Back to Journals » Infection and Drug Resistance » Volume 15
Long-Term Follow-Up Study of COVID-19: Evaluation on Thin-Slice CT
Authors Guan CS, Liu ZJ , Du YN, Chen H, Bai Y, Lv ZB, Xu YL , Xie RM, Chen BD
Received 29 June 2022
Accepted for publication 29 September 2022
Published 18 October 2022 Volume 2022:15 Pages 6029—6037
DOI https://doi.org/10.2147/IDR.S379158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Chun-Shuang Guan,1,* Zhi-Juan Liu,2,* Yan-Ni Du,1,* Hui Chen,1 Yan Bai,1 Zhi-Bin Lv,1 Yan-Li Xu,2 Ru-Ming Xie,1,* Bu-Dong Chen1,*
1Department of Radiology, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Chronic Disease Management Centre, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ru-Ming Xie; Bu-Dong Chen, Tel +8613911320739 ; +8613801358954, Email [email protected]; [email protected]
Purpose: To retrospectively analyse the CT imaging during the long-term follow-up of COVID-19 patients after discharge.
Patients and Methods: A total of 122 patients entered the study group. All patients underwent CT examinations. The CT images, which included distribution and imaging signs, were evaluated by two chest radiologists. Laboratory examinations included routine blood work, biochemical testing, and SARS-CoV-2 antibody screening. Statistical methods include chi-square, Fisher’s exact test, one-way analysis of variance, rank sum test and logistic regression by SPSS 17.0.
Results: There were 22 (18.0%) patients in the mild group, 74 (60.7%) patients in the moderate group, and 26 (21.3%) patients in the severe–critical group. The median follow-up interval was 405 days (378.0 days, 462.8 days). Only monocytes, prothrombin activity, and γ-glutamyltransferase showed significant differences among the three groups. We found that the more severe the patient’s condition, the more SARS-CoV-2 IgG antibodies existed. Only 11 patients (11.0%) showed residual lesions on CT. The CT manifestations included irregular linear opacities in nine cases (9.0%), reticular patterns in six cases (6.0%), and GGOs in five cases (5.0%).
Conclusion: The proportion of residual lesions on CT in COVID-19 patients was significantly reduced after long-term follow-up. The patients’ age and disease conditions were positively correlated with residual lesions.
Keywords: COVID-19, follow-up studies, tomography, X-ray computed, lung
Introduction
The first case of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported on December 31, 2019. At the time of publication, the outbreak of SARS-CoV-2 had lasted for more than two years, and the number of infected patients continues to increase.1 By September 14, 2022, there were more than 607 million confirmed cases and more than 6 million reported deaths worldwide. The three countries with the largest number of confirmed cases were the United States (more than 94 million), India (more than 44 million), and Brazil (more than 34 million).1
Some studies of short- and medium-term follow-up of COVID-19 reported that 30.8–47.3% of 134–353 patients showed complete absorption of the lesions on CT; meanwhile, some lesions remained on CT, including ground-glass opacity (GGO), irregular linear opacities, consolidation, and so on.2–5 Our previous study on a short-term follow-up CT had also demonstrated that the pneumonia had been completely absorbed in 37.7% of 69 COVID-19 patients, and residual pneumonia in 62.3%.6 The remained CT manifestations included GGO (62.3%), irregular lines (20.8%), reticulation (9.4%), consolidation (3.8%), and pleural thickening adhesion (3.8%).6 There was one year follow-up study from the onset of symptoms recently, which showed 75% of 209 patients were completely absorbed, and 25% had residual lesions, including reticular patterns (13.4%) and irregular linear opacities (12.0%).7 Another one-year follow-up study after discharge recently showed that the proportion of residual lesions in the lung was higher, i.e.63.5% (132/208). And the main residual lesions were reticular patterns (25.5%) and GGO (25.0%).8 However, in daily work, we found that the proportion of patients with residual lesions decreased significantly with the increase of follow-up interval, and some laboratory tests returned to normal. Therefore, not only the CT imaging but also the laboratory results of COVID-19 patients were analysed through the long-term follow-up study of more than one year after discharge.
Materials and Methods
Patients
This study was approved by the Review Committee and the Ethics Committee of Beijing Ditan Hospital, Capital Medical University. The requirement of written informed consent was waived for the retrospective analyses by the Institutional Review Board. The patients’ data were kept confidential, without divulging personal privacy, and complied with the Helsinki Declaration. Follow-up data from 133 patients with COVID-19 were collected from July 1, 2021, to December 31, 2021. The inclusion criteria were as follows: 1) patients who had a confirmed SARS-CoV-2 infection with a positive SARS-CoV-2 nucleic acid test or high homology of novel coronavirus on gene sequencing; and 2) an age ≥18 years old. The exclusion criteria were as follows: 1) an age <18 years old; 2) lack of follow-up chest CT examination; and 3) incomplete laboratory data. According to the clinical manifestations and imaging examinations, the patients were divided into the following types: mild, moderate, and severe–critical when hospitalized.9 Mild patients showed mild clinical symptoms and no pneumonia on chest CT. The moderate patients showed fever, respiratory symptoms, etc., and showed pneumonia on chest CT. Severe–critical patients showed progressive aggravation of clinical symptoms, respiratory rate ≥ 30 times/minute, oxygen saturation ≤ 93% when inhaling air in the resting state, partial pressure of arterial blood oxygen (PaO2)/concentration of oxygen uptake (FiO2) ≤ 300mmHg, respiratory failure, shock, combined with other organ failure etc., and chest CT showed that the pneumonia had significantly progressed more than 50% within 24–48 hours. All patients underwent laboratory examination and chest CT examination at the same time during follow-up.
Laboratory Examination
Laboratory examinations included SARS-CoV-2 IgM and IgG antibody tests, white blood cell (WBC), neutrophil (N), lymphocyte (L), monocyte (MO), C-reactive protein (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), lactate dehydrogenase (LDH), prothrombin time (PT), prothrombin activity (PTA), and D-dimer (DD).
Imaging Technique and Methods
Sensation 16-slice CT (Siemens, Forchheim, Germany) was used for all chest examinations. The CT scans were obtained at the end of inhalation without intravenous contrast. The image was from the tip of the lung to the base of the lung. The scans were obtained with the following parameters: 130 kV tube, automatic tube current, and 512×512 matrix. The slice thickness was 1.5 mm, which was reconstructed via a lung algorithm.
Imaging Interpretation
Two chest radiologists with 12 and 15 years of experience retrospectively reviewed the images and resolved discrepancies by consensus. The images were analysed on a picture archiving and communication system (PACS, Carestream Health, US). The images were reviewed on both lung settings (window width 1500 HU, level −700 HU) and mediastinum settings (window width 400 HU, level 40 HU). The window width and level were adjusted to be appropriate for this analysis.
The interpretation of the CT findings was based on the Fleischer Society’s recommendations for classifying pulmonary lesions.10 The CT images were evaluated for distribution and imaging signs. The distributions were divided into the subpleural area, the peribronchovascular bundle, and a mix of subpleural area and peribronchovascular bundle. The lesions involved the pulmonary lobes, including the left upper lobe, left lower lobe, right upper lobe, right middle lobe, and right lower lobe. The imaging signs included GGOs, consolidation, reticular patterns, irregular linear opacities, pleural effusion, and enlarged mediastinal lymph nodes. GGO was defined as a hazy area of increased attenuation without obscuration of the underlying vessels. Consolidation was defined as homogeneous opacification of the parenchyma with obscuration of the underlying vessels. A reticular pattern was defined as a collection of innumerable small linear opacities.10 Enlarged mediastinal lymph nodes were defined as lymph nodes with a short axis diameter greater than 1.0 cm.
Statistical Analysis
All data were analysed by SPSS 17.0 (IBM, Armonk, NY, US). Continuous data were expressed as the mean plus the standard deviation or median (25th percentile, 75th percentile), and categorical data were expressed as the frequency. Chi-square or Fisher’s exact test was used to analyse the categorical variables. One-way analysis of variance or rank sum test was used for the analysis of measurement data. Logistic regression was used to analyse the correlation among residual lesions in the lungs. A p value of <0.05 was considered to be a statistically significant difference. A p value of <0.017 was considered to be a statistically significant difference when comparing the three in pairs.
Results
Clinical Features
We collected 133 COVID-19 follow-up patients in total but excluded 11 patients. The present study was comprised of 122 patients (mean age: 44.590±12.324 years), including 40 females (32.8%, 40/122; mean age: 46.850±12.601 years) and 82 males (67.2%, 82/122; mean age: 43.488±12.111 years). There was no significant difference in age between females and males (p=0.948). There were 22 patients (18.0%, 22/122) in the mild group, 74 patients (60.7%, 74/122) in the moderate group, and 26 patients (21.3%, 26/122) in the severe–critical group. There was significant difference in age between the mild group and the severe–critical group (p=0.001); however, there were no significant differences in age between the moderate and mild groups or the moderate and severe–critical groups (p=0.028 and p=0.070, respectively) (Table 1).
 |
Table 1 COVID-19 Patients’ Clinical Types, Basic Materials, and Follow-Up Intervals |
The median follow-up interval for the 122 patients was 405 days (378.0 days, 462.8 days; range: 367–620 days). There were significant differences in the interval among the three groups (p=0.001). There were significant differences between the severe–critical and mild groups and between the severe–critical and moderate groups (all p values were 0.001), while there was no significant difference between the mild and moderate groups (p=0.374) (Table 1).
Laboratorial Tests
Routine Laboratorial Tests
Among the laboratory tests collected from the 122 patients, only the MO, PTA, and GGT tests showed significant differences among the three groups (p=0.017, 0.041, and 0.008, respectively). There were significant differences in MO and PTA between the mild and moderate groups (p=0.004 and 0.012, respectively). However, only five patients (4.1%, 5/122) had abnormal MO values, which were lower than the normal range, while PTA values were within the normal range. There were significant statistical differences in GGT between the severe–critical and mild groups and between the severe–critical and moderate groups (p=0.014 and 0.003, respectively). Among them, 17 patients (13.9%, 17/122) had abnormal values that were higher than the normal range. There was no significant difference in other laboratory results among the three groups. Moreover, most of the values were in the normal range (Table 2).
 |
Table 2 COVID-19 Patients’ Laboratorial Examination Items’ Values |
SARS-CoV-2 Antibodies
All the 122 patients were examined for SARS-CoV-2 IgM and IgG antibodies. 38 cases were not vaccinated (31.1%, 38/122), including 5 (13.2%) in the mild group, 20 (52.6%) in the moderate group, and 13 (34.2%) in the severe–critical group. Then 84 cases were vaccinated (68.9%, 84/122), including 17 (18.0%) in the mild group, 54 (60.7%) in the moderate group, and 13 (21.3%) in the severe–critical group. There was no statistical difference in patient classification between the vaccinated group and the non-vaccinated group. Because vaccination could affect the antibody value and this study was in order to observe the changes of antibodies in different clinical classifications, the present study only analysed the antibodies of patients who had not been vaccinated. In the non-vaccinated group, three patients (7.9%, 3/38) and 34 patients (89.5%, 34/38) were positive for IgM and IgG antibodies, respectively. There was no significant difference in antibodies among the mild, moderate, and severe–critical groups with non-vaccinated patients (p>0.05), although the more severe the patient’s condition was, the more SARS-CoV-2 IgG antibodies were found (Table 3).
 |
Table 3 SARS-CoV-2 Antibodies Among Different Types of Non-Vaccinated Patients |
Imaging Manifestations on Follow-Up CT
All the 22 patients in the mild group showed no signs of viral pneumonia on chest CT. There were positive signs of viral pneumonia on chest CT in 100 patients, including 74 patients in the moderate group and 26 patients in the severe–critical group. After follow-up, the CT results of 89 patients (89.0%, 89/100) showed that the pneumonia had been completely absorbed. Residual lesions on CT were observed in only 11 patients (11.0%, 11/100), including four patients in the moderate group (5.4%, 4/74) and seven patients in the severe–critical group (26.9%, 7/26). There was a significant difference between the moderate group and the severe-critical group (p=0.006) (Table 4).
 |
Table 4 Chest CT Manifestations of COVID-19 in Moderate and Severe-Critical Group After Follow-Up |
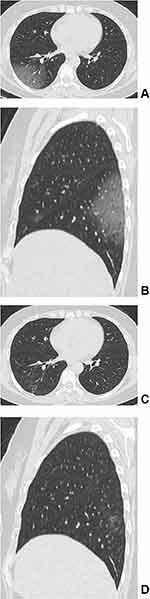
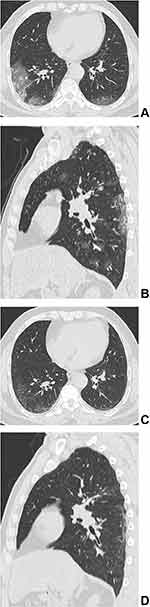
All the 11 patients who presented with residual lesions had them under the subpleural area. An average of two lobes were involved in the moderate group, while an average of 3.571±1.512 lobes were involved in the severe–critical group. There was a significant difference between the two groups (p=0.033). The CT manifestations included irregular linear opacities in nine cases (81.8%, 9/11), reticular patterns in six cases (54.5%, 6/11), and GGOs in five cases (45.5%, 5/11) (Figures 1–2). There was no significant difference in residual imaging between the moderate group and the severe–critical group. Consolidation, pleural effusion, and enlarged lymph nodes were not present. (Table 4).
By logistic regression analysis, the patients’ ages (b=0.085, p=0.013) was positively correlated with the pulmonary residual lesions on CT. That was, the older the patient was, the more likely there was to be residual lesions in the lung after long-term follow-up. The clinical classification of patients was also positively associated with the residual lesions in the lungs (b=1.488, p=0.041). When the patient’s condition worsened, the probability of residual lesions in the lung increased. That was to say, severe-critical patients were more likely to have residual lesions than the moderate patients. Other factors, such as gender, laboratory results, and vaccination status, had no correlation with pulmonary residual lesions (p>0.05).
Discussion
The COVID-19 outbreak has been ongoing worldwide for more than two years. The number of confirmed cases has constantly increased as the mutant strains of SARS-CoV-2 continue to develop; however, there have been few long-term follow-up studies after discharge.7,8 Through more than one year follow-up study, we found that, first, residual lung lesions could be found in both moderate and severe–critical patients, and the proportion of residual lesions in the severe–critical group was significantly higher than that in the moderate group. Second, common residual CT findings included irregular linear opacities, reticular patterns, and GGOs. Third, SARS-CoV-2 IgG increased gradually in mild, moderate, and severe–critical patients. Fourth, there were significant differences in MO, PTA, and GGT among the three clinical classifications, but these values were within the normal ranges.
Imaging manifestations were on the basis of pathological changes. COVID-19 pneumonia mainly showed localized or diffuse alveolar injury, exudative alveolitis, and interstitial inflammation.9 A large amount of serous fluids and fibrin exudates, and a large number of hyaline membranes could be seen in the alveoli. The exudative cells were mainly monocytes and macrophages, and multinucleated giant cells could be seen. The alveolar walls were diffusely thickened due to hyperplasia of fibroblasts and type II alveolar epithelial cells. Congestion, oedema, and mononuclear and lymphocyte infiltration could be seen in the alveolar septum. Thickened interlobular septa were also found. Pulmonary vasculitis, thrombosis, thromboembolism, focal bleeding, and necrosis could all be seen in the lung tissue. In cases with a longer duration of disease, the alveolar exudate was organized, and pulmonary interstitial fibrosis was present.9,11,12 Therefore, after the gradual escalation of alveolar injury in the acute stage of COVID-19 pneumonia, the exudate was gradually absorbed after reaching the peak, and the corresponding imaging manifestations appeared on CT. In the early stage, patchy and quasi-circular pure GGOs were distributed under the pleura. At the advanced stage and the peak stage, the GGOs and consolidation gradually increased, resulting in a typical crazy-paving pattern and air-bronchography. Then, in the absorption stage, the GGOs and consolidation were gradually absorbed, and fibrotic changes appeared, such as irregular linear opacities and reticular patterns.6,13–15 Fibroplasia is a form of tissue injury and repair that results from the proliferation of fibroblasts, vascular endothelial cells, and connective tissue and finally forms fibrous tissue.2 Therefore, in the late stages of COVID-19, fibrosis gradually appeared and was gradually absorbed. It has been reported that residual lesions can be found on CT (52.7–69.2%) at follow-up intervals ranging from 2 weeks to 10 months. The CT manifestations mainly included GGOs (25.9–63.5%), irregular linear opacities (15.9–36.5%), reticular patterns (0.9–9.4%), and consolidation (1.1–9.6%).2–6 Nevertheless, in the two 1-year follow-up studies, the proportion of residual lesions was significantly different (25% vs 63.5%, respectively),7,8 and the most common residual CT findings were different, namely, reticular patterns (13.4%) and irregular linear anomalies (12.0%),8 reticular patterns (25.5%) and GGO (25.0%).7 In this study with more than one year follow-up, only 11.0% of COVID-19 patients had residual lesions, including irregular linear opacities (9.0%), reticular patterns (6.0%), and GGOs (5.0%), but consolidation did not appear. Consolidation was defined as the appearance of acute exudative changes in the alveoli on imaging. With an extensive recovery period, the exudate from the acute phase is basically absorbed, so the consolidation shadow is not visible. There might be many reasons why the present follow-up study is inconsistent with previous studies.2–8 The main reason might be that the follow-up interval of this study was longer, with a median follow-up time of 405 days and a maximum follow-up time of 620 days. With the gradual absorption of inflammation in the lung, a small amount of fibrosis remained. To determine whether the residual fibrosis could be fully absorbed ultimately requires even longer follow-up observation.
Through this follow-up study, the residual lesions were positively correlated with age and disease condition. In short, with increasing age, the condition gradually worsened, and the probability of residual lesions increased, which is consistent with the literature.2–8 In this study, although the follow-up interval in the severe-critical group was longer than that in the moderate group, the proportion of residual lesions in the lung was higher. Therefore, we speculate that the time required for repair after severe lung injury is longer. The other factors, such as gender, laboratory examination, and vaccination status, had no correlation with residual lesions (p>0.05).
Some laboratory results exhibited abnormal values, such as reduced or normal peripheral white blood cells, decreased lymphocytes, increased CRP, ALT, AST, GGT, and LDH in the acute and recovery stages of COVID-19, and increased D-dimer in severe–critical patients.2,3,5,9 There were significant differences in MO, PTA, and GGT among different clinical types in the present study, but we observed that most of the three items, including other laboratory results, were within the normal range. Therefore, we speculate that the laboratory results also gradually returned to the normal range from the acute stage to the recovery stage. The high level of antibodies had a certain correlation with the severity of the disease in the acute stage.16–18 Nevertheless, the levels of SARS-CoV-2 IgM and IgG antibodies had no correlation with the patients’ conditions in this study; however, SARS-CoV-2 IgM and IgG antibodies increased gradually with the severity of illness in non-vaccinated patients, which was consistent with previous literature report.8 In the present study, the proportion of IgM and IgG antibodies positive (7.9% and 89.5%, respectively) was slightly lower than that in the previous 1-year follow-up study (15.4% and 95.9%, respectively).8 We speculate that this might be related to the longer follow-up interval and the smaller sample size.
Our study has some limitations. First, the sample size of this study is smaller than part of the follow-up studies and some data may need a larger sample size to support our conclusions, but the current sample size can also prove the accuracy of the conclusion to a certain extent. Second, at follow-up after more than one year, the COVID-19 patients in this study still had a small amount of residual fibrous lesions. We will continue this study to evaluate if these lesions can be fully absorbed.
Conclusion
In a word, the proportion of residual lesions on CT in COVID-19 patients was significantly reduced after a long-term follow-up, and only a small amount of irregular linear opacities, reticular patterns, and GGOs remained. Patient age and disease conditions were positively correlated with residual lesions. SARS-CoV-2 IgM and IgG antibodies increased with the severity of the disease. Most laboratory results returned to the normal range after long-term follow-up.
Acknowledgments
We are grateful to AJE for providing language editing services.
Funding
The work was supported by Beijing Municipal Science & Technology Commission [grant numbers Z201100007920017] and Health Science Promotion Project of Beijing [grant numbers 2020-TG-001].
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization; 2022 WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/.
2. Zhong L, Zhang S, Wang J, et al. Analysis of chest CT results of coronavirus disease 2019 (COVID-19) patients at first follow-up. Can Respir J. 2020;2020:5328267. doi:10.1155/2020/5328267
3. Zhang S, Liu L, Yang B, et al. Clinical characteristics of 134 convalescent patients with COVID-19 in Guizhou, China. Respir Res. 2020;21:314. doi:10.1186/s12931-020-01580-0
4. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi:10.1016/S0140-6736(20)32656-8
5. Liu HQ, Yuan B, An YW, et al. Clinical characteristics and follow-up analysis of 324 discharged COVID-19 patients in Shenzhen during the recovery period. Int J Med Sci. 2021;18:347–355. doi:10.7150/ijms.50873
6. Guan CS, Lv ZB, Li JJ, et al. CT appearances, patterns of progression, and follow-up of COVID-19: evaluation on thin-section CT. Insights Imaging. 2021;12:73. doi:10.1186/s13244-021-01019-0
7. Pan F, Yang L, Liang B, et al. Chest CT patterns from diagnosis to 1 year of follow-up in patients with COVID-19. Radiology. 2022;302:709–719. doi:10.1148/radiol.2021211199
8. Li D, Liao X, Ma Z, et al. Clinical status of patients 1 year after hospital discharge following recovery from COVID-19: a prospective cohort study. Ann Intensive Care. 2022;12:64. doi:10.1186/s13613-022-01034-4
9. The Central People’s Government of the People’s Republic of China; 2022. Consensus on Guidelines for the Publication of the Ninth Trail Version of the Diagnosis and Treatment Plan of the Novel Coronavirus Coronavirus . Available from: http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm.
10. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi:10.1148/radiol.2462070712
11. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi:10.1016/S2213-2600(20)30076-X
12. Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. doi:10.1016/j.jtho.2020.02.010
13. Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295:715–721. doi:10.1148/radiol.2020200370
14. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi:10.1016/S0140-6736(20)30183-5
15. Varble N, Blain M, Kassin M, et al. CT and clinical assessment in asymptomatic and pre-symptomatic patients with early SARS-CoV-2 in outbreak settings. Eur Radiol. 2021;31:3165–3176. doi:10.1007/s00330-020-07401-8
16. Luo H, Jia T, Chen J, et al. The characterization of disease severity associated igg subclasses response in COVID-19 patients. Front Immunol. 2021;12:632814. doi:10.3389/fimmu.2021.632814
17. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71:2027–2034. doi:10.1093/cid/ciaa344
18. Sun B, Feng Y, Mo X, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9:940–948. doi:10.1080/22221751.2020.1762515
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.