Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 13
Long-Term Outcome Measures of Repeated Non-Animal Stabilized Hyaluronic Acid (Durolane) Injections in Osteoarthritis: A 6-Year Cohort Study with 623 Consecutive Patients
Authors Carney G, Harrison A, Fitzpatrick J
Received 29 July 2021
Accepted for publication 3 September 2021
Published 18 September 2021 Volume 2021:13 Pages 285—292
DOI https://doi.org/10.2147/OARRR.S331562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Georgia Carney,1,2 Andrew Harrison,3 Jane Fitzpatrick1,2,4
1Joint Health Institute, Melbourne, Victoria, Australia; 2Faculty of Medicine, Dentistry and Health Sciences, The University of Melbourne, Parkville, Victoria, Australia; 3Bioventus International, Hoofddorp, 2131 LS, Netherlands; 4Australasian College of Sport and Exercise Physicians, Melbourne, Victoria, Australia
Correspondence: Jane Fitzpatrick Level 7, Alan Gilbert Building
161 Barry Street University of Melbourne, Parkville, Victoria, 3010, Australia
Tel +61 3 9429 6444
Email [email protected] Twitter @sportsdoc
Purpose: To determine the duration of symptom relief following repeated administration of hyaluronic acid injections for osteoarthritis.
Patients and Methods: This was a 6-year observational study with 623 consecutive patients who had received hyaluronic acid injections. The primary outcome measure was the mean time between injections measured in days. Classical one-sample 2-sided t-tests, one-way analysis of variances and post-hoc analyses were performed to determine if there were statistically significant differences between age, gender, radiographic severity and the type of joints injected. All patients were invited to complete an online post-treatment experience and satisfaction survey.
Results: The analysis included 727 joints (mean Kellgren-Lawrence grade, 2.9 ± 0.8 (range 2– 4)) in 623 patients (297 (47.7%) male; mean age at first injection, 57.8 ± 12.7 years (range 21.2– 92.1)). Patients ranged from having 1– 8 injections per joint. The mean time between injections in days was 466.8 ± 321.7 (2nd injection, 157 joints), 400.5 ± 164.7 (3rd injection, 58 joints), 378.2 ± 223.1 (4th injection, 27 joints), 405.3 ± 216.3 (5th injection, 7 joints), 268.4 ± 104.4 (6th injection, 5 joints), 289.8 ± 99.4 (7th injection, 4 joints), and 272.5 ± 33.2 (8th injection, 2 joints). Patients with grades 2 and 3 compared to grade 4 osteoarthritis experienced a longer time between injections (F (2, 154) = 3.53, p = 0.0316). No statistically significant differences were observed between age, gender, or joint groups. The survey included 233 participants (109 (46.8% male)). A total of 144 respondents (64.9%) recommended hyaluronic acid injections for osteoarthritis.
Conclusion: Pain relief from hyaluronic acid injections was sustained for on average 466.8 days post initial treatment. Patients who received subsequent 3rd, 4th, and 5th injections also experienced extended duration of benefit. Patients with grades 2 or 3 osteoarthritis are more likely to experience a longer duration of relief.
Keywords: biological treatment, joint, hyaluronic acid, intra-articular injection, long-term, pain relief
Introduction
Osteoarthritis (OA) is the most common form of arthritis and is a painful cause of debilitation for those who have the condition. Affecting more than 500 million people (7%) of the global population, in 2019 OA was the 15th highest cause of years lived with disability (YLDs).1 OA commonly reduces mobility1,2 with large numbers of joint replacements performed each year attributing an average lifetime cost of $US 140, 300 for individuals with OA in the knee.3
Intra-articular injection of hyaluronic acid (HA), also known as viscosupplementation, is a non-surgical option for the symptomatic treatment of OA. An essential component of synovial fluid, endogenous HA facilitates joint lubrication and shock absorption. As progression of OA disease is often characterized by decreasing endogenous HA in the joint, intra-articular injection of HA is an appealing conservative treatment.4
There are several HA products available for the treatment of OA. Each is distinguished by their source and production method; the molecular size and weight of HA; the amount, concentration, and volume of HA injections per dose; and the number of injections performed. One of the potential clinical limitations of HA injections is the degradation of exogenous HA due to physiological turnover in the treated joint. In order to reduce this, several HA products use chemical cross-linking to increase the molecular size and weight of HA. HA products derived from biological fermentation and with a high molecular weight of >3000 kilodaltons are likely to be superior in the treatment of OA.5
Non-animal stabilized HA (Durolane) is a newly developed HA product made from a bacterial fermentation process. Purified and stabilized using covalent bonds and natural entanglement, Durolane has a high molecular weight and is designed to supplement endogenous HA by forming a viscous three-dimensional gel.6
Durolane injections have been demonstrated to have a half-life of four weeks in the knee joint in rabbit models and human studies.7,8 A systematic review of seven level 1 and four level 2–3 studies indicated that patients can expect pain relief from a single injection to last for at least 182 days or 6 months, and will experience improvements in other functional measures including quality of life scores.9 Safety of repeat Durolane injections has been confirmed10 although effectiveness of the long-term use of Durolane through repeat injections is yet to be determined. A recent systematic review of the long-term safety and efficacy of repeated courses of several HA products involved 17 studies11 but only one of these looked specifically at the effects of Durolane injections.10
The primary aim of this study was to determine the length of symptom relief following repeated administration of Durolane HA injections for OA. The secondary outcome was to determine patient satisfaction with the procedure.
Patients and Methods
Ethical Approval
Ethics approval was obtained from the University of Melbourne Human Research Ethics Committee (reference number: 2021-21209-16823-4). All patients within this clinical dataset analysis provided informed consent for the use of their data and informed consent was obtained from those participants in the patient satisfaction survey. The findings are presented in line with guidelines from The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement on reporting observational studies.12
Patients
The clinical dataset from the medical records of 623 consecutive patients with knee, hip, shoulder, or ankle OA who had received one or more Durolane injections by a single clinician in a single site between May 2013 – December 2019 was analyzed. Data was collected between March 2021 – May 2021. Patients were adults (>18 years) with OA confirmed by radiographic imaging. Inclusion criteria were all patients having a Durolane injection in any joint during the time period. Exclusion criteria were mechanical symptoms in the joint, malalignment or inflammatory signs or symptoms.
Study Design
This study comprised two parts. For Part A of the study, data were extracted onto a spreadsheet and de-identified before analysis. The primary objective was to measure the mean time between patients returning for a second or subsequent HA injections, measured in days. All participants were analyzed by group (whether they had received one, two or more HA injections) and then stratified by baseline age, gender, and KL grade characteristics. For Part B of this study, patients recruited from the Part A pool were invited to participate in an online, anonymous, survey to indicate their satisfaction post HA injection treatment. Patients were contacted via SMS in May 2021 with two reminders sent each a week apart and asked to answer 8 multiple-choice questions. The survey took approximately five minutes to complete.
Statistical Analysis
Analysis was conducted using STATA version 13.1 (Stata Corp. 2016 Stata Statistical Software: Release 13.1. College station, TX: Stata Corp LP) with p-values calculated to <0.05 significance.
Time between injections was presented for each injection group using the mean time in days ± the standard deviation (SD) with 95% confidence intervals (CI) and the range. A classical one-sample 2-sided t-test was performed with the assumption of unknown variance. The null hypothesis was set at 182 days (26 weeks or 6 months) in line with previous clinical trial reports that non-animal stabilized HA is effective up to this point.9
One-way analysis of variance (ANOVA) and post hoc Tukey’s tests were performed to compare the mean time between second or subsequent injections differed depending on gender, age, KL grade and the anatomical location of the joint injected (hip, knee, shoulder, and ankle) groups. Equal variances and an approximately normal distribution were assumed for each category.
Descriptive statistics were used to report survey results using counts and percentages for categorical and dichotomous variables.
Type of Injection
Treatment with HA (Durolane; Bioventus LLC, Durham, NC, USA; 20mg/mL sodium hyaluronate, 3mls) was administered as a single injection by the clinician at a single site in Melbourne, Australia. Patients were administered HA injections intra-articularly using an 18–22 G needle with strict aseptic technique. A prefilled 3mL syringe was used for the knee, hip, and shoulder joints with a 1mL syringe used in patients who received HA injection to their ankle. Prior to injection, subcutaneous local anesthetic (2mls lignocaine) was used as needed and all procedures were performed under ultrasound guidance.
Grading of X-Rays
KL grades were assigned by a senior clinician or a qualified radiologist from the most recent radiological imaging taken prior to patients’ first HA injection. Where the KL grade was reported as between grades this was recorded up, ie, grade 2–3 OA was recorded as grade 3.
Results
Part A
A total of 623 patients (297 (47.7%) male; mean age at first injection, 57.8 ± 12.7 years (range 21.2–92.1 years) involving 727 joints (326 (44.8%) knees; 387 (53.2%) hips; 12 (1.7%) shoulder, and 2 (0.3%) ankles were included in the analysis. Baseline demographics and clinical characteristics are presented in Table 1.
 |
Table 1 Demographic Data |
Participants ranged from having one to eight injections over the six-year period. The average time between patients receiving their first HA injection and receiving a second was 466.8 ± 321.7 days (range 31–1831 days). At injections one, two, three, four and five there was a statistically significant difference from the expected mean time of 182 days to actual return time to treatment (466.8, 400.5, 378.2 and 405.3 days, p = <0.0001, <0.0001, 0.0001 and 0.0341 respectively). At injections six, seven and eight the mean time to return to treatment also was greater than expected although this result was not statistically significant (268.4, 289.8 and 272.5 days, p = 0.1379, 0.1186 and 0.1617 respectively). Data are displayed at each timepoint and overall, in Table 2 and Figure 1.
 |
Table 2 Average Time Gap Between HA Injections in Days |
 |
Figure 1 Graph of duration of symptom relief in days for each injection. x-axis = injection number, y-axis = days. |
A one-way ANOVA test was conducted to stratify patients who had two or more HA injections related to age, gender, KL grade and anatomical location of the joint injected. There was a statistically significant difference between KL grade groups in joints returning for a HA injection (F (2, 154) = 3.53, p-value = 0.0316) (Table 3). A Tukey post-hoc test revealed that time between repeated HA injections was significantly higher in the KL grade 2 group compared to grade 4 (−107.8 ± 43.7 days, p-value = 0.038). However, there were no statistically significant differences between KL grades 2 and 3 (−7.0 ± 39.3 days, p-value 0.983), nor between KL grades 3 and 4 (−100.9 ± 44.6 days, p-value = 0.063) as shown in Table 4.
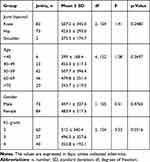 |
Table 3 Average Time Joints Returned for a 2nd HA Injection, in Days, Stratified by Group |
 |
Table 4 Tukey’s Comparison of Average Time Between Injections, in Days, for KL Grade |
Further analysis showed that this was only evident in patients returning for a second injection, and not for subsequent 3rd, 4th, 5th, 6th, 7th, or 8th injections. Also, no statistically significant differences were observed between age, gender, and anatomical location of injected joint groups. Data for these tests are stratified by the number of injections done and provided in the Supplementary Tables.
Part B
All 623 patients were sent the survey link, 233 patients (109 (46.8%) male) consented to participate in the online patient satisfaction survey, a 37.4% response rate. The flow of patients through the survey is outlined in Figure 2.
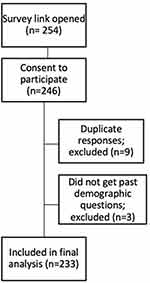 |
Figure 2 Flow of patients through post-treatment experience and satisfaction survey. Abbreviation: n, number of participants. |
Of the 151 participants (64.8%) who stated that they experienced significant relief from their OA symptoms following their first HA injection, 36 participants (23.9%) felt that this relief lasted less than six months, while the remaining 115 (76.1%) of participants indicated that their pain relief was sustained for between 6 to more than 18 months post treatment as shown in Table 5.
 |
Table 5 Patient Experience and Satisfaction with HA Treatment Survey Results |
One-hundred and fifty participants (66.4%) had received concurrent or subsequent treatments for their OA with joint replacement 77 (51.3%) being the most common, followed by physiotherapy 58 (38.7%), arthroscopy 33 (22%), strength-based programs 32 (21.3%), corticosteroid injections 18 (12%) and platelet-rich plasma injections 17 (11.3%).
One hundred and forty-four (64.9%) survey respondents indicated that based on their experience they would recommend HA treatment for OA. An open-text comment was available to report any adverse events: 6 participants (2.7%) indicated that they felt they had experienced an adverse reaction to HA injections.
Discussion
Patients receiving HA injections in this study had a clinical and radiographic diagnosis of OA. In line with recommended patient selection criteria for HA injections, patients were excluded if they had mechanical symptoms in the joint, malalignment or inflammatory signs or symptoms.
The primary analysis showed that patients returned for a 2nd HA injection on average 466.8 ± 321.7 days (approximately 15 months) post initial treatment (Table 2, Figure 1). This is statistically significantly longer than what has been previously reported as the duration of benefit in HA injections of 182 days (26 weeks or 6 months) (p-value <0.0001) (Table 2).9 Similar results were obtained for patients receiving 3rd, 4th, and 5th HA injections (400.5, 378.2 and 405.3 days, p-value <0.0001, 0.0001 and 0.0341 respectively) (Table 2). Sustained benefit was also observed for the smaller cohort of patients who received 6th, 7th, and 8th HA injections (268.4, 2989.8 and 272.5, p-value 0.1379, 0.1186 and 0.1617 respectively) (Table 2).
The results of this study were surprising in that the average duration of pain relief was substantially longer than has previously been reported and sustained for five injection periods. Further, for some patients, the time gap between HA injections was as many as 844, 875, 1162 and 1831 days (between 2.3 and 5.0 years). For 6th and subsequent injections, the time gap between injections was reduced. The smaller number of joints in these later injection timepoints contributes to less statistical power and suggests that after this point patients experienced less relief from HA treatment.
A sub-group analysis found that HA injections had a more sustained benefit in patients with grade 2 and grade 3 OA compared to grade 4 OA consistent with previous studies13,14 with a mean time of 515, 497 and 353 days, respectively, before patients returned for another treatment (F (2, 154) = 3.53, p-value = 0.0316) (Tables 3 and 4). Although previous expert consensus has suggested that patients may experience the effect of HA injections in the knee and hip joints differently due to different joint etiology15 no statistically significant differences were observed between joint categories in our study. There were too few shoulder and ankle joints included in our study to compare these joint types. Previous clinical studies have reported contrasting conclusions from finding that old age is likely to predict response to HA treatment13 to younger patients are more likely to receive benefit.14 Our study did not identify any statistically significant differences between age nor gender groups.
The results of the patient experience and satisfaction survey mirrored results of the clinical dataset in that of participants experiencing relief, 76.1% (115/151) indicated that they felt relief from HA injections for a period greater than 6-months. Our study did not explicitly collect data on adverse events; however, it should be noted that 6 participants (2.7%) responded that they felt they had experienced an adverse reaction to the injection. These experiences are consistent with reports from previous safety studies which have found that HA products were well tolerated in most patients, although arthralgia, joint stiffness, joint swelling, and musculoskeletal discomfort occur in some patients.11 No serious treatment-related adverse events have previously been reported in HA safety trials.10
The practice site had a specific treatment algorithm for the management of OA during the study period. All patients were assessed according to current evidence-based OA management guidelines.16 A psycho-social approach was used to identify and manage attitudes and beliefs relating to OA; individualized load management strategies were used to optimize pain reduction (via either increased or decreased load); physiotherapy or strength-based exercises were encouraged throughout treatment; weight management was an integral component; corticosteroid injections and arthroscopy were discouraged (unless there was inflammatory arthritis or clear mechanical symptoms, eg, locking); HA injections were the first-line injection treatments; Platelet-rich plasma (PRP) or other ortho-biologicals were second-line injection treatments and arthroplasty was considered if all other treatments failed and the patient had radiographic evidence of progressive OA.
In line with this, patients reported use of physiotherapy (38%) and strength programs (21%). As PRP was offered after HA it is likely that the PRP injections (11%) were given to patients who felt that first or subsequent HA injections were not effective. Patients involved in the post treatment survey were able to tick as many other treatments that they had tried for their OA as they felt were applicable. We are not able to report whether these treatments were used before, after or at the same time as patient received HA injections.
Over the study period, 51.3% of patients went on to have a joint arthroplasty. There are a number of retrospective studies which look at whether HA injections delay the time to total joint replacement. Evidence from a large US Health Claims database analysis identified that patients who receive no HA injection have a total knee replacement on average 8 months post diagnosis whilst one and more than five HA injections delay the need for joint replacement by an average of 16 and 43 months, respectively.17 Our findings further support that repeated HA injections provide extended pain relief potentially delaying the need for joint replacement surgery.
The greatest limitation to our study is the observational nature of the study without a control group. Except through the survey, we did not record whether patients received concurrent or subsequent treatments for their OA. Additionally, patients may have reasons other than satisfaction with treatment which impacted on their time taken between injections – eg, convenience, wait list to get an appointment at the specialist clinic, cost. The strength of this study is that the time in days between injections correlates with the patient response data suggesting the time in days between injections is a reasonable measure of length of duration of symptom relief.
The analysis involved a large number of patients and joints with a high response rate of patients to the patient satisfaction survey. Further, patients were excluded if they had inflammatory arthritis or joint effusions, which is an important consideration in patient selection for HA injections.9 Most research on HA injections is focused on the safety and efficacy of a single (or single course) of HA injections. This study is unique in that it reports on the experience of patients who have received one to eight HA injections as an ongoing treatment for OA.
Conclusion
This study showed that HA injections provided patients with symptomatic pain relief for an average 466.8 days (approximately 15 months) post initial treatment, and that this was sustained for subsequent injections. We did not find any statistically significant differences between age, gender, nor the knee, hip, and shoulder joint groups. Clinicians can expect that patients with low to moderate OA are more likely experience sustained duration of benefit from HA injections. We consider that these recommendations are applicable to knee and hip OA patients presenting with pain in the absence of clinical effusion.
Acknowledgments
The authors acknowledge the support of Sally Boyd for her administrative organisation of patient records and retrieval of scans for this research.
Funding
This study was supported by funding from Bioventus.
Disclosure
AH is an employee of Bioventus. JF reports grants from Joint Health Institute Ltd, during the conduct of the study; personal fees for consultancy from Bioventus Inc, grants, personal fees, non-financial support supporting clinical trial from Zimmer Biomet and Orthocell; consulting fees from Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) results. Available from: http://ghdx.healthdata.org/gbd-results-tool.
2. Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–358. doi:10.2105/AJPH.84.3.351
3. Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015;67(2):203–215. doi:10.1002/acr.22412
4. Wen DY. Intra-articular hyaluronic acid injections for knee osteoarthritis. Am Fam Physician. 2000;62(3):565–570, 572.
5. Altman RD, Bedi A, Karlsson J, Sancheti P, Schemitsch E. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2016;44(8):2158–2165. doi:10.1177/0363546515609599
6. Agerup B, Berg P, Akermark C. Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. BioDrugs. 2005;19(1):23–30. doi:10.2165/00063030-200519010-00003
7. Lindqvist U, Tolmachev V, Kairemo K, Aström G, Jonsson E, Lundqvist H. Elimination of stabilised hyaluronan from the knee joint in healthy men. Clin Pharmacokinet. 2002;41(8):603–613. doi:10.2165/00003088-200241080-00004
8. Edsman K, Hjelm R, Lärkner H, et al. Intra-articular duration of Durolane™ after single injection into the rabbit knee. Cartilage. 2011;2(4):384–388. doi:10.1177/1947603511400184
9. Leighton R, Fitzpatrick J, Smith H, Crandall D, Flannery C, Conrozier T. Systematic clinical evidence review of NASHA (Durolane hyaluronic acid) for the treatment of knee osteoarthritis. Open Rheumatol J. 2018;10:43–54.
10. Leighton R, Akermark C, Therrien R, et al. NASHA hyaluronic acid vs. methylprednisolone for knee osteoarthritis: a prospective, multi-centre, randomized, non-inferiority trial. Osteoarthr Cartil. 2014;22(1):17–25. doi:10.1016/j.joca.2013.10.009
11. Altman R, Hackel J, Niazi F, Shaw P, Nicholls M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum. 2018;48(2):168–175. doi:10.1016/j.semarthrit.2018.01.009
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X
13. Bowman EN, Hallock JD, Throckmorton TW, Azar FM. Hyaluronic acid injections for osteoarthritis of the knee: predictors of successful treatment. Int Orthop. 2018;42(4):733–740. doi:10.1007/s00264-017-3731-8
14. Wilder E, Flanagan R, Strauss E, Samuels J. BMI, age, radiographic severity and ultrasound guidance impact the response to hyaluronic acid injections in knee osteoarthritis. Osteoarthr Cartil. 2015;23:A405–A406. doi:10.1016/j.joca.2015.02.751
15. Henrotin Y, Raman R, Richette P, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45(2):140–149. doi:10.1016/j.semarthrit.2015.04.011
16. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–1589. doi:10.1016/j.joca.2019.06.011
17. Altman R, Lim S, Steen RG, Dasa V. Hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: evidence from a large U.S. Health Claims Database. PLoS One. 2015;10(12):e0145776. doi:10.1371/journal.pone.0145776
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
