14.7%, and 19.8% of patients with normal, mild, and moderate renal impairment, respectively, with no clear differences between treatment groups (Table 3).
Discontinuation of study medication
Generally, lower proportions of patients discontinued study treatment, either prematurely or due to an AE, with tiotropium/olodaterol compared with the monocomponents, with no effect of renal impairment category observed (Figure 3).
Safety outcomes in patients with severe renal impairment
Among the 5 patients with severe renal impairment, AEs occurred in 1 of the 2 patients receiving olodaterol and 3 of the 3 patients receiving tiotropium/olodaterol; all patients recovered and all continued taking study medication. One patient receiving tiotropium/olodaterol experienced an AE that was considered by the investigator as possibly related to treatment (dysgeusia, insomnia, sudoresis, and tremor); this was not considered serious, and the patient recovered and continued treatment (Table 4).
Clinically relevant AEs associated with renal impairment, and use of tiotropium/olodaterol or the monocomponents
Renal impairment did not affect the incidence of any specific clinically relevant AE, and there was no difference in AE incidence among patients in each group treated with tiotropium/olodaterol versus tiotropium or olodaterol alone. Notably, exposure-adjusted incidence rates of MACE, renal and urinary tract AEs, and potential anticholinergic effects were not affected by the level of renal impairment or by treatment (Figures 4 and S1A–D).
Discussion
In this subgroup analysis of data from the TONADO studies, the safety profile of tiotropium/olodaterol compared with the monocomponents was similar to the overall results of the study, regardless of the severity of renal impairment. When analyzed by each renal impairment category, the proportion of patients experiencing an AE was comparable to the results of the TONADO studies, in which 74.4% of patients experienced ≥1 AE.7 Importantly, the proportion of patients experiencing an AE did not increase proportionally with the severity of renal impairment, and no increase in the proportion of patients with an AE or SAE was found with tiotropium/olodaterol compared with the monocomponents. Discontinuation of study medication occurred less frequently with tiotropium/olodaterol than with the monocomponents, suggesting better tolerability of the FDC in this patient population.7 Overall, these data support the safety of dual bronchodilation with tiotropium and olodaterol in patients with moderate-to-very severe COPD and renal impairment, with no additional safety concerns found with combination therapy over the monocomponents.
The pharmacokinetics of tiotropium have been well-characterized in preclinical studies using in vitro and in vivo models, and confirmed in clinical studies.19,20 Tiotropium binds to human M3 receptors with high affinity, and its slow dissociation kinetics account for its long duration of action, providing 24-hour bronchodilation.19,20 Similarly, binding, kinetic, and functional data support the 24-hour duration of olodaterol action, confirming the suitability of both agents for once-daily dosing.21 The doses of the monocomponents used in the FDC (tiotropium 5 μg, olodaterol 5 μg) are based on data from dose-finding studies and are consistent with the respective optimum dose when each is used as a monotherapy.22 In a 4-week, Phase II, dose-ranging study, systemic exposure to olodaterol was shown to increase dose-proportionally within the dose range 5–20 μg in patients with moderate-to-severe COPD.23 Olodaterol is excreted renally only at low levels, varying from 0.341% to 0.628% of the inhaled dose after 1 and 29 days of dosing, respectively.23 Olodaterol was associated with a dose-response improvement in lung function, which plateaued at 10–20 μg, with no evidence of dose-dependent safety signals.23 Dose-ranging studies of tiotropium delivered with the Respimat inhaler identified doses of 5 and 10 μg as the most effective and safe, based on the assessment of efficacy, safety, and pharmacokinetics.24 Compared with doses of 5 and 10 μg, 20 μg tiotropium resulted in a much higher systemic exposure, without any additional benefits in efficacy; 5 and 10 μg were therefore investigated in the Phase III trials.24 When administered in combination, the pharmacokinetics of tiotropium and olodaterol are similar to those when either drug is administered alone, with no dose adjustments required for either tiotropium or olodaterol.10 This is in contrast to other LABA/LAMA FDCs.22,25 A Phase I study of aclidinium/formoterol FDC versus the monocomponents in healthy subjects revealed no differences in the relative bioavailability of the FDC compared with the monocomponents; however, systemic exposure, as determined by Cmax and AUC0-t, was 26% and 3% higher with the FDC than with aclidinium alone, and 18% and 11% higher than formoterol alone, respectively.26
Unlike olodaterol, tiotropium is excreted renally, and urinary excretion of tiotropium administered via Respimat increases in a dose-dependent manner.24 Renal impairment has been associated with increased plasma drug concentrations and reduced clearance following inhalation of tiotropium, either as a dry powder (via HandiHaler®) or solution (via Respimat).9 In a study assessing the pharmacokinetics of single-dose tiotropium administered intravenously to patients with varying levels of renal impairment, increasing plasma concentrations of tiotropium were associated with decreasing renal function, with the highest levels observed in individuals with severely impaired renal function.27 This was associated with an increased mean terminal disposition half-life. Although the dose used in that study resulted in a higher systemic exposure compared with the recommended dose of tiotropium, no acute AEs occurred in any individual with renal impairment.27 Thus, no dose adjustment is recommended for tiotropium/olodaterol in patients with moderate-to-severe renal impairment, although it is advised that patients should be monitored closely for potential anticholinergic effects.10 Similarly, no dose adjustments are recommended for the combination therapies indacaterol/glycopyrronium, umeclidinium/vilanterol, or aclidinium/formoterol in patients with renal impairment.25,28 Aclidinium is rapidly hydrolyzed, and a Phase I study showed that very low levels (<1%) are excreted unchanged renally, regardless of the presence of varying levels of renal impairment, suggesting that a dose adjustment is not needed in this population.29 In patients with severe renal impairment, there was no evidence of an increase in systemic exposure of either umeclidinium or vilanterol when administered in combination, with umeclidinium given at twice the recommended dosage.30
A database analysis suggested that tiotropium administered via the Respimat device may confer an increased risk of mortality among patients with stage 3–5 chronic kidney disease, defined as an estimated glomerular filtration rate (GFR) <60 mL/min/1.73 m2 body surface area, while no increased risk was suggested in patients with normal renal function (adjusted hazard ratio [HR] 1.52; 95% CI: 1.02–2.28 versus HR 0.84; 95% CI: 0.25–2.85, respectively).31 However, in a pooled analysis of 10,805 patients with renal impairment enrolled in 22 placebo-controlled trials of tiotropium (HandiHaler 18 μg and Respimat 5 μg), no association was found between the incidence of AEs, SAEs, or fatal SAEs with tiotropium and mild-to-moderate renal impairment, defined as CLcr ≥60 and ≥30 mL/min, respectively,17,32 consistent with the findings of the present analysis.
The long-term general and cardiovascular safety of tiotropium and olodaterol in patients with COPD has been confirmed in several large clinical trials.4,33–36 Anticholinergics such as tiotropium inhibit parasympathetic-induced bronchoconstriction by blocking the action of acetylcholine through binding to muscarinic receptors in the airways.37 Although administered via an inhalation device, the use of inhaled LAMAs carries a potential risk of systemic anticholinergic side effects, including dry mouth, constipation, glaucoma, urinary retention, hallucinations, and cardiac effects, at doses higher than the therapeutic doses, or under situations of markedly reduced elimination, particularly in elderly patients.38 In the TONADO studies, there was a low incidence of potential anticholinergic effects overall.7 Similarly, in the present analysis, the use of tiotropium/olodaterol was not associated with any increased risk of anticholinergic side effects compared with the monocomponents, regardless of the level of renal impairment at baseline.
Longitudinal studies have shown that renal function, as measured by estimated GFR or CLcr, declines with advancing age, with a faster rate of decline observed in older individuals and in those with lower baseline renal function.39–41 As the prevalence of COPD increases with advancing age, it is likely that a high proportion of patients with COPD also have reduced renal function.1 Indeed, 1% and 7% of patients with COPD enrolled in Phase III studies have been estimated to have mild and moderate renal impairment, respectively.27 Although advancing age has been associated with reduced renal clearance of tiotropium, increased systemic exposure to the drug was not found.10 Thus, the findings of this analysis support the safety of tiotropium/olodaterol in older patients with COPD and impaired renal function.
Generally, data from randomized clinical trials regarding the safety of tiotropium and olodaterol in patients with renal impairment are lacking, owing to the exclusion criteria of the trials. Therefore, a strength of the present analysis is that the large size of the TONADO trials (>5,000 patients) permits subgroup comparisons regarding renal safety;7 however, the number of patients with severe renal impairment at baseline remained low, likely reflecting the exclusion criteria of the trials. A limitation of this analysis is that CLcr was estimated as per usual practice18 at baseline and was not repeated during follow-up; therefore, changes in renal function over the course of the study period were not considered. The Cockcroft–Gault equation was developed based on the observation that CLcr decreases linearly with age, and predicts CLcr based on measured serum creatinine, age, and weight. However, these initial observations were based on males and assumed that renal function was stable, and the calculation was not adjusted for body surface area.42 Although recommended by the US FDA for use in pharmacokinetics studies, the ability of the Cockcroft–Gault equation to correctly estimate GFR has been questioned, and it has been shown to be imprecise in reflecting GFR based on the measurement of creatinine secretion, particularly in patients aged ≥65 years, who may have a reduced GFR despite normal serum creatinine levels.43–45 Nevertheless, the results of the present analysis are reassuring, with no safety signals observed in patients with COPD and varying levels of renal impairment, as determined by means of CLcr.
Conclusion
In this subgroup analysis of patients with renal impairment participating in the TONADO studies, there was no increase in the proportion of patients with AEs or SAEs with tiotropium/olodaterol versus tiotropium or olodaterol alone, and the presence of renal impairment did not affect the incidence of AEs in any treatment group. These findings indicate that the safety and tolerability of tiotropium/olodaterol FDC delivered via Respimat are comparable to the monocomponents regardless of the level of renal impairment, with no additional safety signals observed.
Acknowledgments
Medical writing assistance was provided by Lisa Jolly of MediTech Media, which was contracted and compensated by Boehringer Ingelheim International.
Author contributions
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors. All authors contributed toward data analysis and interpretation, drafting and revising the paper, and agree to be accountable for all aspects of the work. The authors received no compensation related to the development of the manuscript.
Disclosure
RB reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Roche, and Teva, as well as grants to Mainz University from Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Roche, outside the submitted work. ED’s clinical department has received financial support from Boehringer Ingelheim and Novartis to perform clinical studies; he has participated in advisory boards for Boehringer Ingelheim, Chiesi, Cipla, Novartis, and AstraZeneca, for which a fee was given (not related to this work); he has received travel grants from Boehringer Ingelheim, GlaxoSmithKline, and AstraZeneca to attend international congresses and has received speaker’s fees from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Novartis. UB, IMK, and MT are employees of Boehringer Ingelheim International. CLF has received financial support from Boehringer Ingelheim to perform clinical studies. The authors report no other conflicts of interest in this work.
References
Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. | ||
World Health Organization. Chronic Respiratory Diseases. Available from: http://www.who.int/respiratory/copd/burden/en/. Accessed June 8, 2017. | ||
Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2016 Report. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed April 27, 2016. | ||
Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. | ||
Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2–4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629–645. | ||
Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat® versus placebo and formoterol twice daily in patients with GOLD 2–4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:697–714. | ||
Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4). Eur Respir J. 2015;45(4):969–979. | ||
Kunz C, Luedtke D, Unseld A, et al. Pharmacokinetics and safety of olodaterol administered with the Respimat Soft Mist inhaler in subjects with impaired hepatic or renal function. Int J Chron Obstruct Pulmon Dis. 2016;11:585–595. | ||
Boehringer Ingelheim Pharmaceuticals I. Spiriva® Respimat® (tiotropium bromide) inhalation spray, for oral inhalation. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva%20Respimat/spirivarespimat.pdf. Accessed February 12, 2017. | ||
Boehringer Ingelheim International GmbH. Spiolto Respimat 2.5 microgram/2.5 microgram, inhalation solution. Summary of Product Characteristics, labelling and package leaflet 2015. Available from: http://mri.medagencies.org/download/NL_H_3157_001_FinalPI.pdf. Accessed March 1, 2016. | ||
Vande Griend JP, Linnebur SA. Inhaled anticholinergic agents and acute urinary retention in men with lower urinary tract symptoms or benign prostatic hyperplasia. Ann Pharmacother. 2012;46(9):1245–1249. | ||
Afonso AS, Verhamme KM, Stricker BH, Sturkenboom MC, Brusselle GG. Inhaled anticholinergic drugs and risk of acute urinary retention. BJU Int. 2011;107(8):1265–1272. | ||
Stephenson A, Seitz D, Bell CM, et al. Inhaled anticholinergic drug therapy and the risk of acute urinary retention in chronic obstructive pulmonary disease: a population-based study. Arch Intern Med. 2011;171(10):914–920. | ||
Afonso AS, Verhamme KM, Sturkenboom MC, Brusselle GG. COPD in the general population: prevalence, incidence and survival. Respir Med. 2011;105(12):1872–1884. | ||
Miravitlles M, Price D, Rabe KF, Schmidt H, Metzdorf N, Celli B. Comorbidities of patients in tiotropium clinical trials: comparison with observational studies of patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:549–564. | ||
Freeman D, Lee A, Price D. Efficacy and safety of tiotropium in COPD patients in primary care–the SPiRiva Usual CarE (SPRUCE) study. Respir Res. 2007;8:45. | ||
Halpin DM, Dahl R, Hallmann C, Mueller A, Tashkin D. Tiotropium HandiHaler® and Respimat® in COPD: a pooled safety analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:239–259. | ||
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function–Study Design, Data Analysis, and Impact on Dosing and Labeling. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm204959.pdf. Accessed June 7, 2017. | ||
Casarosa P, Bouyssou T, Germeyer S, Schnapp A, Gantner F, Pieper M. Preclinical evaluation of long-acting muscarinic antagonists: comparison of tiotropium and investigational drugs. J Pharmacol Exp Ther. 2009;330(2):660–668. | ||
Hohlfeld JM, Sharma A, van Noord JA, et al. Pharmacokinetics and pharmacodynamics of tiotropium solution and tiotropium powder in chronic obstructive pulmonary disease. J Clin Pharmacol. 2014;54(4):405–414. | ||
Casarosa P, Kollak I, Kiechle T, et al. Functional and biochemical rationales for the 24-hour-long duration of action of olodaterol. J Pharmacol Exp Ther. 2011;337(3):600–609. | ||
Aalbers R, Maleki-Yazdi MR, Hamilton A, et al. Randomized, double-blind, dose-finding study for tiotropium when added to olodaterol, administered via the Respimat® inhaler in patients with chronic obstructive pulmonary disease. Adv Ther. 2015;32(9):809–822. | ||
Maleki-Yazdi MR, Beck E, Hamilton AL, Korducki L, Koker P, Fogarty C. A randomised, placebo-controlled, Phase II, dose-ranging trial of once-daily treatment with olodaterol, a novel long-acting beta2-agonist, for 4 weeks in patients with chronic obstructive pulmonary disease. Respir Med. 2015;109(5):596–605. | ||
Caillaud D, Le Merre C, Martinat Y, Aguilaniu B, Pavia D. A dose-ranging study of tiotropium delivered via Respimat Soft Mist Inhaler or HandiHaler in COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2(4):559–565. | ||
AstraZeneca UK Limited. Duaklir Genuair 340 micrograms/12 micrograms inhalation powder. Summary of Product Characteristics. Available from: https://www.medicines.org.uk/emc/medicine/29652. Accessed July 17, 2017. | ||
Fuhr R, Leselbaum A, Aubets J. Pharmacokinetics of aclidinium bromide/formoterol fumarate fixed-dose combination compared with individual components: a phase 1, open-label, single-dose study. Clin Pharmacol Drug Dev. 2016;5(2):109–117. | ||
Turck D, Weber W, Sigmund R, et al. Pharmacokinetics of intravenous, single-dose tiotropium in subjects with different degrees of renal impairment. J Clin Pharmacol. 2004;44(2):163–172. | ||
Novartis Pharmaceuticals UK Ltd. Ultibro® Breezhaler 85 micrograms/43 micrograms, inhalation powder hard capsules. Summary of Product Characteristics. Available from: https://www.medicines.org.uk/emc/medicine/29533. Accessed July 17, 2017. | ||
Schmid K, Pascual S, Gil EG, Ortiz S, Jansat JM. Pharmacokinetics and safety of aclidinium bromide, a muscarinic antagonist, in adults with normal or impaired renal function: a phase I, open-label, single-dose clinical trial. Clin Ther. 2010;32(10):1798–1812. | ||
GlaxoSmithKline UK. Relvar Ellipta 184 micrograms/22 micrograms inhalation powder, pre-dispensed. Summary of Product Characteristics. Available from: https://hcp.gsk.co.uk/products/relvar.html. Accessed July 17, 2017. | ||
Verhamme KM, van Blijderveen N, Sturkenboom MC. Tiotropium and the risk of death in COPD. N Engl J Med. 2014;370(5):481–482. | ||
Tashkin D, Metzdorf N, Hallmann C, Könen-Bergmann M, Kupas K, Dahl R. Safety of tiotropium in renally impaired patients. Eur Respir J. 2014;44:P923. | ||
Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369(16):1491–1501. | ||
Tashkin DP, Leimer I, Metzdorf N, Decramer M. Cardiac safety of tiotropium in patients with cardiac events: a retrospective analysis of the UPLIFT® trial. Respir Res. 2015;16(1):65. | ||
Ferguson GT, Karpel JP, Clerisme-Beaty E, Gronke L, Voss F, Buhl R. Efficacy and safety of tiotropium + olodaterol maintenance treatment in patients with COPD in the TONADO® and OTEMTO® studies: a subgroup analysis by age. Int J Chron Obstruct Pulmon Dis. 2016;11:2701–2710. | ||
Buhl R, Magder S, Bothner U, et al. Long-term general and cardiovascular safety of tiotropium/olodaterol in patients with moderate to very severe chronic obstructive pulmonary disease. Respir Med. 2017;122:58–66. | ||
Alagha K, Palot A, Sofalvi T, et al. Long-acting muscarinic receptor antagonists for the treatment of chronic airway diseases. Ther Adv Chronic Dis. 2014;5(2):85–98. | ||
Mintzer J, Burns A. Anticholinergic side-effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–462. | ||
Cohen E, Nardi Y, Krause I, et al. A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol. 2014;27(6):635–641. | ||
Baba M, Shimbo T, Horio M, et al. Longitudinal study of the decline in renal function in healthy subjects. PLoS One. 2015;10(6):e0129036. | ||
Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):278–285. | ||
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. | ||
Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009;54(1):33–42. | ||
Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis. 2005;46(2):233–241. | ||
Pedone C, Corsonello A, Incalzi RA, GIFA Investigators. Estimating renal function in older people: a comparison of three formulas. Age Ageing. 2006;35(2):121–126. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





