Back to Journals » Cancer Management and Research » Volume 12
Long Noncoding RNA SNHG7 Accelerates Proliferation, Migration and Invasion of Non-Small Cell Lung Cancer Cells by Suppressing miR-181a-5p Through AKT/mTOR Signaling Pathway
Authors Li L, Ye D, Liu L, Li X, Liu J, Su S, Lu W, Yu Z
Received 16 April 2020
Accepted for publication 12 August 2020
Published 10 September 2020 Volume 2020:12 Pages 8303—8312
DOI https://doi.org/10.2147/CMAR.S258487
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Liping Li, Dan Ye, Liang Liu, Xiaoju Li, Jun Liu, Shengtian Su, Wenjing Lu, Zhigao Yu
Department of Oncology, Xiantao First People’s Hospital, Xiantao, Hubei, People’s Republic of China
Correspondence: Zhigao Yu
Department of Oncology, Xiantao First People’s Hospital, No. 29, Middle Section of Mianzhou Avenue, Xiantao, Hubei 433000, People’s Republic of China
Tel +86 13477410999
Email [email protected]
Purpose: Non-small cell lung cancer (NSCLC) is a typical epithelial lung cancer with high metastasis, incidence and mortality. In recent years, long noncoding RNA small nucleolar RNA host gene 7 (SNHG7) has been identified as significant regulator in different cancer types, including NSCLC. However, the underlying molecular mechanism of SNHG7 during NSCLC tumorigenesis and progression remains largely unclear.
Methods: SNHG7 and miR-181a-5p expression in NSCLC tumors and cells were detected by qRT-PCR. Cell viability, migration, invasion and apoptosis were evaluated by CCK-8, transwell and flow cytometry assay, respectively. A549 and NCI-H1299 xenograft mice model was constructed by subcutaneously injecting cells stably transfected with sh-SNHG7 and sh-NC. The interaction between SNHG7 and miR-181a-5p was validated by luciferase reporter system, RIP and RNA pull down assay. Protein expression of cleaved caspase 3, proliferating cell nuclear antigen (PCNA), AKT, p-AKT, mammalian target of rapamycin (mTOR) and p-mTOR was analyzed by Western blot.
Results: SNHG7 expression was up-regulated while miR-181a-5p expression was down-regulated in NSCLC tumors, especially those from patients at Phase III+IV, compared with normal tissues. However, SNHG7 depletion attenuated tumor growth in vitro and in vivo. Moreover, miR-181a-5p inhibitor abolished SNHG7 silencing induced inhibition on proliferation, migration and invasion in NSCLC. Subsequently, we found SNHG7 modulated cell progression by targeting miR-181a-5p and activating AKT/mTOR signaling pathway.
Conclusion: SNHG7 accelerates proliferation, migration and invasion of NSCLC by suppressing miR-181a-5p through AKT/mTOR signaling pathway, thus presenting desirable biomarkers for NSCLC therapy.
Keywords: NSCLC, SNHG7, miR-181a-5p, AKT/mTOR pathway, progression
Introduction
Non-small cell lung cancer (NSCLC) which categorized as epithelial lung cancer is characterized by high metastasis, incidence and mortality.1,2 Despite multiple intervention strategies have improved the therapeutic outcomes of NSCLC patients at early stage, the long-term survival rates remain undesirable due to distant metastasis and delayed diagnosis.3–5 Recently, targeted therapy has been accepted as an effective individualized therapy for a variety of cancers, including NSCLC.6 Therefore, discovery of novel biomarkers for targeted therapy is urgently needed.
Long noncoding RNAs (lncRNAs) are significant modulators during tumorigenesis, cell cycle, infiltration, growth, inflammation, apoptosis and autophagy.7–9 Despite with limited protein-encoding ability, they participate in cell regulation by alteration of gene expression at post-transcriptional level, including translation, splicing, and intracellular/extracellular trafficking.10–12 Ectopic expression of lncRNAs plays pivotal roles in the pathogenesis of various diseases, including cardiac hypertrophy and cancer.13 LncRNA small nucleolar RNA host gene 7 (SNHG7), with 984 bp in length, functioned as oncogene in multiple cancers, such as neuroblastoma, hypopharyngeal and breast cancer.14,15 For instance, SNHG7 was involved in tumorigenesis and progression of breast cancer in vitro and in vivo by initiating epithelial to mesenchymal transition (EMT) and activating the Notch-1 pathway via sponging miR-34a.16 However, the underlying mechanism of SNHG7 in NSCLC aggravation requires further investigation.
MicroRNAs refer to small non-coding RNAs with 18–25 endogenous nucleotides in length.17 They play essential regulatory roles in many physiological and pathological processes by base pairing the target messenger RNA (mRNA) and leading to gene expression alteration at post-transcriptional level, including mRNA degradation and protein translation suppression.18–20 As tumor promotor or suppressor, miR-181a-5p is frequently diagnosed in multiple cancers. For example, overexpression of miR-181a-5p in cervical cancer facilitated proliferation, migration and repressed apoptosis via regulation of INPP5A.21 Conversely, miR-181a-5p was down-regulated in hepatocellular carcinoma as well as NSCLC and abundance of miR-181a-5p attenuated cell proliferation, migration and invasion by targeting Egr1 and Kras, respectively.22,23 However, the functional role of miR-181a-5p in NSCLC is still controversial.
In our study, we aimed to clarify the underlying regulatory mechanism of SNHG7/miR-181a-5p axis on NSCLC progression. Our results revealed that SNHG7 could accelerate proliferation, migration and invasion of NSCLC cells by targeting miR-181a-5p and activating AKT/mTOR signaling pathway.
Methods
Tissue Samples
A total of 36 NSCLC patients (phase I+II=16, phase III+IV=20) were recruited from Xiantao First People’s Hospital. NSCLC tumor tissues and the matched healthy tissues were obtained by surgery and immediately stored at −80°C until use. The participants have signed informed consent and the experiments were approved by Ethics Committee of Xiantao First People’s Hospital.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
NSCLC tumor tissues, normal tissues and cells were incubated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) to extract total RNA. The cDNA was synthesized by All-in-One™ First-Strand cDNA Synthesis Kit (FulenGen, Guangzhou, China). Then, qRT-PCR was performed using SYBR green (Applied Biosystems, Foster City, CA, USA). The expression of SNHG7 and miR-181a-5p was normalized by GAPDH and U6, respectively. The primers for SNHG7 miR-181a-5p, U6 and GAPDH were listed as follows: SNHG7 (Forward, 5ʹ-TTGCTGGCGTCTCGGTTAAT-3ʹ; Reverse, 5ʹ-GGAAGTCCATCACAGGCGAA-3ʹ); miR-181a-5p (Forward, 5ʹ-GCCGAACATTCAACGCTGTCG-3ʹ; Reverse, 5ʹ-GTGCAGGGTCCGAGGT-3ʹ); U6 (Forward, 5ʹ-ACCCTGAGAAATACCCTCACAT-3ʹ; Reverse, 5ʹ-GACGACTGAGCCCCTGATG-3ʹ); GAPDH (Forward, 5ʹ-TCTACATGTTCCAGTATGACTC-3ʹ; Reverse, 5ʹ-ACTCCACGACATACTCAGCACC-3ʹ).
Cell Culture and Transfection
NSCLC cell lines A549, NCI-H1299 and human bronchial epithelial cells BEAS-2B were purchased from ATCC (Manassas, VA, USA). Those cells were cultured in DMEM medium (Gibco, Carlsbad, CA, USA) containing 10% FBS and 0.05% penicillin/streptomycin. Small interfering RNA (siRNA) targeting SNHG7 (si-NHG7), small hairpin RNA (shRNA) targeting SNHG7 (sh-NHG7), siRNA negative control (si-NC), shRNA negative control (sh-NC), pcDNA-SNHG7 and pcDNA negative control (pcDNA-NC) were synthesized by Genepharma (Shanghai, China). The miRNA mimics (miR-181a-5p), miR-181a-5p inhibitor (anti-miR-181a-5p) and miRNA negative control inhibitor (anti-NC) were purchased from RIBOBIO (Guangzhou, China). All the vectors were transfected in A549 and NCI-H1299 cells using Lipofectamine 2000 (Invitrogen).
CCK-8, Flow Cytometry and Transwell Assay
Cell viability was measured by CCK-8 assay. Transfected A549 and NCI-H1299 cells were seeded in 96-well plates with density of 5000 cells/well for 24 h, 48 h and 72 h. Next, 10 μL CCK-8 reagent (Beyotime, Shanghai, China) was added for 2 h and OD value at 450 nm was measured by a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Cell apoptosis was detected by flow cytometry assay. In brief, A549 and NCI-H1299 cells were collected at 48 h post transfection and co-stained with Annexin V-FITC and PI using Apoptosis Detection Kit (Vazyme, Nanjing, China). The apoptotic rate was analyzed by BD FACS Canto II flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA). Cell migration and invasion abilities were evaluated by transwell assay. Transfected A549 and NCI-H1299 cells were placed on the upper chamber pre-treated with Matrigel (without Matrigel treatment for migration assay) (Becton Dickinson, Franklin Lakes, NJ, USA) for 48 h. Then, the cells at lower chamber were stained with 0.1% crystal violet (Sigma, St. Louis, MO, USA) for 10 min. The migration and invasion rate was counted using a microscope.
Murine Xenograft Assay
Male nude mice aged 4–5 weeks were purchased from Vital River Laboratory Animal Technology (Beijing, China). A549 and NCI-H1299 cells stably transfected with sh-NHG7 and sh-NC were subcutaneously injected in mice to establish xenograft mice model. Tumor volume was measured every 5 days and tumor weight was measured after the mice were sacrificed. All the animal experiment protocols were approved by the Xiantao First People’s Hospital Animal Ethics Committee. In addition, the animal experiments followed the ARRIVE guidelines and National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Luciferase Reporter Assay
Wild type (SNHG7-wt) and mutant type luciferase vectors (SNHG7-mut) were constructed. A549 and NCI-H1299 cells were co-transfected with SNHG7-wt or SNHG7-mut and miR-181a-5p mimics or NC for 24 h using Lipofectamine 2000 transfection reagent. Luciferase activities were evaluated by dual-luciferase assay.
RNA Immunoprecipitation (RIP) Assay
RIP assay was conducted using Magna RNA immunoprecipitation kit (Millipore, Temecula, California, USA). In brief, transfected A549 and NCI-H1299 cells were lysed by RIP buffer. Then, cell lysis was incubated with magnetic beads coated with anti-Ago2 or IgG antibody. The enrichment of SNHG7 and miR-181a-5p was subjected to qRT-PCR.
RNA Pull Down Assay
Biotinylated miR-181a-5p (Bio-miR-181a-5p) and negative control (Bio-NC) were transfected into A549 and NCI-H1299 cells for 48 h, followed by incubation with Dynabeads M-280 Streptavidin (Invitrogen) for 10 min. The expression of SNHG7 was measured by qRT-PCR.
Western Blot Assay
Western blot assay was performed following the standard protocol. The primary antibodies against cleaved caspase 3, AKT, p-AKT, mTOR and p-mTOR were purchased from Abcam (Cambridge, MA, USA) and HRP-conjugated secondary antibody was obtained from Sangon (Shanghai, China).
Statistical Analysis
Our experiments were repeated at least 3 times and the data were analysed by SPSS software (SPSS, Chicago, Illinois, USA) and GraphPad Prism 7 (GraphPad Inc., San Diego, CA, USA). The correlation between SNHG7 and miR-181a-5p was analyzed by Pearson’s correlation coefficient. P value less than 0.05 (P<0.05) was considered as statistically significant.
Results
Up-Regulation of SNHG7 and Down-Regulation of miR-181a-5p in NSCLC Tissues and Cells
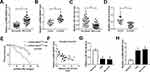
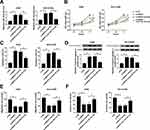
The expression of SNHG7 and miR-181a-5p in NSCLC tumors and the corresponding normal tissues were detected by qRT-PCR to illuminate the role of SNHG7 and miR-181a-5p. As illustrated in Figure 1A, H, SNHG7 expression was up-regulated in NSCLC tumors and cells (A549, NCI-H1299) compared with normal tissues and cells (BEAS-2B). On the contrary, miR-181a-5p expression was down-regulated in NSCLC tumors and cells in comparison with normal tissues and cells (Figure 1C, G). More specifically, SNHG7 expression was extremely higher in NSCLC patients at phase III+IV than that at phase I+II (Figure 1B). However, miR-181a-5p expression showed the opposite trend (Figure 1D). In addition, NSCLC patients with high SNHG7 expression showed a shorter 5-year overall survival than that with low SNHG7 expression (Figure 1E). After analyzed by Person’s correlation coefficient, we discovered that SNHG7 was correlated with miR-181a-5p inversely (R2=0.2778, P=0.001) (Figure 1F). Altogether, we assumed that SNHG7 could regulate miR-181a-5p negatively in NSCLC.
SNHG7 Depletion Suppresses Proliferation, Migration, Invasion and Promotes Apoptosis in NSCLC Cells
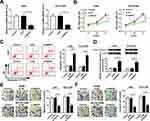
Loss-of-function experiments were performed by silencing SNHG7 to explore the effects of SNHG7 on NSCLC cell proliferation, migration, invasion and apoptosis. As exhibited in Figure 2A, SNHG7 expression was decreased in A549 and NCI-H1299 cells transfected with si-SNHG7 with respect to si-NC group, indicating the transfection efficiency was relatively high. CCK-8 and flow cytometry results revealed that SNHG7 silencing attenuated proliferation and facilitated apoptosis of NSCLC cells (Figure 2B–C). As expected, the expression of apoptosis associated protein cleaved caspase 3 was elevated after SNHG7 knockdown (Figure 2D). On consistent with CCK-8 assay, transwell assay demonstrated that cell migration and invasion abilities were retarded after SNHG7 knockdown in NSCLC cells (Figure 2E and F). These findings clarified that SNHG7 might act as an oncogene in NSCLC.
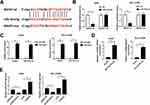
Interference of SNHG7 Inhibits Tumor Growth
The influences of SNHG7 on NSCLC cell progression were evaluated in vivo by monitoring tumor growth of sh-SNHG7 xenograft mice. As displayed in Figure 3A and C, tumor growth was attenuated within 30 d in xenograft mice harboring sh-SNHG7 compared with sh-NC group. Meanwhile, tumor weight of sh-SNHG7 group was obviously lower than that of sh-NC group (Figure 3B and D). In addition, we noticed that SNHG7 level was reduced dramatically in tumor tissues from sh-SNHG7 xenograft mice (Figure 3E). To disclose the underlying mechanism, we analyzed the expression of proliferation-related protein PCNA and apoptosis-related protein cleaved caspase 3 by Western blot. Apparently, PCNA was reduced whereas cleaved caspase 3 was enhanced in tumor tissues after SNHG7 silencing (Figure 3F–G). Taking together, SNHG7 knockdown suppresses tumor growth in vivo by inhibition of proliferation and acceleration of apoptosis in NSCLC.
SNHG7 is a Sponger of miR-181a-5p
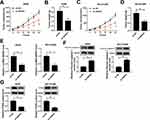
Based on bioinformatics analysis by starBase v2.0, miR-181a-5p contains the binding sites of SNHG7 (Figure 4A). To confirm the prediction, dual-luciferase reporter system was constructed by co-transfecting SNHG7-wt or SNHG7-mut and miR-181a-5p or NC in A549 and NCI-H1299 cells. A considerable decrease of luciferase activity was found in NSCLC cells co-transfecting with SNHG7-wt and miR-181a-5p. However, luciferase activity showed negligible change in SNHG7-mut group (Figure 4B). More importantly, SNHG7 and miR-181a-5p were largely enriched by Ago2 antibody in comparison with IgG antibody, implicating SNHG7 could directly bind to miR-181a-5p (Figure 4C). Additionally, the enrichment of SNHG7 was increased in NSCLC cells transfected with Bio-miR-181a-5p (Figure 4D). We further found that abundance of SNHG7 reduced miR-181a-5p expression while depletion of SNHG7 enhanced miR-181a-5p expression in A549 and NCI-H1299 cells (Figure 4E). All the data demonstrated SNHG7 was a sponger of miR-181a-5p.
SNHG7 Regulates Proliferation, Migration, Invasion and Apoptosis by Sponging miR-181a-5p in NSCLC Cells
To investigate the regulatory effects of SNHG7/miR-181a-5p axis on NSCLC cell progression, A549 and NCI-H1299 cells were transfected with si-SNHG7, si-SNHG7+anti-miR-181a-5p, si-SNHG7+anti-NC or si-NC. The expression of miR-181a-5p was boosted by SNHG7 silencing and repressed by miR-181a-5p inhibitor in A549 and NCI-H1299 cells (Figure 5A). The rescue experiments revealed that miR-181a-5p inhibitor restored SNHG7 silencing induced inhibition on NSCLC cell proliferation (Figure 5B). Moreover, overexpression of miR-181a-5p-induced apoptosis, but miR-181a-5p inhibitor reversed the effects (Figure 5C). As expected, protein expression of cleaved caspase 3 was up-regulated by SNHG7 silencing and down-regulated by miR-181a-5p inhibitor (Figure 5D). Additionally, cell migration and invasion ability were inhibited by SNHG7 silencing and rescued by miR-181a-5p inhibitor (Figure 5E–F). Collectively, SNHG7 promotes proliferation, migration and invasion but inhibits apoptosis by targeting miR-181a-5p in NSCLC cells.
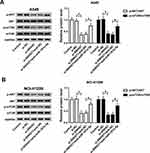
SNHG7 Regulates Cell Progression in NSCLC by Targeting miR-181a-5p Through AKT/mTOR Pathway
It is well acknowledged that AKT/mTOR pathway plays fundamental roles in the growth of a variety of cancers. Therefore, we assumed that SNHG7/miR-181a-5p axis modulates cell progression in NSCLC by regulation of AKT/mTOR pathway. Western blot results showed that the expression of p-mTOR and p-AKT was significantly decreased in A549 and NCI-H1299 cells after transfection with miR-181a-5p (Figure S1). Moreover, protein expression of p-AKT and p-mTOR were repressed in A549 and NCI-H1299 cells transfected with si-SNHG7. Interestingly, miR-181a-5p inhibitor abolished SNHG7 silencing induced inhibition on p-AKT and p-mTOR protein expression (Figure 6A and B). Therefore, we considered that SNHG7 regulated cell progression in NSCLC by targeting miR-181a-5p through AKT/mTOR pathway.
Discussion
Accumulating evidences have identified that SNHG7 which mapped on chromosome 9q34.3 contributed to carcinogenesis, development and poor prognosis of many cancers, like renal cell carcinoma, hepatocellular carcinoma and lung cancer.24 For example, SNHG7 facilitated proliferation, migration and invasion of pancreatic and breast cancer by interacting with miR-342-3p/ID4 axis and microRNA-186, respectively.25,26 Consistently, SNHG7 contributed to cell progression in osteosarcoma by inhibition of p53 expression through targeting DNMT1.27 By contrast, decreased expression of SNHG7 repressed bladder cancer cell proliferation, migration and G0/G1 cell cycle arrest through activation of Wnt/β-catenin pathway.28 Similarly, knockdown of SNHG7 hindered proliferation and induced apoptosis processes by suppressing BDNF in thyroid cancer cells.29 Thereby, we supposed that SNHG7 participates in NSCLC cell progression through interacting with the target gene.
Bioinformatics analysis tools starBase v2.0 predicted that miR-181a-5p contains the binding sites of SNHG7. In recent years, miR-181a-5p has been identified as vital diagnostic and prognostic biomarker in different diseases, such as pregnancy-related disease, brucellosis and cancer.30–32 For instance, up-regulation of miR-181a-5p expression enhanced cell growth and development in gastric cancer by targeting RASSF6 and activating MAPK signaling.33 Likewise, miR-181a-5p served as Wnt-signaling inducer in acute lymphoblastic leukemia to accelerate cell progression.34 Oppositely, miR-181a-5p functioned as tumor suppressor to inhibit motility, invasion and branching morphogenesis of hepatocellular carcinoma by regulating c-Met.35 Therefore, the regulatory effects of miR-181a-5p in NSCLC proliferation, migration, invasion and apoptosis require in-depth understanding.
We hypothesized that SNHG7 accelerates cell growth in NSCLC by targeting miR-181a-5p. The expression of SNHG7 and miR-181a-5p was measured by qRT-PCR to discover the role of them in NSCLC. Up-regulation of SNHG7 and down-regulation of miR-181a-5p were observed in NSCLC tumors and cells compared with the matched normal tissues and cells. As expected, SNHG7 was correlated with miR-181a-5p inversely. Subsequently, loss-of-function experiments were conducted by SNHG7 knockdown to reveal the function of SNHG7. We found that cell growth was attenuated while apoptosis was improved in vitro and in vivo after SNHG7 silencing in NSCLC. In addition, luciferase reporter system, RIP and RNA pull down assay validated that SNHG7 was directly interacted with miR-181a-5p. What is more, the rescue experiments clarified that miR-181a-5p inhibitor reversed the suppressive effects of SNHG7 silencing on proliferation, migration and invasion of NSCLC cells. Interestingly, we found SNHG7 participated in NSCLC cell regulation by targeting miR-181a-5p to alter AKT/mTOR signaling pathway, further disclosed the underlying molecular mechanism.
Conclusion
In conclusion, we demonstrated that SNHG7 promoted proliferation, migration and invasion but hampered apoptosis by interacting with miR-181a-5p in NSCLC cells. SNHG7 depletion suppressed cell growth and induced apoptosis both in vitro and in vivo. Moreover, the functions of SNHG7/miR-181a-5p axis were exerted by regulation of AKT/mTOR signaling pathway. Our study illuminated the underlying regulatory mechanism of SNHG7/miR-181a-5p axis, thereby providing novel biomarkers for the therapy of NSCLC.
Acknowledgments
The authors would like to thank the participants in this study.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Huang J, Xie N, Huang H, et al. Long noncoding RNA STXBP5-AS1 inhibits cell proliferation, migration, and invasion via preventing the PI3K/AKT against STXBP5 expression in non-small-cell lung carcinoma. J Cell Biochem. 2018. doi:10.1002/jcb.28023
2. Li D, Luo Y, Gao Y, et al. piR-651 promotes tumor formation in non-small cell lung carcinoma through the upregulation of cyclin D1 and CDK4. Int J Mol Med. 2016;38(3):927–936. doi:10.3892/ijmm.2016.2671
3. Giroux Leprieur E, Vieira T, Antoine M, et al. Sonic Hedgehog Pathway Activation Is Associated With Resistance to Platinum-Based Chemotherapy in Advanced Non-Small-Cell Lung Carcinoma. Clin Lung Cancer. 2016;17(4):301–308. doi:10.1016/j.cllc.2015.12.007
4. Mi J, Zou Y, Lin X, et al. Dysregulation of the miR-194-CUL4B negative feedback loop drives tumorigenesis in non-small-cell lung carcinoma. Mol Oncol. 2017;11(3):305–319. doi:10.1002/1878-0261.12038
5. Qiao W, Han Y, Jin W, et al. Overexpression and biological function of TMEM48 in non-small cell lung carcinoma. Tumour Biol. 2016;37:2575–2586. doi:10.1007/s13277-015-4014-x
6. Xu J, Du Y, Liu XJ, et al. Recombinant EGFR/MMP-2 bi-targeted fusion protein markedly binding to non-small-cell lung carcinoma and exerting potent therapeutic efficacy. Pharmacol Res. 2017;126:66–76. doi:10.1016/j.phrs.2017.04.001
7. Andresini O, Rossi MN, Matteini F, et al. The long non-coding RNA Kcnq1ot1 controls maternal p57 expression in muscle cells by promoting H3K27me3 accumulation to an intragenic MyoD-binding region. Epigenetics Chromatin. 2019;12(1):8. doi:10.1186/s13072-019-0253-1
8. He C, Lu X, Yang F, et al. LncRNA UCA1 acts as a sponge of miR-204 to up-regulate CXCR4 expression and promote prostate cancer progression. Biosci Rep. 2019:39. doi:10.1042/BSR20181465
9. Kong L, Wu Q, Zhao L, et al. Upregulated lncRNA-UCA1 contributes to metastasis of bile duct carcinoma through regulation of miR-122/CLIC1 and activation of the ERK/MAPK signaling pathway. Cell Cycle. 2019:1–17. doi:10.1080/15384101.2019.1593647
10. Ke N, Pi LH, Liu Q, et al. Long noncoding RNA SNHG7 inhibits high glucose-induced human retinal endothelial cells angiogenesis by regulating miR-543/SIRT1 axis. Biochem Biophys Res Commun. 2019;514(2):503–509. doi:10.1016/j.bbrc.2019.04.141
11. Shao J, Pan X, Yin X, et al. KCNQ1OT1 affects the progression of diabetic retinopathy by regulating miR-1470 and epidermal growth factor receptor. J Cell Physiol. 2019;234(10):17269–17279. doi:10.1002/jcp.28344
12. Gao J, Liu L, Li G, et al. LncRNA GAS5 confers the radio sensitivity of cervical cancer cells via regulating miR-106b/IER3 axis. Int J Biol Macromol. 2019;126:994–1001. doi:10.1016/j.ijbiomac.2018.12.176
13. Jing L, Li S, Wang J, et al. Long non-coding RNA small nucleolar RNA host gene 7 facilitates cardiac hypertrophy via stabilization of SDA1 domain containing 1 mRNA. J Cell Biochem. 2019;120(9):15089–15097. doi:10.1002/jcb.28770
14. Chi R, Chen X, Liu M, et al. Role of SNHG7-miR-653-5p-STAT2 feedback loop in regulating neuroblastoma progression. J Cell Physiol. 2019;234(8):13403–13412. doi:10.1002/jcp.28017
15. Wu P, Tang Y, Fang X, et al. Metformin Suppresses Hypopharyngeal Cancer Growth by Epigenetically Silencing Long Non-coding RNA SNHG7 in FaDu Cells. Fron Pharmacol. 2019;10:143. doi:10.3389/fphar.2019.00143
16. Sun X, Huang T, Liu Z, et al. LncRNA SNHG7 contributes to tumorigenesis and progression in breast cancer by interacting with miR-34a through EMT initiation and the Notch-1 pathway. Eur J Pharmacol. 2019;856:172407. doi:10.1016/j.ejphar.2019.172407
17. Cheng X, Du J, Shen L, et al. MiR-204-5p regulates C2C12 myoblast differentiation by targeting MEF2C and ERRgamma. Biomed Pharmacother. 2018;101:528–535. doi:10.1016/j.biopha.2018.02.096
18. Gao W, Wu Y, He X, et al. MicroRNA-204-5p inhibits invasion and metastasis of laryngeal squamous cell carcinoma by suppressing forkhead box C1. J Cancer. 2017;8(12):2356–2368. doi:10.7150/jca.19470
19. Mokabber H, Najafzadeh N, Mohammadzadeh Vardin M. miR-124 promotes neural differentiation in mouse bulge stem cells by repressing Ptbp1 and Sox9. J Cell Physiol. 2019;234(6):8941–8950. doi:10.1002/jcp.27563
20. Fujii T, Shimada K, Asano A, et al. MicroRNA-331-3p Suppresses Cervical Cancer Cell Proliferation and E6/E7 Expression by Targeting NRP2. Int J Mol Sci. 2016:17. doi:10.3390/ijms17081351
21. Yang M, Zhai X, Ge T, et al. miR-181a-5p Promotes Proliferation and Invasion and Inhibits Apoptosis of Cervical Cancer Cells via Regulating Inositol Polyphosphate-5-Phosphatase A (INPP5A). Oncol Res. 2018;26(5):703–712. doi:10.3727/096504017X14982569377511
22. Ma Z, Qiu X, Wang D, et al. MiR-181a-5p inhibits cell proliferation and migration by targeting Kras in non-small cell lung cancer A549 cells. Acta Biochim Biophys Sin (Shanghai). 2015;47(8):630–638. doi:10.1093/abbs/gmv054
23. Bi JG, Zheng JF, Li Q, et al. MicroRNA-181a-5p suppresses cell proliferation by targeting Egr1 and inhibiting Egr1/TGF-β/Smad pathway in hepatocellular carcinoma. Int J Biochem Cell Biol. 2019;106:107–116. doi:10.1016/j.biocel.2018.11.011
24. Xiang H, Yan H, Sun B, et al. Decreased expression of long non-coding RNA SNHG7 cause recurrent spontaneous abortion through suppression proliferation and invasion of trophoblast cells via miR-34a. Am J Transl Res. 2019;11(1):463–472.
25. Cheng D, Fan J, Ma Y, et al. LncRNA SNHG7 promotes pancreatic cancer proliferation through ID4 by sponging miR-342-3p. Cell Biosci. 2019;9(1):28. doi:10.1186/s13578-019-0290-2
26. Luo X, Song Y, Tang L, et al. LncRNA SNHG7 promotes development of breast cancer by regulating microRNA-186. Eur Rev Med Pharmacol Sci. 2018;22(22):7788–7797. doi:10.26355/eurrev_201811_16403
27. Zhang GD, Gai PZ, Liao GY, et al. LncRNA SNHG7 participates in osteosarcoma progression by down-regulating p53 via binding to DNMT1. Eur Rev Med Pharmacol Sci. 2019;23(9):3602–3610. doi:10.26355/eurrev_201905_17782
28. Chen Y, Peng Y, Xu Z, et al. Knockdown of lncRNA SNHG7 inhibited cell proliferation and migration in bladder cancer through activating Wnt/beta-catenin pathway. Pathol Res Pract. 2019;215(2):302–307. doi:10.1016/j.prp.2018.11.015
29. Wang YH, Peng Y, Xu Z, et al. Knockdown of long noncoding RNA SNHG7 inhibits the proliferation and promotes apoptosis of thyroid cancer cells by downregulating BDNF. Eur Rev Med Pharmacol Sci. 2019;23(11):4815–4821. doi:10.26355/eurrev_201906_18067
30. Wu L, Song WY, Xie Y, et al. miR-181a-5p suppresses invasion and migration of HTR-8/SVneo cells by directly targeting IGF2BP2. Cell Death Dis. 2018;9(2):16. doi:10.1038/s41419-017-0045-0
31. Corsetti PP, De Almeida LA, Goncalves ANA, et al. miR-181a-5p Regulates TNF-alpha and miR-21a-5p Influences Gualynate-Binding Protein 5 and IL-10 Expression in Macrophages Affecting Host Control of Brucella abortus Infection. Front Immunol. 2018;9:1331. doi:10.3389/fimmu.2018.01331
32. Meijer LL, Garajova I, Caparello C, et al. Plasma miR-181a-5p Downregulation Predicts Response and Improved Survival After FOLFIRINOX in Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018. doi:10.1097/SLA.0000000000003084
33. Mi Y, Zhang D, Jiang W, et al. miR-181a-5p promotes the progression of gastric cancer via RASSF6-mediated MAPK signalling activation. Cancer Lett. 2017;389:11–22. doi:10.1016/j.canlet.2016.12.033
34. Lyu X, Li J, Yun X, et al. miR-181a-5p, an inducer of Wnt-signaling, facilitates cell proliferation in acute lymphoblastic leukemia. Oncol Rep. 2017;37(3):1469–1476. doi:10.3892/or.2017.5425
35. Korhan P, Erdal E, Atabey N. MiR-181a-5p is downregulated in hepatocellular carcinoma and suppresses motility, invasion and branching-morphogenesis by directly targeting c-Met. Biochem Biophys Res Commun. 2014;450(4):1304–1312. doi:10.1016/j.bbrc.2014.06.142
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.