Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Long-Term Follow-Up and Clinical Relevance of Incidental Findings of Fibrin Sheath and Thrombosis on Computed Tomography Scans of Cancer Patients with Port Catheters
Authors Lichtenstein T , Mammadov K , Rau K, Große Hokamp N , Do TD , Maintz D, Chang DH
Received 22 October 2020
Accepted for publication 11 January 2021
Published 27 January 2021 Volume 2021:17 Pages 111—118
DOI https://doi.org/10.2147/TCRM.S287544
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Thorsten Lichtenstein,1 Kamal Mammadov,2 Karin Rau,1 Nils Große Hokamp,1 Thuy D Do,3 David Maintz,1 De-Hua Chang1,3
1Department of Radiology, University Hospital of Cologne, Cologne, Germany; 2Department of Radiology, Klinikum Aschaffenburg, Aschaffenburg, Germany; 3Department of Diagnostic and Interventional Radiology, University Medical Center Heidelberg, Heidelberg, Germany
Correspondence: Thorsten Lichtenstein
Institut für Diagnostische und Interventionelle Radiologie, Universitätsklinikum Köln (AöR), Kerpener Str. 62, Cologne 50937, Germany
Tel +49 221 478-82035
Email [email protected]
Purpose: This retrospective study examined the incidence, progression, and clinical relevance of catheter-related thrombosis (CRT) and/or fibrin sheaths presenting as incidental findings on routine staging computed tomography (CT) scans performed in cancer patients.
Patients and Methods: Patients who underwent central venous port catheter (CVC) placement in a tertiary care hospital between September 2010 and August 2013 were followed up for up to five years. Two radiologists assessed the presence of fibrin sheath and thrombosis in consensus in staging CT scan. Patient demographics, type of cancer, preoperative comorbidities, date of CVC placement and CTs, preexisting anticoagulation, as well as the type and treatment of catheter-related complications were determined from the electronic medical record.
Results: A total of 194 patients with 530 CT scans and a mean follow-up time of 394 days were included. Fibrin sheaths and thromboses were seen on 46 scans (8.7%) in 30 patients and 80 scans (15.1%) in 35 patients. The incidence of fibrin sheaths and thromboses was found to be 15.5% and 18%, respectively. The comparison to initial CT reports results indicated that fibrin sheaths or thromboses were missed in 106 examinations (20%). Catheter-associated complications were reported in 14 patients (21.5%) without specific therapy.
Conclusion: Fibrin sheaths and CRTs are often overlooked on routine CT scans when patients are asymptomatic. The subsequent high complication rate demonstrates the clinical relevance of the initial incidental finding on CT scan. Further studies should elucidate the effect of thrombolytic agents and interventional radiologic treatment in asymptomatic patients.
Keywords: catheter-related complications, central venous catheters, port-a-cath, imaging, staging
Introduction
Implantable venous access ports are widely used in patients with inadequate peripheral veins or those requiring long-term therapy and frequent administration of chemotherapy drugs, antibiotics, parenteral nutrition, or blood sampling. Numerous complications associated with the use of central venous catheters (CVCs) have been described in literature, including infections, leakage, and catheter-related thrombosis (CRT).1,2 Besides morbidity and mortality resulting from the catheter-associated complication itself, catheter dysfunction may necessitate treatment interruption, negatively affecting the treatment outcome.3
Different studies showed that complete or partial catheter occlusion affects 16–58% of patients after two years of CVC placement.4–6 The main cause of catheter dysfunction is deep vein thrombosis, the development of fibrin sheaths around the tip of the catheter and/or intraluminal thrombi.3 The formation of fibrin sheaths occurs between 24 h and 3–7 days of after CVC placement as a result of direct endothelial damage.7 Autopsy studies in animal models showed that fibrin sleeves had developed after 4–17 weeks on all catheters examined, encasing the entire catheter circumference.8,9 Over time, fibrin sheaths can increase in size and cause catheter dysfunction, infectious complications, and thromboembolisms.10,11
While asymptomatic CRTs occur in up to 14%–18%, symptomatic CRTs are reported in approximately 5% only.2 The incidence of fibrin sheaths may be as high as 100% and are thought to have a direct clinical effect on large-bore catheters used in hemodialysis, as their presence is generally accompanied by a notable reduction in flow volume. Normal flow volumes in dialysis catheters often exceed 400 mL/min, and a malfunction is indicated by achievable flow rate of 300 mL/min or less.12 Such clear-cut criterion does not exist for low-flow CVCs. The presence of a fibrin sheath in asymptomatic patients becomes apparent only with the inability to aspirate blood. This aspiration failure is generally attributed to a one-way valve mechanism created in the catheter opening holes. The negative pressure generated during aspiration suctions in loose parts of the fibrin sheath, which clog up the catheter opening holes. The ability to infuse and flush remains unchanged, except in cases of full catheter occlusion.
In symptomatic patients, the management of CRTs and fibrin sheaths can be achieved either pharmacologically or mechanically. Pharmacologic therapy regularly involves the use of local or systemic thrombolytic agents.13 Mechanical therapy includes transfemoral percutaneous intravascular stripping techniques or sheath disruption via balloon catheter, followed by catheter exchange.10,14–16 In cancer patients with CVCs, fibrin sheaths and CRTs are common incidental findings in computed tomography (CT) scans performed for oncologic follow-up. To date, there are no standard treatment guidelines available for the management of incidentally found fibrin sheaths and CRTs in asymptomatic patients.
The objective of this study was to examine the incidence, progression, and clinical relevance of asymptomatic, incidental CRTs and/or fibrin sheaths on routine staging CT scans performed in cancer patients.
Patients and Methods
Our study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the medical faculty of the University of Cologne (Registration Number 19–1015), which waived the requirement for written informed consent because of the retrospective, observational character of the study. A total of 194 patients who underwent CVC placement in the University Clinic of Cologne between September 2010 and August of 2013 were identified by a query of the institute’s Picture Archiving and Communication System and collected for this retrospective single-center study. All patients undergoing CVC placement for systemic chemotherapy, antibiotic therapy, parenteral nutrition, and/or photophoresis were included in the study. Patients with CVCs placed due to other therapeutic considerations and those who did not seek oncological treatment in our facility were excluded. The maximum follow-up period was defined as five years after CVC placement.
Patient data were extracted from the electronic medical records and de-identified. Patient demographics, type of cancer, preoperative comorbidities, date of CVC placement, preexisting anticoagulation, and dates of CT scans were documented. When present, the type of catheter-associated malfunction and treatment were also recorded.
CT Analysis
All CT scans done after the CVC placement as part of oncological surveillance and routine follow-up appointments were evaluated by one board-certified radiologist with >5 years of experience. All positive findings were confirmed by a second, subspecialty-trained, board-certified interventional radiologist. CT scans were evaluated according to image quality with regards to the research question and rated as either sufficient or insufficient. The presence of fibrin sheaths and/or thromboses (present/absent) and progression over time (progression/stable/partial regression/full regression) were recorded. Fibrin sheaths and thromboses were defined as localized hypodense areas along the length of the catheter ≤2 mm and >2 mm in diameter, respectively. Further, thromboses were graded with regards to extent of vessel occlusion. Thrombosis was deemed minor or moderate in cases with a vessel lumen diameter of ≤50% or >50% and <100%, respectively. The rate of missed diagnoses of fibrin sheaths and CRTs was determined by the comparison of the initial CT results of the aforementioned analysis.
Statistical analysis was performed with the Mann–Whitney U or Pearson’s chi-squared test for group comparison using IBM SPSS Statistic version 27. P < 0.05 was considered statistically significant. Descriptive data are presented as mean and standard deviation unless otherwise stated.
Results
Patient Characteristics
A total of 194 patients (106 [55%] females 88 males [45%]) met the inclusion criteria and were followed for an average of 393 days (min: 0, max: 1803 days) after port CVC placement. The mean age was 57.4 years at the time of port placement (range: 18–83 years), with 145 (74.7%) devices implanted via the left subclavian vein (SCV) and 49 (25.3%) via the right SCV. The clinical characteristics of the patients with and without fibrin sheath or thrombosis are shown in Table 1. The most frequent cancer types included Non-Hodgkin lymphoma (14.9%), lung cancer (12.9%), and breast cancer (11.9%).
 |
Table 1 Clinical Characteristics of Patients with (WFT) and without Fibrin Sheath or Thrombosis (WOFT) in Comparison |
CT Scan Analyses
A total of 530 CT scans were evaluated, of which 21 (4%) demonstrated insufficient image quality with regard to the research question. CVC-associated complications were found on 126 scans; fibrin sheaths and thromboses were found on 46 CT scans (8.7%) in 30 patients and 80 scans (15.1%) in 35 patients (Figures 1 and 2). In five patients, the presence of thromboses and fibrin sheath alternated at different time points during the follow-up. The presence of fibrin sheaths or thrombosis was overlooked in 106 scans (20%) in 50 patients, as compared to the initial report. Of these, 44 were fibrin sheaths and 62 were thromboses. Thirteen patients (28.3%) in whom the presence of thrombosis or fibrin sheaths had previously been missed on CT scans subsequently developed clinical complications.
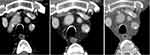
 |
Figure 1 Presence of thrombosis (arrow) on the initial CT scan (left) and on follow-up imaging after seven weeks (right). Note its rapid progression with respect to vessel occlusion. |
The incidence of fibrin sheaths and thrombosis was found to be 15.5% and 18%, respectively. The grades of overlooked thromboses in all minor, moderate, and complete vessel occlusion were 49 (79.0%), 11 (17.4%), and 2 (3.2%), respectively. Follow-up imaging regarding thrombosis and fibrin sheaths was available for 32 patients. The evolution of fibrin sheath and thrombosis, independent of the specific therapy as seen on the last available imaging, is illustrated in Figure 3.
 |
Figure 3 Evolution of fibrin sheath and thrombosis as seen on last available imaging. Both full and partial regressions occurred in patients spontaneously. |
Catheter-Associated Complications
In 16 patients who experienced symptomatic catheter-associated complications, nine presented with arm swelling, four experienced catheter dysfunctions, and two suffered from pulmonary embolism. In one patient, multiple complications occurred at three different time points. In this patient, arm swelling was followed by catheter dysfunction approximately 1.5 years later, and another episode of arm swelling 5 months afterwards. Notably, this particular patient was on anticoagulative therapy (phenprocoumon). Pertaining to the latter, throughout all 16 patients with symptomatic complications, 15 patients (7.7%) had no preexisting medications, while one patient (0.5%) took oral anticoagulation medication (Figure 4).
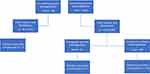 |
Figure 4 Systemic anticoagulation and catheter-associated complications. Two additional complications (arm swelling) occurred without the evidence of fibrin sheath/thrombosis on CT scan. |
In patients with symptomatic catheter-associated complications, fibrin sheaths (n = 7) and/or thromboses (n = 10) were found in image review; in two patients, the presence of neither fibrin sheath nor thrombosis was confirmed. These two patients developed symptomatic arm swelling, possibly due to a thoracic inlet venous obstruction exacerbated by the catheter.
Treatment Regimens for Thrombosis or Fibrin Sheaths
The 16 patients with symptomatic, port-associated complications were treated with either heparins (n = 4), rivaroxaban (n = 3), or local urokinase lysis (n = 2). These treatments were successful (ie, partial or full regression) in six patients. One patient presenting with multiple complications underwent several, consecutive treatments (phenprocoumon, local lysis with urokinase, and heparin). Eventually, port explantation due to symptomatic, port-associated complications was performed in three patients (Table 2).
 |
Table 2 Effect of Different Treatment Types on Clinical Course of Fibrin Sheath and Thrombosis in Symptomatic Patients |
Only nine of the remaining 46 asymptomatic patients with fibrin sheath or thrombosis visible on CT scans received treatment according to the hospital medical records reviewed. Seven patients (four with thrombosis and three with fibrin sheath) were prescribed systemic anticoagulation medication, while two patients (one with thrombosis and one with fibrin sheath) underwent catheter removal.
Discussion
This is the first study to evaluate the long-term clinical course of fibrin sheaths and thromboses detected on CT scans routinely prescribed for oncologic treatment. The incidence of fibrin sheaths and thrombosis in our population is comparable to previous studies, at 15.5% and 18.0%, respectively.16,17 We were able to show that the size of fibrin sheaths often remain constant and rarely progresses over time. In fact, the study results indicate that fibrin sheaths are apt to decrease in size spontaneously, even in patients not on preexisting anticoagulation. Similar outcomes were found for the presence of thrombosis. For this reason, the clinical relevance of fibrin sheath and thrombosis in asymptomatic patients is still debatable.17
Interestingly, catheter-associated complications occurred mainly independent of the progression of findings in the further course. The discrepancy between the imaging and clinical course may be explained by the large time intervals between the CT follow-ups, ranging from several weeks to months. In conclusion, routine CT staging seems to be an excellent method for the detection of fibrin sheaths and CRTs. However, they are inappropriate for monitoring dynamic thrombogenic event, in addition to radiation safety reasons. In the case of an initial incidental finding on CT, close clinical monitoring by ultrasound would be advisable in clinical practice. This approach is supported by our observation that almost one-third of the patients without specific therapy, where the presence of thrombosis or fibrin sheaths had previously been missed on CT scans, subsequently developed clinical complications.
While treatment regimens are defined for CVC patients with symptomatic fibrin sheaths and CRTs, the procedure for asymptomatic patients is uncertain. Jones et al demonstrated that despite no treatment in 31 children with asymptomatic CV-related thrombosis, none had an acute complication, and only one child had mild long-term sequelae. In comparison to the study of Jones et al, the overall complication rate was higher in our study at 8.2%. Whether this figure already suggests or justifies drug or interventional therapy for all asymptomatic patients remains questionable. A generally applicable solution does not seem to work because of the extremely heterogeneous patient population. Patients differ in age, underlying disease, indication for port implantation, comorbidities, previous therapy, and thrombus burden to name a few. Future studies are needed to identify the patients at high risk for catheter-associated complications. In this way, reasonable stratification of therapy and personalized medicine will become possible.
Patients with preexisting anticoagulation were about twice as likely to develop fibrin sheaths or CRTs compared with patients without medication. At first glance, the high rate seems contradictory. However, all patients received therapeutic anticoagulation because of preexisting thrombosis, so it can be assumed that these patients were at increased risk of thrombosis. On the other hand, only two patients with preexisting anticoagulation suffered from catheter-related complications. Because of the small number of cases in our study, a conclusive assessment of the prophylactic effect of anticoagulation on the development of a complication is not possible.
In addition, several studies examining the prophylactic effect of different thrombolytic agents on fibrin sheath and thrombosis development in patients with CVCs have shown contradictory results. A meta-analysis of eight studies (1428 patients) examining the effectiveness of thromboprophylaxis in CVC patients was not able to find an additional benefit for patients receiving anticoagulation prophylaxis.18 In contrast, the Cochrane Systematic Review published in 2011 showed that low-dose vitamin K antagonists were associated with a statistically significant reduction in asymptomatic CRT (RR 0.42%; 95% CI 0.28–0.61).19 The results for unfractionated heparin and low-molecular weight heparin were not significant in asymptomatic CRT patients. The 2018 Cochrane Systematic Review update included 13 randomized controlled trials enrolling 3420 participants and found moderately certain evidence that low-molecular weight heparin reduces the incidence of CRTs.20 However, the updated review did not differentiate the incidences of all CRT from asymptomatic CRT as outcomes. Furthermore, the transferability of the results found in the literature to this study may be questioned. Diagnostic procedures, CRT definitions, study populations, CVC subtypes, and placement methods varied in all studies included in the 2018 review. Increased bleeding risk and thrombocytopenia are potential side effects of thromboprophylaxis that must be considered when prescribing anticoagulants for CRT prevention.
This study was mainly limited by the relatively small sample size. Furthermore, there was no progression data available for many patients who only had one CT scan during the course of their chemotherapy. In asymptomatic patients, the decision to treat may be done externally with a primary care physician, so the documentation of treatment may be lacking in some cases.
Conclusion
Fibrin sheaths and CRTs are very common findings affecting almost one-third of the patients included in this study. However, these are often overlooked on routine CT scans taken during an oncological therapy when patients with CVCs are asymptomatic. Further studies need to identify the patients at high risk for subsequent complications in order to initiate early and targeted prophylactic therapies.
Funding
Source(s) of support in the form of grants, equipment, drugs, or other assistance, whether from public or private sources: None.
Disclosure
Nils Große Hokamp reports grants and personal fees from Philips Healthcare, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Liu C, Chen L, Kong D, et al. Incidence, risk factors and medical cost of peripheral intravenous catheter-related complications in hospitalised adult patients. J Vasc Access. 2020;112972982097812. doi:10.1177/1129729820978124.
2. Kreuziger LB, Jaffray J, Carrier M. Epidemiology, diagnosis, prevention and treatment of catheter-related thrombosis in children and adults. Thromb Res. 2017;157:64–71. doi:10.1016/j.thromres.2017.07.002
3. Baskin JL, Pui CH, Reiss U, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet. 2009;374:159–169. doi:10.1016/S0140-6736(09)60220-8
4. Wei T, Li XY, Yue ZP, et al. Catheter dwell time and risk of catheter failure in adult patients with peripheral venous catheters. J Clin Nurs. 2019;28:4488–4495. doi:10.1111/jocn.15035
5. Marsh N, Webster J, Larson E, et al. Observational study of peripheral intravenous catheter outcomes in adult hospitalized patients: a multivariable analysis of peripheral intravenous catheter failure. J Hosp Med. 2018;13:83–89. doi:10.12788/jhm.2867
6. Chou PL, Fu JY, Cheng CH, et al. Current port maintenance strategies are insufficient: view based on actual presentations of implanted ports. Medicine (Baltimore). 2019;98:e17757. doi:10.1097/MD.0000000000017757
7. Crain MR, Horton MG, Mewissen MW. Fibrin sheaths complicating central venous catheters. AJR. 1998;171:341–346. doi:10.2214/ajr.171.2.9694448
8. Wang LH, Wei F, Jia L, et al. Fibrin sheath formation and intimal thickening after catheter placement in dog model: role of hemodynamic wall shear stress. J Vasc Access. 2015;16:275–284. doi:10.5301/jva.5000358
9. Florescu MC, Runge J, Flora M, et al. Location and structure of fibrous sheath formed after placing a tunneled hemodialysis catheter in a large pig model. J Vasc Access. 2018;19:484–491. doi:10.1177/1129729818760978
10. Faintuch S, Salazar GM. Malfunction of dialysis catheters: management of fibrin sheath and related problems. Tech Vasc Interv Radiol. 2008;11:195–200. doi:10.1053/j.tvir.2008.09.008
11. Tang S, Beigel R, Arsanjani R, Larson B, Luthringer D, Siegel R. Infective endovascular fibrin sheath vegetations – a new cause of bacteremia detected by transesophageal echocardiogram. Am J Med. 2015;128:1029–1038. doi:10.1016/j.amjmed.2015.03.019
12. Daugirdas JT, Depner TA, Inrig J; National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66:884–930. doi:10.1053/j.ajkd.2015.07.015
13. Chang DH, Mammadov K, Hickethier T, et al. Fibrin sheaths in central venous port catheters: treatment with low-dose, single injection of urokinase on an outpatient basis. Ther Clin Risk Manag. 2017;13:111–115. doi:10.2147/TCRM.S125130
14. Mohamad Ali A, Uhwut E, Liew S, et al. Dialysis catheter fibrin sheath stripping: a useful technique after failed catheter exchange. Biomed Imaging Interv J. 2012;8:e8. doi:10.2349/biij.8.1.e8
15. Kennard AL, Walters GD, Jiang SH, et al. Interventions for treating central venous haemodialysis catheter malfunction. Cochrane Database Syst Rev. 2017;10:CD011953. doi:10.1002/14651858.CD011953.pub2
16. Jones S, Butt W, Monagle P, et al. The natural history of asymptomatic central venous catheter-related thrombosis in critically ill children. Blood. 2019;133:857–866. doi:10.1182/blood-2018-05-849737
17. Boddi M, Villa G, Chiostri M, et al. Incidence of ultrasound-detected asymptomatic long-term central vein catheter-related thrombosis and fibrin sheath in cancer patients. Eur J Haematol. 2015;95:472–479. doi:10.1111/ejh.12519
18. Chaukiyal P, Nautiyal A, Radhakrishnan S, et al. Thromboprophylaxis in cancer patients with central venous catheters. A systematic review and meta-analysis. Thromb Haemost. 2008;99:38–43. doi:10.1160/TH07-07-0446
19. Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for patients with cancer and central venous catheters. Cochrane Database Syst Rev. 2011;CD006468.
20. Kahale LA, Tsolakian IG, Hakoum MB, et al. Anticoagulation for patients with cancer and central venous catheters. Cochrane Database Syst Rev. 2018;CD006468.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.