Back to Journals » International Journal of Women's Health » Volume 7
Long-term efficacy, safety, and patient acceptability of ibandronate in the treatment of postmenopausal osteoporosis
Authors Inderjeeth C, Glendenning P, Ratnagobal S, Inderjeeth D, Ondhia C
Received 8 September 2014
Accepted for publication 16 October 2014
Published 17 December 2014 Volume 2015:7 Pages 7—17
DOI https://doi.org/10.2147/IJWH.S73944
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Charles A Inderjeeth,1,2 Paul Glendenning,2,3 Shoba Ratnagobal,1 Diren Che Inderjeeth,1 Chandni Ondhia1
1Department of Geriatric Medicine and Rheumatology, North Metropolitan Health Service, 2School of Medicine and Pharmacology, University of Western Australia, 3Department of Clinical Biochemistry, PathWest Royal Perth Hospital, Perth, WA, Australia
Abstract: Several second-generation bisphosphonates (BPs) are approved in osteoporosis treatment. Efficacy and safety depends on potency of farnesyl pyrophosphate synthase (FPPS) inhibition, hydroxyapatite affinity, compliance and adherence. The latter may be influenced by frequency and route of administration. A literature search using “ibandronate”, “postmenopausal osteoporosis”, “fracture”, and “bone mineral density” (BMD) revealed 168 publications. The Phase III BONE study, using low dose 2.5 mg daily oral ibandronate demonstrated 49% relative risk reduction (RRR) in clinical vertebral fracture after 3 years. Non-vertebral fracture (NVF) reduction was demonstrated in a subgroup (pretreatment T-score ≤ -3.0; RRR 69%) and a meta-analysis of high annual doses (150 mg oral monthly or intravenous equivalent of ibandronate; RRR 38%). Hip fracture reduction was not demonstrated. Long-term treatment efficacy has been confirmed over 5 years. Long term safety is comparable to placebo over 3 years apart from flu-like symptoms which are more common with oral monthly and intravenous treatments. No cases of atypical femoral fracture or osteonecrosis of the jaw have been reported in randomized controlled trial studies. Ibandronate inhibits FPPS more than alendronate but less than other BPs which could explain rate of action onset. Ibandronate has a higher affinity for hydroxyapatite compared with risedronate but less than other BPs which could affect skeletal distribution and rate of action offset. High doses (150 mg oral monthly or intravenous equivalent) were superior to low doses (oral 2.5 mg daily) according to 1 year BMD change. Data are limited by patient selection, statistical power, under-dosing, and absence of placebo groups in high dose studies. Ibandronate treatment offers different doses and modalities of administration which could translate into higher adherence rates, an important factor when the two main limitations of BP treatment are initiation and adherence rates. However, lack of consistency in NVF reduction and absence of hip fracture data limits more generalized use of this agent.
Keywords: fracture, ibandronate, risedronate, zoledronic acid, alendronate
Background
Multiple antiresorptive treatments are available and can be used for the prevention or treatment of osteoporosis. When administered with adequate calcium and vitamin D replacement, antiresorptive treatments improve bone mineral density (BMD), reduce bone turnover, and reduce both vertebral and non-vertebral osteoporotic fractures when administered orally (daily, weekly, monthly, or intermittently), subcutaneously every 6 months, or intravenously (2 monthly, 3 monthly, or annually).1–3
Osteoporosis is a chronic disease and optimal management necessitates that prescribed therapies are taken regularly for as long as the condition is active. It is important that treatments are effective in low- as well as high-risk groups and that onset of efficacy is early and durable.2,3 Long-term therapy may significantly increase pill burden as adequate calcium and vitamin D supplementation is necessary for full efficacy to be achieved. Adherence rates are universally poor for self-administered medications and chronic diseases regardless of disease type, severity, and accessibility to resources. It is not surprising that poor adherence also extends to patients receiving osteoporosis medications.4
Bisphosphonates (BPs) are currently the most common prescribed treatment for the prevention of osteoporotic fracture.5 However the choice of BP is complicated by differences in efficacy and safety profiles. There is also a need to align these specific factors with patient preference, which may help improve adherence.4,6 Alendronate, ibandronate, risedronate, and zoledronic acid are widely used second-generation nitrogen-containing BPs that are approved for use in patients with osteoporosis5–7 and while they are all nitrogen-containing BPs1,8 they differ in terms of pharmacological structure and potency. Table 1 ranks nitrogen-containing BPs by hydroxyapatite binding affinity and potency.1,8–10 The rank order for binding affinity is zoledronic acid > alendronate > ibandronate > risedronate. Higher affinity BPs will bind more avidly to the bone surface and penetrate less into the bone surface. Lower affinity BPs will distributed more widely in bone and probably have a quicker offset of action after cessation of therapy. Nitrogen-containing BPs also differ in their potency of inhibition of farnesyl pyrophosphate synthase (FPPS), the main target enzyme of action, with the rank order of potency being zoledronic acid > risedronate > ibandronate > alendronate.8–10 Binding affinity plus the potency of inhibition of FPPS may both explain differences in speed of onset of anti-fracture effect and whether there is an effect on non-vertebral sites.1,2 Their clinical efficacy and safety depends largely on compliance and adherence to therapy, which is influenced by both dosing regimen and route of administration, with more frequent dosing demonstrating higher rates of suboptimal outcome.4,11,12 Poor adherence results in a negative impact on fracture risk, health-care costs, and quality of life.12
  | Table 1 Available forms of nitrogen-containing bisphosphonates |
Ibandronate, a newer BP, has the greatest number of options in terms of route and frequency of administration.8 Ibandronate has the potential advantage in comparison to other BP options of offering patients and clinicians a highly variable choice that may align most closely with an individual’s preference and as a consequence could improve compliance and adherence.4,11,12 However demonstration of efficacy in terms of durable and consistent fracture reduction is essential. The purpose of this review is to provide a summary of the efficacy, safety, and adherence rates noted with ibandronate as provided by randomized controlled trial (RCT) data.
Methods
We performed a literature search of PubMed and Medline® using the keywords “ibandronate”, “postmenopausal osteoporosis”, “fracture”, and “bone mineral density” for relevant publications. The search was limited to humans, only included articles available in English, and was limited to the past 10 years (January 2003–February 2013). Older papers were referred to for cross-referencing where appropriate. A total of 168 relevant papers were identified and reviewed. The principal publications which are summarized in this review were limited to peer-reviewed RCT studies utilizing placebo or active-comparator controls, meta-analyses, and relevant observational studies. The emphasis of this review was on current, commonly recommended dosage regimens available including daily 2.5 mg orally, monthly 150 mg orally, and 3-monthly 3 mg intravenous treatment regimens.1
Efficacy of ibandronate dosing regimens studied
Oral
Daily and intermittent dosing
The pivotal 3-year oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe (BONE) study13 assessed two oral dosage regimens. Efficacy was assessed in 2,946 women aged 55–80 years who were 5 or more years postmenopause with 1–4 prevalent vertebral fractures (VFs) (T4–L4) and a lumbar spine (LS) BMD T-score of −2 to −5 in at least one lumbar vertebra (L1–4).13 Subjects with upper gastrointestinal (GI) disorders were not excluded. Subjects were block randomized to either continuous 2.5 mg daily oral ibandronate, 20 mg every other day for 12 doses every 3 months of oral ibandronate (intermittent treatment), or placebo. The study medication was taken 1 hour before food in the morning and all participants received 500 mg of calcium and 400 IU of cholecalciferol daily. The primary endpoint was new morphometric vertebral fractures (MVFs) after 3 years of treatment with secondary outcomes including the rate of worsening VFs, clinical VFs, non-vertebral fractures (NVFs); and change in BMD, bone turnover, or height. VFs were assessed by annual lateral spine radiographs and a VF was defined as a 20% loss of height and an absolute reduction of at least 4 mm in vertebral height from baseline.
Approximately 65% of participants completed the study, with more completers receiving ibandronate than placebo. Daily oral ibandronate 2.5 mg and intermittent oral 20 mg ibandronate reduced the risk of new MVFs, ie, relative risk reduction (RRR) by 62% (4.7% vs 9.6%; P<0.01) and 50% (4.9% vs 9.6%; P<0.01) respectively compared with placebo over 3 years. Clinical vertebral fractures (CVFs) were risk reduced by 49% and 48%, respectively, in both treatment groups compared with placebo (P<0.05). There was no difference between the two active treatment arms suggesting the equivalent efficacy of daily oral compared with intermittent oral ibandronate therapy. There were similar and significant reductions in VFs at 2 years (daily oral 61%, [P<0.01], and intermittent oral 56% [P<0.05]). The reduction in incidence of new VFs at 1 year was 58% which showed a similar trend but did not meet statistical significance (P=0.0561). Similar findings were noted for CVF reduction with a RRR of 49% for the daily oral 2.5 mg group and 48% for the intermittent oral 20 mg treatment group compared with placebo after 3 years (P=0.02 for both compared with placebo). The incidence of clinical osteoporotic NVFs was low and similar in both the active treatment and placebo groups: daily 9.1%, intermittent 8.9%, and placebo 8.2%. This study was not powered to assess NVF outcomes.
Post-hoc analysis14 of the daily oral ibandronate cohort (977 vs 975 placebo subjects) reported significant reduction in new moderate or severe incident VFs: 1-year RRR was 59% (P<0.05); 2-year was 59% (P<0.01), and 3-year was 59% (P<0.01). A subsequent analysis identified that there was no significant age interaction for MVFs in women aged $70 compared with women aged <70 years in the BONE study.15 There was a significant interaction reported between baseline femoral neck (FN) BMD and NVFs for daily but not intermittent oral therapy in the subgroup with FN BMD T-scores <−3.0: a RRR of 69% was obtained with daily oral 2.5 mg ibandronate (P=0.02) and of 37% for intermittent oral 20 mg ibandronate (P=0.22).13
Comparing daily with weekly dosing
Cooper et al assessed 235 women aged 53–80 years who were 3 or more years postmenopause with a LS BMD T-score of −2.0 or less.16 This non-inferiority study compared daily oral 2.5 mg ibandronate with weekly oral 20 mg ibandronate. Primary outcome was the relative change in LS (L1–4) BMD after 48 weeks. Both treatments increased BMD at 48 weeks with lumbar BMD increasing by 3.4% with daily oral compared with 3.5% with weekly oral, and total hip BMD increasing by 2.1% with daily oral compared with 1.7% with weekly oral ibandronate. Fracture outcomes were not reported but the study was underpowered to detect any difference in fracture rates between treatments.
Comparing daily with monthly dosing
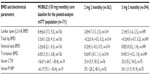
Monthly Oral iBandronate In LadiEs (MOBILE)17–19 was a 2-year, multinational, randomized, double-blind, non-inferiority study which enrolled 1,609 women with postmenopausal osteoporosis to one of four oral regimens: 2.5 mg daily oral, 50 mg × two doses 1 day apart monthly, 100 mg monthly oral, or 150 mg monthly oral with a matching placebo to maintain treatment blinding. All participants received 500 mg of calcium and 400 IU of cholecalciferol daily. Subjects were aged 55–80 years, at least 5 years postmenopause, with osteoporosis (LS BMD T-score between −2.5 and −5.1). The primary outcome was % change in BMD at 1 year at the LS with secondary outcomes of % change in proximal femoral BMD (total hip, femur and trochanter) at 1 year. LS BMD increased by 3.9%, 4.3%, 4.1%, and 4.9%, in the 2.5 mg daily oral, 50/50 mg monthly oral, 100 mg monthly oral, and 150 mg monthly oral active treatment groups, respectively. All monthly regimens were non-inferior and the 150 mg monthly oral treatment arm was superior to the daily oral treatment group (P<0.01). Hip BMD increased by a similar magnitude in all four groups (2%–3%).
A long-term extension study included those on oral 100 mg monthly orally (n=358) and 150 mg monthly orally (n=361) for 5 years. In the pooled 5-year analyses, LS BMD increased relative to baseline by 8.2% (95% confidence interval [CI] 7.2–9.2) in the 100 mg monthly oral group and 8.4% (95% CI 7.5–9.4) in the 150 mg monthly oral treatment arm. The mean increases in LS BMD at years 3, 4, and 5 in the 150 mg monthly oral group were significant at 1.3%, 1.8%, and 2%, respectively. At 1, 2, and 3 years, hip BMD increased by 3.4% (95% CI 2.8–4.0) in the 100 mg monthly oral ibandronate group and 4.1% (95% CI 3.5–4.7) in the 150 mg monthly oral ibandronate group. After 5 years, hip BMD remained significantly elevated at 3.0% and 3.5% in the 100 mg and 150 mg monthly oral ibandronate groups, respectively.
Intravenous dosing
The Dosing IntraVenous Administration (DIVA) study20,21 was a 2-year non-inferiority RCT study comparing two intravenous (IV) regimens (2 mg IV every 2 months or 3 mg IV every 3 months) with 2.5 mg daily oral ibandronate. The DIVA study enrolled 1,395 women aged 55–80 years who were at least 3 years postmenopause and had a LS BMD (L2–4) T-score of less than −2.5. The primary outcome was the mean % change in LS BMD with mean % change in proximal femoral BMD (total hip, FN, or trochanter) or bone turnover as assessed by serum carboxy-terminal collagen crosslinks (CTX) providing secondary outcome data. After 1 year of treatment, LS BMD increased by 5.1% in the 2 mg IV 2-monthly group, 4.8% in the 3 mg IV 3-monthly group, and 3.8% in the 2.5 mg daily oral ibandronate group. Both IV treatment groups were non-inferior but, more importantly, both IV treatment arms were superior to daily oral ibandronate (P<0.01). After 1 year’s treatment, FN BMD was 2.6%, 2.4%, and 1.8% in the 2 mg IV 2-monthly, 3 mg IV 3-monthly, and 2.5 mg daily oral ibandronate groups, respectively. Both IV treatment arms were superior to daily oral therapy (P=0.05).
In the open-label 5-year long-term extension, 781 women were allocated to one of two open-label IV treatment arms: 2 mg IV every 2 months (n=381) or 3 mg IV every 3 months (n=400). Of these, 756 were included in the intent-to-treat (ITT) analysis (362 vs 394). Pooled 5-year analysis reported LS BMD increases over 5 years of 8.4% and 8.1% in the 2 mg IV every 2 months and 3 mg IV every 3 months groups, respectively, relative to baseline BMD for each cohort. Total hip BMD increased at 1, 2, and 3 years relative to baseline with a plateau in change between 2 and 3 years in both treatment groups. In the 2 mg IV every 2 months and 3 mg IV every 3 months groups, reported mean increase in LS BMD at 3 years was 3.6% (95% CI 3.3–4.1) and 3.2% (95% CI 2.6–3.8), and at 5 years, mean increase in total hip BMD was 3.0% (95% CI 2.4–3.5) and 2.8% (95% CI 2.1–3.5), respectively. In the 3-year extension, mean LS BMD increased at all time points relative to baseline (2 years) with a significant mean increase in LS BMD at 5 years of 2.0% and 2.1% in the 2 mg IV every 2 months and 3 mg IV every 3 months groups, respectively.
Meta-analyses
Three meta-analyses of pooled data from the pivotal ibandronate studies have been published.22–24 Two studies22,23 utilized individual patient data from the Phase III ITT populations rather than mean values for each study with at least 2 years’ follow-up (BONE, MOBILE and DIVA).13,18,21 Since oral and IV doses and the bioavailability of each exposure differed, an annual cumulative exposure (ACE) was calculated in these studies utilizing drug dose in milligrams factored by the total number of annual doses and an absorption factor of 0.6% for oral and 100% for IV doses. Subjects were then grouped into high ACE (>10.8 mg), mid ACE (5.5–7.2 mg), or low ACE (2.0–4.0 mg) categories. Since all studies did not include a placebo arm (active-comparator studies), Harris et al22 compared the placebo arm from the pivotal BONE study with patients receiving a high ACE dose (combining 150 mg oral monthly, 3 mg IV 3-monthly, and 2 mg IV 2-monthly doses) and a low ACE dose (2.5 mg oral daily). Six major NVFs (clavicle, humerus, wrist, pelvis, hip, and leg), all NVFs, and all clinical fractures were examined.
The first meta-analysis included 8,710 patients and utilized Cox proportional-hazards models to estimate the adjusted RRR for fracture with ibandronate versus placebo, and time to fracture was compared using log-rank tests.22 The high-dose ACE group showed significant reductions in the adjusted RRR of major NVFs (34.4%, P=0.032), all NVFs (29.9%, P=0.041), and clinical fractures (28.8%, P=0.010) relative to placebo (BONE study indirect placebo comparison). The high-dose ACE group had significantly longer time to fracture versus placebo for key NVFs (P=0.031), all NVFs (P=0.025), and clinical fractures (P=0.002). Several study limitations were noted including that not all studies were placebo controlled and a limited number of baseline characteristics were available for multivariate analyses.
Cranney et al23 utilized individual patient data from eight randomized controlled studies (n=9,753) to assess NVF incidence. Patients with high ACE (150 mg monthly oral, 3 mg IV 3 monthly, and 2 mg IV 2 monthly) had a significant reduction in NVFs in comparison to the low ACE group (2.5 mg daily oral ibandronate). Combining higher ACE doses in comparison to low ACE doses from two trials resulted in a hazard ratio of 0.62 (95% CI 0.396–0.974, P=0.038).
The findings by Cranney et al23 were verified in another meta-analysis by Sebba et al24 who pooled data from 8,710 patients from the four Phase III clinical trials of ibandronate (IV fracture prevention study,25 BONE, MOBILE, and DIVA) to assess the relationship between ibandronate dose, changes in BMD, and rates of both clinical fractures and NVFs. LS and total hip BMD increased with increasing ibandronate dose and the incidence of all clinical fractures decreased as LS BMD increased. A statistically significant inverse linear relationship was observed between % change in LS BMD and the rate of clinical fracture (P=0.005). An inverse, nonlinear relationship was noted between the change in total hip BMD and NVFs which was not significant.
Comparison data
There are limited RCT comparison data. No prospective long-term comparator trials comparing the anti-fracture efficacy of BPs have been conducted, making direct comparisons difficult. The Monthly Oral Therapy with Ibandronate for Osteoporosis intervention (MOTION)26 study compared clinical outcomes of once-monthly 150 mg oral ibandronate with once-weekly 70 mg oral alendronate. After 12 months there was no significant difference in the increase in BMD or VF incidence (0.6% in both groups). The incidence of NVFs with alendronate and ibandronate was 1.4% and 1.6%, respectively.
The eValuation of IBandronate Efficacy (VIBE) study was a 12-month observational study using two US databases and comparing fracture rates of more than 64,000 patients newly treated with either monthly ibandronate or weekly oral alendronate or risedronate.27 The incidence of any clinical fracture was lower (P=0.052) in patients receiving ibandronate therapy compared with in patients on weekly oral BPs (adjusted relative risk 0.82, 95% CI 0.66–1.00). The incidence of VFs was significantly lower in patients receiving ibandronate compared with patients on weekly BPs (adjusted relative risk 0.36, 95% CI 0.18–0.75, P=0.006). There was no difference in hip fractures or NVFs. In an ITT analysis, which included all patients who received at least one prescription, there were no differences in fracture incidence.
A major review of studies of adherence, ie, patient preference and compliance, suggested patients prefer less frequent oral dosing such as monthly compared with daily or weekly oral therapy and, in some settings, IV compared with oral dosing regimens.4
Safety
The BONE study13 compared tolerability of daily oral ibandronate, intermittent therapy, and placebo. There were no significant differences between treatments or in comparison to placebo in any treatment-related adverse events (AEs) (20%, 19%, and 18% in the daily oral ibandronate, intermittent therapy, and placebo groups, respectively), serious adverse events (SAEs) (0.3%, 0.7%, and 0.3%), withdrawal due to AEs (7.5%, 7.2%, and 8.1%), or gastrointestinal dyspepsia (11.4%, 9.0%, and 9.0%).
Miller et al17 compared the tolerability of daily oral 2.5 mg with monthly oral 150 mg ibandronate over 12 months. Monthly treatment was as well tolerated as daily when any AE (70.0% vs 89.0%), any drug-related AE (33.0% vs 30.0%), withdrawal due to AEs (5.8% vs 7.3%), any SAE (7.0% vs 5.0%), or any drug-related SAE (0.0% vs 0.3%) was compared. Upper GI AEs in the total cohort (17% vs 18%) and in those receiving concomitant nonsteroidal anti-inflammatory drugs (18%) were similar. Those with a prior history of upper GI disorder reported more upper GI AEs with daily oral (38%) compared with monthly oral therapy (20%). Influenza-like symptoms, occurring within 72 hours and lasting no longer than 72 hours, were more common in the monthly oral group (8%) compared with the daily oral group (3%). No adverse renal deterioration and no reports of osteonecrosis of the jaw (ONJ) or atypical fractures were reported with longer term 5-year treatment with monthly dosing.19 However occasional post-marketing case reports28 and US Food and Drug Administration adjudication suggest a possible but infrequent association.
The DIVA study20 compared oral daily to IV 3-monthly therapy over 12 months. Patients had similar rates of any AE (76.0% vs 77.0%), any treatment-related AE (39.0% vs 33.0%), withdrawal due to AEs (6.6% vs 4.5%), any SAE (0.4% vs 0.2%), or deaths (0.4% vs 0.2%). The most commonly reported AEs were dyspepsia (3.4%–4.1%), upper abdominal pain (3.0%–4.1%), arthralgia (2.4%–3.6%), and flu-like illness (0.9%–4.1%). Renal AEs were few and similar in the oral and IV treatment groups (2%) with no change in mean serum creatinine. In the long-term extension to 5 years21 (all IV active treatment arms), influenza-like symptoms were reported in 4.5% in the 3-monthly group, 8.3% in the 2-monthly group, and GI symptoms in 14% of patients in the 3-monthly group and in 20% in patients in the 2-monthly group. The study reported no new or unexpected side effects compared with the original core study. Creatinine clearance remained stable with a mean rate of decrease of 1.5 mL/min/year. No cases of ONJ were reported.
Summary of results
Fracture outcomes
Tables 2 and 3 summarize the 3-year published fracture outcomes. The BONE study demonstrated a significant 50%–62% reduction in MVFs at 2 and 3 years and in CVFs of 48%–49% at 3 years. There was a nonsignificant trend with 59% reduction in VFs as early as 1 year (P=0.056). NVF reduction has only been demonstrated in the subgroup with T-scores of less than or equal to −3.0 (RRR 69%). However, in three meta-analyses, higher ACE doses demonstrated an NVF reduction of 30%–38% and a clinical fracture reduction of 29%. There was a significant increase in BMD with increasing dose and an inverse association between lumbar BMD increase and clinical fracture rate.
Early (12-month) BMD outcomes
Table 4 summarizes the early changes in BMD. Increases in LS BMD were significant at 12 months compared with placebo in the pivotal BONE study as well as in the non-inferiority studies of oral monthly and IV treatments. Changes in FN of total hip BMD were not available in all studies and, when available, the changes were not always significant. These data suggest a dose-dependent effect on BMD with greater magnitude of increase in BMD with higher annual cumulative dosing.
Long-term bone mineral density outcomes
Table 5 summarizes the long-term benefits of ibandronate. Long-term outcomes are restricted to BMD outcomes as studies were inadequately powered for fracture outcomes. BMD increased with monthly oral and intermittent IV following 3 years’ treatment and was maintained at 5 years.
Safety data
Table 6 summarizes both short- and long-term safety data of oral daily and intermittent therapies in comparison to placebo for 3 years. Monthly oral dosing has been demonstrated to be comparable to daily oral in regards overall and gastrointestinal (GIT) safety. Flu-like symptoms were more common with monthly oral treatment in the first 12 months and declined significantly by 5 years. The IV regimen reported rates of 4.1% and 4.5% of flu-like symptoms at 1 year and 5 years, respectively. No cases of atypical fracture or ONJ were reported in any of the pivotal published studies. There were no significant adverse cardiovascular outcomes.
Discussion
BPs are common treatments used in the prevention and management of osteoporosis. However adherence, the composite of compliance and persistence, is universally poor.4 Poor adherence results in suboptimal outcomes in terms of fracture prevention. The main reasons for poor adherence include dosing regimen (fasting and frequency), side effects, and inconvenience.1 Patient preference for a treatment is influenced by efficacy, side effects, dose frequency, and formulation.3 Not all nitrogen-containing BPs are the same with differences in potency of inhibition of FPPS, affinity for hydroxyapatite binding, and route and means of administration.
Ibandronate has the distinct advantage in comparison to other BPs of being available in multiple dosing formulations and the frequency of administration can be varied, which offers patients more flexibility (Table 1).1 Ibandronate is available as oral and IV formulations. Oral formulations can be administered daily, weekly, intermittently in 3-month cycles, or monthly. IV formulations have the advantage of a 15- to 30-second administration compared with the 15- to 30-minute slow infusion required for zoledronic-acid administration to reduce potential nephrotoxicity. Furthermore, neither oral nor IV ibandronate treatment schedules have demonstrated significant nephrotoxicity.6–21,29 The acute-phase flu-like cytokine syndrome is less common in patients administered ibandronate than in those administered zoledronic acid according to one retrospective, non-randomized study.30 Cases of ONJ have been reported in patients being treated for osteoporosis or cancer. However, ONJ is far more common in cancer patients, as higher cumulative doses of potent bisphosphonates or the receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor denosumab have been associated with more frequent cases of ONJ.31 Although unproven, the risk of ONJ could be lower with less potent BPs, such as ibandronate, which have a weaker binding affinity than zoledronic acid or alendronate and less potency of inhibition of FPPS than zoledronic acid.32 Oral BPs are associated with an increased risk of GI irritation1 and absorption, and hence potency, is reduced by poor compliance.1,4,33 Most study reports regarding GI intolerance have examined alendronate26,34–36 and risedronate use.37 Ibandronate, may cause similar or less GI irritation as reported in the BONE trial over 3 years of treatment compared with placebo, but larger, longer term studies are required to clarify these findings.13 GI AEs with high-dose oral ibandronate appear to be similar to daily oral treatment when compared with placebo treatment.13–19 In the MOTION study, GI tolerability of once-monthly high-dose oral ibandronate was similar to once-weekly oral alendronate treatment.26,36
Pivotal placebo-controlled RCTs of ibandronate confirm efficacy in VF reduction with oral daily doses and intermittent oral or IV treatment. However, the lack of statistical significance of VF reduction at 1 year is notable in the BONE study and may reflect the daily oral dose chosen in this pivotal Phase III study. Differences in time to onset of action have been demonstrated in previous studies comparing alendronate and risedronate and probably reflect the hierarchy of potency of inhibition of FPPS.1 The daily oral dose of 2.5 mg of ibandronate has been demonstrated to be inferior to monthly 150 mg oral dosing in the MOBILE study17 and inferior to IV ibandronate in the DIVA study20 when assessed according to the primary outcome of each study (the % change in spinal BMD). Similar findings were demonstrated in meta-analyses which conclude that higher dose treatments (150 mg monthly oral or IV ibandronate) are associated with longer time to fracture and lower fracture rates compared with low-dose daily oral 2.5 mg ibandronate.
With higher monthly oral doses of ibandronate, the MOTION and VIBE studies demonstrate non-inferiority in the former and superiority in the latter for monthly oral ibandronate in comparison to weekly alendronate or risedronate. Unfortunately, direct drug comparison trials of RCTs with sufficient power to examine anti-fracture efficacy are not feasible as the sample sizes required are too large. Data collected from medical claims databases offer some real world clinical insight into patient comparisons and the potential benefit of treatment. Although inherent patient-selection biases may have a significant bearing on outcomes, these can to some degree be minimized by the use of appropriate statistical methods. Hence, while reassuring, the data from MOTION and VIBE are not conclusive.38
Longer term oral19 and IV21 studies confirm benefit in BMD improvement and maintenance, but no fracture outcomes are available since these studies are relatively underpowered. Data on NVF reduction is limited to post-hoc analysis and meta-analyses of studies with higher ACEs and lower BMD (T-score less than or equal to −3.0), respectively. This should be viewed in the context of the fact that the NVF efficacy of the available BPs is also limited and not as effective as the VF reduction.39
In the era of RCT data and the need to demonstrate fracture efficacy outcomes, ibandronate as a treatment is disadvantaged by poor study design, patient selection, and dosage in pivotal studies. The only adequately powered placebo-controlled RCT study (BONE) assessed arguably the least effective daily cumulative dose. Studies using potentially more effective monthly oral or quarterly IV doses (higher ACE), have also been generally underpowered and assessed against active, albeit less efficacious, comparators (lower ACE) from pivotal studies rather than against placebo, compounding the data deficit. Patients enrolled had osteopenia rather than osteoporosis at the hip, with the latter predicting lower NVF risk. However, the demonstration of equivalence in terms of BMD response compared with treatment regimens used in the pivotal studies suggest it is effective and has a role in the treatment of osteoporosis. This beneficial role is supported by a good safety profile and infrequent complications of long-term BP use, namely ONJ and atypical, subtrochanteric fractures. Ibandronate is, at worst, equivalent in terms of GIT safety compared with other existing oral BPs and, at best, may have lower rates of GIT toxicity which could make it a more desirable therapy. This is further supported by the potentially lower rates of acute-phase reaction and fewer nephrotoxicity concerns with ibandronate compared with those of the more frequently used IV BP zoledronic acid.
Conclusion
Despite a large amount of data, the only conclusive evidence for efficacy with ibandronate therapy is on VF risk reduction. There is limited and inconclusive evidence for NVF risk reduction, and no evidence that hip fracture rates are reduced.
Ibandronate studies have significant limitations and there is a paucity of placebo-controlled RCT and fracture-outcome data. This is compounded by a number of study-design flaws including patient selection, limited statistical power, under-dosing in the pivotal Phase III BONE study and the absence of placebo groups in studies of the more effective higher dose oral (150 mg monthly) and IV (3 mg 3-monthly) treatments.
With its multiple dosing options and routes of administration, simplicity of IV delivery, and potentially lower rates of side effects, ibandronate may offer advantages over other oral or IV BP treatments that could translate into higher rates of adherence – an important factor in 2014 when the two main limiting factors which impact on future fracture rates are treatment initiation and adherence to therapy.
Appropriately designed placebo or active-comparator RCTs are needed to define if ibandronate reduces non-vertebral and/or hip fractures. In the absence of such data, and in view of the diverse alternative dosing and frequency options provided by the currently available BPs, ibandronate could be considered a second-line treatment option when poor adherence or renal impairment are present and vertebral but not NVF risk reduction is needed.
Disclosure
The authors report no conflicts of interest in this work.
References
Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555–1565. | |
Inderjeeth CA, Chan K, Kwan K, Lai M. Time to onset of efficacy in fracture reduction with current anti-osteoporosis treatments. J Bone Miner Metab. 2012;30(5):493–503. | |
Inderjeeth CA, Foo AC, Lai MM, Glendenning P. Efficacy and safety of pharmacological agents in managing osteoporosis in the old old: review of the evidence. Bone. 2009;44(5):744–751. | |
Lee S, Glendenning P, Inderjeeth CA. Efficacy, side effects and route of administration are more important than frequency of dosing of anti-osteoporosis treatments in determining patient adherence: a critical review of published articles from 1970 to 2009. Osteoporos Int. 2011;22(3):741–753. | |
Cole Z, Dennison E, Cooper C. Update on the treatment of post-menopausal osteoporosis. Brit Med Bull. 2008;86(1):129–143. | |
Rizzoli R. Bisphosphonates for post-menopausal osteoporosis: are they all the same? QJM. 2011;104(4):281–300. | |
Di Munno O, Delle Sedie A. Efficacy of ibandronate: a long term confirmation. Clin Cases Miner Bone Metab. 2010;7(1):23–26. | |
Russell RG. Bisphosphonates: mode of action and pharmacology. Paediatrics. 2007;119 Suppl 2:150–162. | |
Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38(5):617–627. | |
Lawson MA, Xia Z, Barnett BL, et al. Differences between bisphosphonates in binding affinities for hydroxyapatite. J Biomed Mater Res B Appl Biomater. 2010;92(1):149–155. | |
Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18(8):1023–1031. | |
Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21(9):1453–1460. | |
Chesnut CH III, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241–1249. | |
Felsenberg D, Miller P, Armbrecht G, Wilson K, Schimmer RC, Papapoulos SE. Oral ibandronate significantly reduces the risk of vertebral fractures of greater severity after 1, 2, and 3 years in postmenopausal women with osteoporosis. Bone. 2005;37(5):651–654. | |
Ettinger MP, Felsenberg D, Harris ST, et al. Safety and tolerability of oral daily and intermittent ibandronate are not influenced by age. J Rheumatol. 2005;32(10):1968–1974. | |
Cooper C, Emkey R, McDonald RH, et al. Efficacy and safety of oral weekly ibandronate in the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2003;88(10):4609–4615. | |
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20(8):1315–1322. | |
Reginster J, Adami S, Lakatos P, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006;65(5):654–661. | |
Miller PD, Recker RR, Reginster JY, et al. Efficacy of monthly oral ibandronate is sustained over 5 years: the MOBILE long-term extension study. Osteoporos Int. 2012;23(6):1747–1756. | |
Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum. 2006;54(6):1838–1846. | |
Bianchi G, Czerwinski E, Kenwright A, Burdeska A, Recker RR, Felsenberg D. Long-term administration of quarterly IV ibandronate is effective and well tolerated in postmenopausal osteoporosis: 5-year data from the DIVA study long-term extension. Osteoporos Int. 2012;23(6):1769–1778. | |
Harris ST, Blumentals WA, Miller PD. Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studies. Cur Med Res Opin. 2008;24(1):237–245. | |
Cranney A, Wells GA, Yetisir E, et al. Ibandronate for the prevention of nonvertebral fractures: a pooled analysis of individual patient data. Osteoporos Int. 2009;20(2):291–297. | |
Sebba AI, Emkey RD, Kohles JD, Sambrook PN. Ibandronate dose response is associated with increases in bone mineral density and reductions in clinical fractures: results of a meta-analysis. Bone. 2009;44(3):423–427. | |
Recker R, Stakkestad JA, Chesnet CH 3rd, et al. Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone. 2004;34(5):890–899. | |
Miller PD, Epstein S, Sedarati F, Reginster JY. Once-monthly oral ibandronate compared with weekly oral alendronate in postmenopausal osteoporosis: results from the head-to-head MOTION study. Curr Med Res Opin. 2008;24(1):207–213. | |
Harris ST, Reginster JY, Harley C, et al. Risk of fracture in women treated with monthly oral ibandronate or weekly bisphosphonates: the eValuation of IBandronate Efficacy (VIBE) database fracture study. Bone. 2009;44(5):758–765. | |
Shane E, Burr D, Ebeling PR, et al. A typical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294. | |
Miller PD, Ragi-Eis S, Mautalen C, Ramirez F, Jonkanski I. Effects of intravenous ibandronate injection on renal function in women with postmenopausal osteoporosis at high risk for renal disease – the DIVINE study. Bone. 2011;49(6):1317–1322. | |
Sieber P, Lardelli P, Kraenzlin CA, Kraenzlin ME, Meier C. Intravenous bisphosphonates for postmenopausal osteoporosis: safety profiles of zoledronic acid and ibandronate in clinical practice. Clin Drug Investig. 2013;33(2):117–122. | |
Wang X, Yang KH, Wanyan P, Tian JH. Comparison of the efficacy and safety of denosumab versus bisphosphonates in breast cancer and bone metastases treatment: A meta-analysis of randomized controlled trials. Oncol Lett. 2014;7(6):1997–2002. | |
Pazianas M, Cooper C, Ebetino FH, Russell RG. Long-term treatment with bisphosphonates and their safety in postmenopausal osteoporosis. Ther Clin Risk Manag. 2010;6:324–343. | |
Schimmer RC, Bauss F. Effect of daily and intermittent use of ibandronate on bone mass and bone turnover in postmenopausal osteoporosis: a review of three phase II studies. Clin Ther. 2003;25(1):19–34. | |
de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, et al. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335(14):1016–1021. | |
Ettinger B, Pressman A, Schein J. Clinic visits and hospital admissions for care of acid-related upper gastrointestinal disorders in women using alendronate for osteoporosis. Am J Manag Care. 1998;4(10):1377–1382. | |
Emkey R, Delmas PD, Bolognese M, et al. Efficacy and tolerability of once-monthly oral ibandronate (150 mg) and once-weekly oral alendronate (70 mg): additional results from the Monthly Oral Therapy With Ibandronate For Osteoporosis Intervention (MOTION) study. Clin Ther. 2009;31(4):751–761. | |
Lanza FL, Hunt RH, Thomson AB, Provenza JM, Blank MA. Endoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal women. Gastroenterology. 2000;119(3):631–638. | |
Rossini M, Orsolini G, Adami S, Kunnathully V, Gatti D. Osteoporosis treatment: why ibandronic acid? Expert Opin Pharmacother. 2013; 14(10):1371–1381. | |
Boonen S, Laan RF, Barton IP, Watts NB. Effect of osteoporosis treatments on risk of non-vertebral fractures: review and meta-analysis of intention-to-treat studies. Osteoporos Int. 2005;16(10):1291–1298. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.