Back to Journals » Journal of Inflammation Research » Volume 15
Long-Term Efficacy and Low Adverse Events of Methylprednisolone Pulses Combined to Low-Dose Glucocorticoids for Systemic Sclerosis: A Retrospective Clinical Study of 10 Years’ Follow-Up
Authors Cheng H , Yu Z, Yan CL, Yang HD, Gao C, Wen HY
Received 4 May 2022
Accepted for publication 26 July 2022
Published 4 August 2022 Volume 2022:15 Pages 4421—4433
DOI https://doi.org/10.2147/JIR.S373387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Hao Cheng,1 Zhen Yu,1 Cheng-lan Yan,1 Hui-dan Yang,1 Chong Gao,2 Hong-yan Wen1
1Department of Rheumatology, Shanxi Medical University, the Second Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 2Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Correspondence: Hong-yan Wen, Email [email protected]
Background: Patients with systemic sclerosis (SSc) have poor prognosis without cure methods. We began, 10 years ago, to relieve active SSc using short-term intravenous high-dose methylprednisolone pulse (MP-Pulse) and then maintain remission using long-term and low-dose oral glucocorticoids (LTLD-GC).
Methods: Total 46 of SSc patients with interstitial lung disease (ILD) and induration of skin during January 2006 to December 2019 were analyzed retrospectively, who were followed up for 10 years or more. The patients were treated with MP-Pulse (15 mg/kg/day, 4 days/week, for 2 weeks) with (n=21) or without (n=25) LTLD-GC (prednisone 5– 10 mg/day or methylprednisolone 4– 8 mg/day). The biographic and clinical data, including occurrence of infection or any adverse reactions, were collected at baseline, 6 months, 1 year, and annually through 10 years after treatment.
Results: From baseline to 10 years, compared with MP-Pulse alone, MP-Pulse/LTLD-GC significantly reduced skin and lung fibrosis and improved lung function: Rodnan skin score (mRSS: 22.1± 12.4 to 8.16± 2.5, P< 0.001), forced vital capacity (FVC: 71.7% to 89.83%, P< 0.001), forced expiratory volume in the first second (FEV1: 75.7% to 87.88%, P< 0.001), diffusing capacity of the lung for carbon monoxide (DLCO: 63.4% to 87.73%, P< 0.001), and high-resolution chest computerized tomography scan (HRCT score: 3.96± 2.81 to 1.42± 0.83, P< 0.001). None of the 46 patients had femoral head necrosis, compression fracture, death, or life-threatening adverse events.
Conclusion: These outcomes indicate that intravenous MP-Pulse combined with oral LTLD-GC could achieve significant remission and better long-term (10 years) efficacy without severe adverse effects in SSc patients with ILD and induration of skin.
Keywords: glucocorticoids, low-dose, pulse, systemic sclerosis, safety
A Letter to the Editor has been published for this article.
Introduction
Systemic sclerosis or scleroderma (SSc) is a highly heterogeneous autoimmune disease with unknown etiology and characterized by fibrosis of the skin and internal organs and vasculopathy.1,2 Although systemic sclerosis is uncommon, it has high morbidity and mortality. Studies have found inflammatory and autoimmune responses play a critical pathogenic role in SSc. Anti-inflammatory and immunosuppressive were helpful in SSc.3–5 However, there is currently no known cure for SSc, exploring new and effective treatments is extremely important.
Glucocorticoids (GCs) have anti-inflammatory and immunosuppressive actions.6 Glucocorticoids (GC) utilization in systemic sclerosis (SSc) is still controversial for the lack of evidence and their related adverse events. Some studies have found that pulsed intravenous methylprednisolone (MP-Pulse) is a feasible therapy for active SSc.7,8 However, repeated MP-Pulse therapy can increase the risk of adverse reactions.9,10 The adverse effects of glucocorticoids are extensive and can involve many organ systems. Some studies have found that glucocorticoid-induced osteoporosis (GIOP) develops in a time- and dose-dependent manner.11 GCs have been part of the therapeutic strategy in managing ILD, diffuse cutaneous disease, or myositis. However, awareness of the risk of SRC should persist, especially in the use of glucocorticoids (GC), especially in higher doses above 15 mg/day, which has long been known as an essential negative factor for the development of SRC. A daily dosage of >15 mg is associated with a fourfold increase in the result of scleroderma renal crisis (SRC). So, the use of GC dosages above 15 mg/day is discouraged.12–15
In theory, a low dose of GC has a less adverse effect and can be used for a relatively long time.16 Studies have been found physiologic doses of hydrocortisone have shown to improve mild-to-moderate psychosocial disturbances.17 So, short-term MP-Pulse combined with long-term and low-dose GCs should have complementary advantages, providing better and sustained efficacy for SSc. Unfortunately, there has been no comprehensive study on the effects of this combined therapy in SSc, although a high frequency of GCs utilization has been undoubtedly recorded. Therefore, we developed an MP-Pulse/oral LD-GC treatment on the basis of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) more than a decade ago: allowing a rapid remission of disease by short-term and high-dose MP-Pulse and then maintaining with long-term and low-dose oral glucocorticoids (close to physiological measures).
Although glucocorticoid therapy is often used for SSc and has confirmed its efficacy and adverse effects, it still has room to optimize, especially in long-term and low-dose observation. So, we first described that we developed an MP-Pulse/oral LD-GC therapy based on conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). This study investigated whether MP-Pulse/low-dose GCs in the long-range treatment is effective and safe for SSc patients with ILD and skin induration. This article aims to provide novel therapeutic strategies and some options for the problematic treatment of SSc patients.
Patients and Methods
Patients
Inclusion and Exclusion Criteria
The study included 46 SSc patients from the rheumatology clinic of the Second Hospital of Shanxi Medical University who were enrolled from January 2006 to December 2009. But they came from different medical teams. These patients who were followed for more than 10 years and enrolled in the study were aged between 18 and 80 years and had the active disease (Rodnan Skin Score (mRSS) units ≥10). SSc-ILD patients were defined as those with evidence of fibrosis on high-resolution CT (HRCT) findings and a predicted forced vital capacity (FVC) of at least 50%18. A flow chart of the patients is depicted in Figure 1. Meanwhile, they come from different medical groups. The diagnosis of SSc was based on American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) recommendations.19,20 The patients were excluded from this study if they with thrombosis, pregnancy, malignant disease, had a history of malignancy, a recent clinically significant infection, or had any other connective tissue disease, pulmonary interstitial lesions due to other causes, such as idiopathic and pharmaceutical, or coal dust and asbestos asthma. Involvement of other organs except skin and lung lesions of SSc patients was also excluded. This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University and obtained an exemption from informed consent of the Ethics committee (No. 2019YX009).
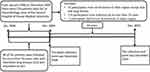 |
Figure 1 A flow chart of the patients. |
Treatment
The study is a single-center retrospective study. Our medical center is divided into multiple medical groups. Different medical groups differ in the application of glucocorticoid therapy. Based on similar cs-DMARD treatment, 46 patients received intravenous (i.v.) MP-Pulse therapy (15 mg/kg/day for 4 days/week, 2 weeks).21,22 And 21 of them were followed by long-term (10 years) oral LTLD-GC (prednisone 5–10 mg/day or methylprednisolone 4−8 mg/day at 8:00 am), that is, small doses of glucocorticoid prednisone or an equivalent amount of prednisolone.9
Follow-Up Assessment
The clinical follow-up assessment mainly included dosage, frequency and duration of treatments, disease activity, occurrence of infection, and any other adverse reactions at baseline, 6 months, annually through 10 years after treatment. Clinical and laboratory data were obtained retrospectively from medical records. Data for each patient was assessed by the same investigator throughout the study using the following outcome measures.
Outcomes
The primary outcome was ≥10 years of follow-up. Safety end points were serious AEs (SAEs).
Clinical Evaluations
Skin
Skin thickness was evaluated using a modified Rodnan skin score (mRSS). mRSS were obtained retrospectively from medical records. The sclerotic degree of the following 17 parts was evaluated, including the face, chest, abdomen, bilateral fingers, back of hand, forearm, upper arm, thigh, calf and back of foot. Each part was assessed as follows: normal = 0, mild = 1, moderate = 2, and severe = 3.23
Lung
Chest HRCT: ILD diagnosis was determined by a review of a high-resolution computed tomography scan (HRCT, GE DiscoveryRT). CT features most likely associated with SScILD were as follows: fibrosis inside focal GGOs in the upper lobes; fibrosis in lower-lobe GGOs; and RETs in the lower lobes, exceptionally if bilateral/symmetrical or with signs of fibrosis. About usual interstitial pneumonia (UIP), HRCT showed subpleural reticular dense and giant capsule honeycomb with interlobular septal thickening, distraction bronchiectasis, and the range of slope from the tip of the lung to the bottom of the lung; most cases may also appear a dense shadow of glass, but the content is limited. For non-specific interstitial pneumonia (NISP), HRCT showed a symmetrical distribution of bilateral subpleural abnormalities, including patchy glazing-like dense irregular line-like or dense reticular shadow scattered in tiny nodules.24 Lung involvement and fibrosis were evaluated using HRCT, which have four features: single ground-glass opacity (GGO), ground-glass lesions and reticular nodular lesions (mixed pattern), single reticular nodular lesions (reticula-nodular) and honeycomb changes. Three representative levels of aortic arch, bronchial bifurcation and diaphragm top were used, each of which was scored separately. The range of these lesions is quantified separately according to their percentage of the area of each layer of lung tissue, 0: no changes above; 1: <25%; 2: 25–49%; 3: 50–74%; 4: ≥75%. Add up the scores of all four regions as lung HRCT scores.25
PFT: Standard PFTs were performed at baseline, 1, 3, and 6 months in all patients, including assessments of forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and carbon monoxide diffusing capacity (DLCO) corrected for hemoglobin concentration. PFT parameters are expressed as a percentage of normal predicted values based on age, sex and height. All tests were performed at the Second Hospital of Shanxi Medical University (PFT, Geratherm, Spirostik Complete).26
sPAP: Systolic pulmonary artery pressure (sPAP) was assessed by color Doppler echocardiography (ECO, PHILIPS EPIQ7C).27
Safety Assessments
Femoral head necrosis and compression fracture were diagnosed by a specialist orthopedic surgeon and a rheumatologist. The clinical follow-up assesses this radiograph of the pelvis every 1–2 years. Liver dysfunction was defined as more than twice the upper limit of normal (AST, ALT≤40.0 U/L). Hyperglycemia is defined as a fasting blood glucose of at least 6.10 mmol/L. Dyslipidemia is defined as the value exceeding these normal ranges (mmol/L): cholesterol (CHOL) 3.10–6.00, triglycerides (TRIG) 0.45–1.60, high-density lipoprotein (HDL) 0.80–2.35, and low-density lipoprotein (LDL) 1.68–4.50. Renal dysfunction was defined as an increase in serum creatinine concentration of more than 30% above baseline levels at any study time point. Patients had scleroderma renal crisis (SRC), serum creatinine ≥2.0 mg/day, or a doubling of serum creatinine above the value at baseline, in the absence of another defined cause and/or malignant hypertension systolic blood pressure (BP) ≥160 mmHg or diastolic BP ≥110 mmHg on at least 2 occasions, a minimum of 12 hours apart.12–15,17
Sample Size Determination
The sample size calculations were based on detecting treatment differences of on the main outcome, assuming a two-tailed test, a effect size of 0.5, an alpha level (α) of 0.05 and a desired power (β) of 80%. The estimated desired sample size was calculated to be at least 17 subjects per group. A dropout percentage of 10% was expected, so at least 19 patients were included in each group.
Statistical Analysis
Data were statistically significant at a value of P<0.05. Statistical analyses were performed by SPSS version 23.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism version 8.01. Normal distributed variables’ descriptive data were presented as mean and standard deviation and non-normal distributed variables were presented as median with range. Categorical variables were reported as numbers. Paired-samples t-test or paired-samples Wilcoxon test was used for comparison of changes before and after treatment. Independent-samples t-test or Mann–Whitney U-test was used to compare the differences between two groups. ROC curves were prepared using SPSS. The predictive the therapeutic effect of FVC, FEV1 and DLCO were also assessed with ROC curves in SSc and aid visual comparison.
Result
The Selection of Patients
After screening by inclusion and exclusion criteria, a total of 43 participants were excluded due to involvement of other organs except skin and lung lesions. Within the remaining 176 participants, 128 cases were followed up for less than 10 years, and 2 cases had severe dysfunction of major organs; 46 patients were used for data analysis (Figure 1).
Baseline Characteristics
The baseline characteristics of the 46 SSc patients in the two groups (MP-Pulse alone and MP-Pulse/LTLD-GC) at the beginning of the therapy are summarized in Table 1. Before treatment, there were 5 patients (10.86%) with diabetes and 4 (8.70%) with hypertension: their conditions had been controlled. Between the two group, the differences were not statistically significant in the general conditions and disease indicators, including those for skin hardness score and lung evolvement as well as basic disease and laboratory test (P>0.05). The two groups were comparable.
 |
Table 1 Baseline Characteristics of 46 Patients with SSc Treated with Two Different Therapies and Basic Treatment |
All patients were treated with MP-Pulse and 21 of them were followed by oral LTLD-GC for 10 years. In the period, 7 patients (13.04%) twice, and 4 (8.70%) thrice as disease was active or relapsed. Because fewer of patients had received two or three MP-Pulse treatment, the difference between the two groups were not statistically significant. All patients received intermittent csDMARDs, including methotrexate, cyclophosphamide, leflunomide, and hydroxychloroquine. Because of the long history of collection, most patients were adjusted a variety of immunosuppressant treatment schemes. Approximate 90% of the patients received a combination of drugs: the combination with coined CYC (cyclophosphamide) accounted for 67.40%. There was no statistically significant difference in MTX, LEF and other immunosuppressant monotherapy and various combinations between the two groups (P>0.05). The two groups were comparable (Table 1).
Short-Term Efficacy
First, we compared the short-term (1 year) efficacy of MP-Pulse with or without the combination with LTLD-GC. Compared with the baseline, from 6 months to 1 year, there were significant improvements in skin hardness score and lung function test FVC after both treatments (p<0.05) (Figure 2A and B). The lung function indicators – FEV1 and DLCO – of both groups showed an upward trend (Figure 2C and D); meanwhile the lung HRCT score of both groups showed a downward trend (Figure 2E), although there was no statistical significance. SPAP had no significant difference between the two groups of patients (Figure 2F).
Long-Term Efficacy
Next, compared to baselines, 3 to 10 years of MP-Pulse/LTLD-GC treatment improved all indicators except sPAP. Particularly, lung HRCT imaging showed the reduced lung lesion compared with those before MP-Pulse/LTLD-GC (Figure 3). Furthermore, there were statistically significant improvements in skin hardness, lung fibrosis and function: lung HRCT score (3.96±2.81 to 1.42±0.83), FEV1 (75.7% to 87.88%), DLCO (63.4% to 87.73%), and FVC (71.7% to 89.83%) (Figure 4A–F). Notably, before and after treatment of MP-Pulse alone as controls, there was no significant difference in each index (Figure 4A–F).
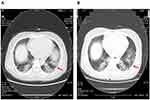 |
Figure 3 Represent HRCT showed the reduced lung lesion in a patient after the 9-years treatment of MP-Pulse/LTLD-GC. Notes: (A) Before June 2010 and (B) After September 2019. |
At the different timepoint (year) after treatment, we compared skin/lung lesion and lung function indicators between MP-Pulse/LTLD-GC and MP-Pulse alone. Long-term treatment (6th and 10th year) of MP-Pulse/LTLD-GC, but not MP-Pulse alone, reduced significantly skin (mRSS, p<0.05 or p<0.001) and lung fibrosis (HRCT, p<0.05), and concomitantly improved lung function: increasing FVC (P<0.05 or P<0.001), FEV1 (p<0.05), and DLCO (p<0.05) (Figure 5A–F). Interestingly, FVC (AUC: 0.854) and FEV1 (AUC: 0.906) could predict the therapeutic effect of MP-Pulse/LTLD-GC in SSc (Figure 5G).
Treatment Safety
In 1 year, all 46 patients using MP-Pulse therapy had no severe adverse events. Patients with a history of hypertension and diabetes had elevated blood pressure and elevated serum glucose during MP-Pulse treatment, which all reverted to normal by adjusting therapeutic regimen. Eight patients (17.40%) had infection (including pulmonary, urinary tract, digestive system, and skin infection); all infected cases were cured successfully by anti-infective treatment. No new arrhythmias or ischemic events were noted on ECG monitoring for all subjects and 1 patient (2.17%) had chest tightness, but no accompanying vital sign or ECG changes (Table 2).
 |
Table 2 Adverse Events from 1 Year to 10 Years of Therapy (n = 46) |
In the long-term (10 years) of the treatment, none of these patients had femoral head necrosis and compression fracture, which should profit partially from the fact that the most of them were treated in regular with anti-osteoporosis medications (Table 1). Compared to baseline level, 5 patients (10.87%) had a 30% increase in creatinine level; 1 (2.18%) increased by 50% in liver enzymes; 1 (2.18%) developed diabetes requiring treatment after 6 years of treatment; 2 (4.34%) presented hypertension requiring treatment after an average of 3.5 years of treatment; 2 (4.34%) showed hyperlipidemia requiring treatment after an average of 7.8 years; after an average 6.8 years of treatment, 7 (15.22%) was found with peripheral neuropathy such as limb numbness, but no limb tremor; 9 (19.57%) had infection (including pulmonary, urinary tract, digestive system infect, and skin infection), one of whom developed herpes zoster infection. All infected cases were cured successfully by anti-infective treatment. There was no statistically significant difference in the incidence of infection and other adverse reactions between the two groups (P>0.05) in the long-term of 10 years (Table 2).
Discussion
Although corticosteroids have been used as the primary treatment for SSc, their efficacy is unsatisfactory. High-dose corticosteroid therapy increases the risk of complications by renal crisis, but it still has room to optimize, especially in long-term and low-dose observation. So, the combination of low-dose and long-term corticosteroids with different immunosuppressive drugs has been considered a novel therapeutic strategy and option in treating SSc.3,6
Glucocorticoids (GCs) are remarkably efficacious in managing many of autoimmune disorders and represent a mainstay in the therapeutic strategies of many autoimmune diseases.6,7 They can be used as low- or high-dose, short- or long-term, and pulsed high-dose intravenous infusion of methylprednisolone (MP-Pulse) depending on clinical requirement. The obvious side effects by long-term and high-dose GC applications have been confirmed, whereas short-term GCs cannot delay the progress of SSc. Notably, MP-Pulse can be used in conditions where rapid immunosuppression and anti-inflammatory effect is desired. For SSc patients, the efficacy of GCs in published studies is limited and controversial, which should associate with different dose, duration and route of administration in these studies.8–10,15 In particular, in the study on CTD-ILD by Yamano et al, two courses of pulse dose methylprednisolone therapy followed by prednisone and oral tacrolimus appeared to be well tolerated, and to have multidimensional efficacy.9 These studies suggest that short-term intravenous high-dose methylprednisolone pulse (MP-Pulse) and low-dose oral glucocorticoids (LTLD-GC) may be effective in the short term.
Since repeated MP-Pulse therapy can lead to increased risk of the adverse reactions,8 we began, 10 years ago, to relieve active SSc patients using short-term intravenous high-dose methylprednisolone pulse (MP-Pulse) and then maintain remission using long-term and low-dose oral glucocorticoids (LTLD-GC). To our knowledge, this is the first study to assess the long-term therapeutic effect and adverse events of intravenous MP-Pulse together with oral LTLD-GC for SSc.
Our study showed short (6 month-1 year) after MP-Pulse with or without LTLD-GC reduced the skin fibrosis and improved lung function, especially the later. However, long-term efficacy of MP-Puls/LTLD-GCs was significantly better than that of MP-Pulse alone. Starting 3 years after MP-Pulse/LTLD-GC treatment, all indicators were improved as compared with those before treatment but not MP-Pulses alone. It significantly reduced skin and lung fibrosis and improved lung function as compared to either baseline or MP-pulse alone. FVC and FEV1 can predict the therapeutic effect of this treatment. In addition, a study showed that the combination therapy of low-dose dexamethasone and atorvastatin protected endothelial cell function.28 In addition to glucocorticoids, some new drugs have been used to treat SSc-ILD, such as nintedanib, an intracellular inhibitor of tyrosine kinases, which has been approved in many countries for the treatment of idiopathic pulmonary fibrosis (IPF) and SSc‐ILD.29,30 These results suggest that nintedanib has a clinically relevant benefit on the progression of SSc-ILD. Whether it can replace glucocorticoids or reduce glucocorticoid doses, and then reduce the occurrence of adverse events, is worth our further study.
All the patients, including MP-Pulse alone group and MP-Pulse/LTLD-GC group, had accepted csDMARDs treatment for the whole 10 years, among which methotrexate and cyclophosphamide mainly were used. Currently, conventional therapies for SSc include the immunosuppressive agents such as methotrexate, azathioprine, mycophenolate mofetil (MMF), and cyclophosphamide (CYC). However, none of the disease-modifying antirheumatic drugs (DMARDs) has proven efficacy for SSc skin fibrosis. And there remain no FDA-approved medications, some of which are off-label, for cutaneous fibrosis in SSc.31,32 These immunosuppressive medications are generally associated with improved lung function and skin scores but have potentially serious side effects. Our research showed csDMARDs had no apparent difference between the groups. Therefore, better efficacy in MP-Pulse/LTLD-GC group should be contributed by LTLD-GC treatment. In other studies, GC combined with immunosuppressive drugs can improve lung function and skin involvement.33 But they do not explain if adding GCs to csDMARDs could lead to better control of the disease because the follow-up time of these studies was shorter, their uncontrolled design, or the small number of patients enrolled.
Importantly, none of the patients showed a life-threatening adverse event associated with the combined therapy. At the beginning, we were concerned whether MP-Pulse/LTLD-GC could result in scleroderma-related renal crisis, as indeed reported in the literature.12 As a result, no episode of renal impairment in the 21 patients was observed after MP-Pulse/LTLD-GC. In this period of treatment, there were 1 new diabetes mellitus and 2 new hypertensions. Because of the small-sample number, the long follow-up time, and multiple effecting factors, the events were not considered as adverse effects caused by drugs for the time being. However, to prevent from the adverse effect risk of GCs treatment, screening carefully patient’s glucose levels and monitoring blood pressure should be performed prior to MP-Pulse administration. In addition, preventing from infection should be considered.
Despite the retrospective nature, our results indicate that MP-Pulse/LTLD-GC therapy for 10 years reduced significantly the skin/lung sclerosis and improved lung function, which was better than MP-Pulse alone as the control group. No patient was interrupted by the drug side effects of this treatment. Importantly, it demonstrated multidimensional effects on patients with SSc. Therefore, this therapy is safe, feasible, and low cost and may become the optimal regimen for the treatment of SSc that delays or even reverses the disease.
There are limits to our study. This was a follow-up and single-center analysis, and assessments were not blinded to the treatment protocol. Due to the long follow-up time, the number of patients able to follow-up is limited, and some confounding factors were poorly controlled during this period. Therefore, more studies combined with prospective and retrospective clinical and laboratory studies using a large sample are needed to confirm the improvement of pulmonary function and skin fibrosis and the occurrence of possible adverse events. Furthermore, we only selected patients with conventional immunosuppressants as comparators; specific csDMARDs combined with MP-Pulse/long-term LTLD-GC should be optimized in the future.
Conclusion
Our study shows for the first time that intravenous high-dose MP-Pulse might be considered a treatment for rapidly relieving active SSc. More importantly, long-term and low-dose (≤physiological dose) oral glucocorticoids not only maintain remission and prevent relapse but may have slowed and even reversed these skin and organ fibrosis trends without severe adverse events during the whole 10 years of treatment. However, further study should be done using large samples, and the specific mechanism needs further investigation.
Abbreviations
ACR, American College of Rheumatology; BP, blood pressure; CTD, connective tissue disease; CHOL, cholesterol; CYC, cyclophosphamide; cs-DMARDs, conventional synthetic disease-modifying antirheumatic drugs; DMARDs, disease-modifying antirheumatic drugs; DLCO, diffusing capacity of the lung for carbon monoxide; DBP, diastolic blood pressure; EULAR, European League Against Rheumatism; ECO, echocardiography; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; GCs, glucocorticoids; GGO, single ground-glass lesions; HDL, high-density lipoprotein; HRCT, high-resolution CT; I.v, intravenous; LDL, low-density lipoprotein; LTLD-GC, long-term and low-dose oral glucocorticoids; ILD, interstitial lung disease; LEF, leflunomide; MP-Pulse, methylprednisolone pulse; mRSS, Rodnan skin score; MTX, methotrexate; SSc; systemic sclerosis; SRC, scleroderma renal crisis; sPAP, systolic pulmonary artery pressure; SBP, systolic blood pressure; TRIG, triglycerides; UIP, usual interstitial pneumonia; VC, vital capacity.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The Ethics Committee of the Second Hospital of Shanxi Medical University approved the study and obtained an exemption from informed consent of the Ethics committee (No. 2019YX009). The reason for the waiver is that the research using medical records and biological specimens obtained in previous clinical diagnoses and treatment, and meeting all of the following conditions: (1) the research purpose is important; (2) the risk of the study is not greater than the minimum risk; (3) exemption from informed consent will not adversely affect the rights and health of patients; (4) patients’ privacy and personal identifiable information are protected; (5) the study will not be conducted if informed consent is required. We have strict confidentiality of patient data and compliance with the Declaration of Helsinki.
Acknowledgments
We would like to thank Dr Chong Gao for reviewing the manuscript for English language editing and Dr Lu He for analyzing clinical levels.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Key Scientific Research Project of Medical Science of Shanxi Province (2021XM08), Basic Research Youth Project of Shanxi Province (202103021223442) and Basic Research Project of Shanxi Province (20210302123268).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Varga J, Trojanowska M, Kuwana M, et al. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord. 2017;2:137–152. doi:10.5301/jsrd.5000249
2. Smith V, Scirè CA, Talarico R, et al. Systemic sclerosis: state of the art on clinical practice guidelines. RMD Open. 2018;4(Suppl 1):e000782. doi:10.1136/rmdopen-2018-000782
3. Ruaro B, Sulli A, Pizzorni C, et al. Correlations between blood perfusion and dermal thickness in different skin areas of systemic sclerosis patients. Microvasc Res. 2018;115:28–33. doi:10.1016/j.mvr.2017.08.004
4. Maehara T, Kaneko N, Perugino CA, et al. Cytotoxic CD4+ T lymphocytes may induce endothelial cell apoptosis in systemic sclerosis. J Clin Invest. 2020;130(5):2451–2464. doi:10.1172/JCI131700
5. Asano Y. Systemic sclerosis. J Dermatol. 2018;45(2):128–138. doi:10.1111/1346-8138.14153
6. Iudici M, van der Goes MC, Valentini G, Bijlsma JWJ. Glucocorticoids in systemic sclerosis: weighing up the benefits and risks -A systematic review. Clin Exp Rheumatol. 2013;31(2 Suppl 76):157.
7. Griffiths B, Miles S, Moss H, Robertson R, Veale D, Emery P. Systemic sclerosis and interstitial lung disease: a pilot study using pulse intravenous methylprednisolone and cyclophosphamide to assess the effect on high resolution computed tomography scan and lung function. J Rheumatol. 2002;29(11):2371–2378.
8. Vanthuyne M, Blockmans D, Westhovens R, et al. A pilot study of mycophenolate mofetil combined to intravenous methylprednisolone pulses and oral low-dose glucocorticoids in severe early systemic sclerosis. Clin Exp Rheumatol. 2007;25(2):287–292.
9. Yamano Y, Taniguchi H, Kondoh Y, et al. Multidimensional improvement in connective tissue disease-associated interstitial lung disease: two courses of pulse dose methylprednisolone followed by low-dose prednisone and tacrolimus. Respirology. 2018;23(11):1041–1048. doi:10.1111/resp.13365
10. Iudici M, Fasano S, Iacono D, Russo B, Cuomo G, Valentini G. Prevalence and factors associated with glucocorticoids (GC) use in systemic sclerosis (SSc): a systematic review and meta-analysis of cohort studies and registries. Clin Rheumatol. 2014;33(2):153–164. doi:10.1007/s10067-013-2422-0
11. Adami G, Saag KG. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int. 2019;30(6):1145–1156. doi:10.1007/s00198-019-04906-x
12. Steen VD, Medsger TA
13. EUSTAR collaborators, Bütikofer L, Varisco PA, Distler O, et al. ACE inhibitors in SSc patients display a risk factor for scleroderma renal crisis-A EUSTAR analysis. Arthritis Res Ther. 2020;22(1):59. doi:10.1186/s13075-020-2141-2
14. Iudici M, van der Goes MC, Valentini G, Bijlsma JW. Glucocorticoids in systemic sclerosis: weighing the benefits and risks - A systematic review. Clin Exp Rheumatol. 2013;31(2):157–165.
15. Iudici M. What should clinicians know about the use of glucocorticoids in systemic sclerosis? Mod Rheumatol. 2017;27(6):919–923. doi:10.1080/14397595.2016.1270796
16. Kavanaugh A, Wells AF. Benefits and risks of low-dose glucocorticoid treatment in the patient with rheumatoid arthritis. Rheumatology. 2014;53:1742–1751. doi:10.1093/rheumatology/keu135
17. Yasir M, Goyal A, Bansal P, Sonthalia S. Corticosteroid Adverse Effects. StatPearls; 2021.
18. Thiebaut M, Launay D, Rivière S, et al. Efficacy and safety of rituximab in systemic sclerosis: French retrospective study and literature review. Autoimmun Rev. 2018;17(6):582–587. doi:10.1016/j.autrev.2017.12.010
19. Kowal-Bielecka O, Fransen J, Avouac J, et al. EUSTAR Coauthors. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327–1339. doi:10.1136/annrheumdis-2016-209909
20. Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–1755. doi:10.1136/annrheumdis-2013-204424
21. Weusten BL, Jacobs JW, Bijlsma JW. Corticosteroid pulse therapy in active rheumatoid arthritis. Semin Arthritis Rheum. 1993;23:183–192. doi:10.1016/S0049-0172(05)80039-3
22. Kimberly RP. Pulse methylprednisolone in SLE. Clin Rheum Dis. 1982;8(1):261–278. doi:10.1016/S0307-742X(21)00212-5
23. Khanna D, Furst DE, Clements PJ, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord. 2017;2:11–18. doi:10.5301/jsrd.5000231
24. Orlandi M, Landini N, Sambataro G, et al. The role of chest CT in deciphering interstitial lung involvement: systemic sclerosis versus COVID-19. Rheumatology. 2022;61(4):1600–1609. PMID: 34320649. doi:10.1093/rheumatology/keab615
25. Wangkaew S, Euathrongchit J, Wattanawittawas P, Kasitanon N. Correlation of delta high-resolution computed tomography (HRCT) score with delta clinical variables in early systemic sclerosis (SSc) patients. Quant Imaging Med Surg. 2016;6(4):381–390. doi:10.21037/qims.2016.08.08
26. Adler S, Huscher D, Siegert E, et al.; EUSTAR co-workers on behalf of the DeSScipher project research group within the EUSTAR network. Systemic sclerosis associated interstitial lung disease - individualized immunosuppressive therapy and course of lung function: results of the EUSTAR group. Arthritis Res Ther. 2018;20(1):17. doi:10.1186/s13075-018-1517-z
27. Chan KL, Currie PJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol. 1987;9(3):549–554. doi:10.1016/S0735-1097(87)80047-5
28. Fan Y, Wang D, Rao C, et al. Atorvastatin combined with low-dose dexamethasone treatment protects endothelial function impaired by chronic subdural hematoma via the transcription factor KLF-2. Drug Des Devel Ther. 2020;14:3291–3299. doi:10.2147/DDDT.S256050
29. Distler O, Highland KB, Gahlemann M, et al.; SENSCIS Trial Investigators. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380(26):2518–2528. PMID: 31112379. doi:10.1056/NEJMoa1903076
30. Maher TM, Mayes MD, Kreuter M, et al. Effect of nintedanib on lung function in patients with systemic sclerosis-associated interstitial lung disease: further analyses of a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2021;73(4):671–676. doi:10.1002/art.41576
31. Wigley FM, Boin F. Clinical features and treatment of scleroderma. In: Kelley and Firestein’s Textbook of Rheuma-Tology.
32. Connolly MK. Systemic sclerosis (scleroderma): remaining challenges. Ann Transl Med. 2021;9(5):438. doi:10.21037/atm-20-5449
33. German Network for Systemic Scleroderma Centers, Hunzelmann N, Moinzadeh P, Genth E, et al. High frequency of corticosteroid and immunosuppressive therapy in patients with systemic sclerosis despite limited evidence for efficacy. Arthritis Res Ther. 2009;11(2):R30. doi:10.1186/ar2634
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



