Back to Journals » Cancer Management and Research » Volume 12
Long Non-Coding RNA SNHG14 Regulates SPIN1 Expression to Accelerate Tumor Progression in Non-Small Cell Lung Cancer by Sponging miR-382-5p
Authors Chen X, Song P, Yao Y, Yang Y
Received 22 February 2020
Accepted for publication 26 June 2020
Published 25 September 2020 Volume 2020:12 Pages 9113—9123
DOI https://doi.org/10.2147/CMAR.S250893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Xiaoliang Chen, Pingan Song, Yuan Yao, Yang Yang
Department of Thoracic Surgery, Gansu Gem Flower Hospital, Lanzhou 730060, Gansu, People’s Republic of China
Correspondence: Xiaoliang Chen Department of Thoracic Surgery
Gansu Gem Flower Hospital, 733 Welfare East Road, Xigu District, Lanzhou City, Gansu Province 730060, People’s Republic of China
Tel +86-931-7995570
Email [email protected]
Background: Non-small cell lung cancer (NSCLC) is the most common type of lung carcinoma. Long non-coding RNA (lncRNA) small nucleolar RNA host gene 14 (SNHG14) was identified to participate in tumor progression. However, the mechanism and functions of SNHG14 were rarely reported in NSCLC progression.
Methods: The relative gene expression was tested by qRT-PCR. Cell viability, apoptosis, migration and invasion were measured by MTT assay, flow cytometry, and transwell migration and invasion assays, respectively. The interactions between miR-382-5p and SNHG14 or SPIN1 were predicted by starBase and confirmed by the dual-luciferase reporter assay and RNA pull-down assay. The protein level of SPIN1 was evaluated by Western blot assay.
Results: The levels of SNHG14 and SPIN1 were significantly increased, while the level of miR-382-5p was apparently reduced in NSCLC tissues and cells. SNHG14 was verified to sponge miR-382-5p and SPIN1 was identified as a direct target of miR-382-5p. SNHG14 depletion repressed cell viability, migration and invasion, but induced the apoptotic rate by targeting miR-382-5p. miR-382-5p overexpression blocked cell viability, metastasis and promoted cell apoptosis by regulating SPIN1. SNHG14 silencing down-regulated SPIN1 expression by sponging miR-382-5p.
Conclusion: SNHG14 facilitated NSCLC progression by regulating SPIN1 expression via targeting miR-382-5p.
Keywords: lncRNA SNHG14, miR-382-5p, SPIN1, tumor progression, NSCLC
Introduction
Lung cancer, the most leading causes of cancer-associated death overall the world, is divided into two groups: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) which accounted for about 80–85% in all lung cancer patients.1,2 Despite the improvements in surgeries and therapies in recent decades, the 5-year overall survival of NSCLC is just approximately 15%.3 One of the most important reasons is metastasis.4 Hence, it is crucial to explore the mechanism of NSCLC.
Long non-coding RNAs (lncRNAs), a form of long RNA (>200 nucleotides (nt)) with no translation capacity, have been identified to affect target gene expression at posttranscriptional stage and to implicate in the processes of tumor development.5 In NSCLC, lots of lncRNAs were documented to aberrant expression. For instance, a study indicated that MALAT1 was elevated in NSCLC, and its depletion suppressed metastasis and cell growth in vitro and also restrained xenograft tumor growth in vivo.6 Liu et al reported that the high level of HOTAIR in NSCLC promoted cell metastasis.7 The similar results of ANRIL8 and UCA19 were also reported in NSCLC. Small nucleolar RNA host gene 14 (SNHG14) is located on human chromosome 15 and reported to accelerate tumor development in many types of cancers, including colorectal cancer,10 ovarian cancer,11 bladder cancer,12 and gastric cancer.13 However, the mechanism of SNHG14 was rarely reported in NSCLC.
MicroRNAs (miRNAs) are a family of small RNA (~22 nt) without translation ability and could target message RNA (mRNA) to decrease target gene expression.14 Also, many miRNAs were reported to be dysregulated in NSCLC. For example, miR-451 was significantly down-regulated in NSCLC, and its overexpression triggered apoptosis but inhibited cell growth.15 The similar results of miR-145,15 miR-193a-3p/5p16 were reported in NSCLC. Chen et al documented that miR-382 was strikingly reduced in NSCLC, and miR-382 overexpression blocked cell growth, metastasis by targeting SETD8 in NSCLC.17 Nevertheless, the mechanism of miR-382-5p was not clear in NSCLC.
Spindlin-1 (SPIN1) is located on human chromosome 9, and the ectopic expression of SPIN1 was documented to be associated with tumor progression. A research in glioma implied that SPIN1 facilitated cell growth while constrained apoptosis regulated by miR-489.18 Another reporter in breast cancer demonstrated that SPIN1 enhanced cell growth, metastasis mediated by miR-1271 in vitro.19 However, the mechanism of SPIN1 in NSCLC was scarcely documented. In this research, the mechanism of SNHG14 in NSLSC was our main study point.
Materials and Methods
Tissue Samples
Fifty NSCLC tissue samples were collected from Gansu Gem Flower Hospital, as well as the corresponding adjacent normal tissue samples. The clinicopathologic features of these patients are presented in Table 1. All collected tissue samples were immediately frozen in −80°C refrigerator until used. The project was authorized by the Ethics Committee of Gansu Gem Flower Hospital and carried out according to the Declaration of Helsinki Principles. All patients were provided informed consents.
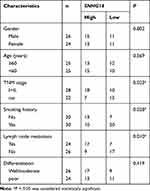 |
Table 1 Correlation Between Clinicopathological Characteristics and SNHG14 Expression Level in NSCLC Patients |
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The RNA samples were extracted from NSCLC tissues and cells using TriQuick Reagent (Solarbio, Beijing, China). The random primers (Solarbio) were used for reverse transcription, and the quantitative PCR was performed using SYBR mix (TaKaRa, Dalian, China). The level of SNHG14 and SPIN1 was normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) while miR-382-5p was normalized via small nuclear RNA U6, and processed with the method of 2−ΔΔCt. The primers were obtained from Songon (Shanghai, China) and exhibited as follows: SNHG14: (GeneBank accession No. NR_146177; F, 5ʹ-ACCTGCAAGCTTTTTGACCC-3ʹ, and R, 5ʹ-AGCAGACAAAGAAAAACCCCAAT-3ʹ); miR-382-5p: (GeneBank accession No. NR_029874.1; F, 5ʹ-TAAAATAGGAGTACTGTCTAA-3ʹ, and R, 5ʹ-ATTAGTAAATTGGCTGCTGCAG-3ʹ); SPIN1: (GeneBank accession No. NM_006717.3; F, 5ʹ-GAAGGTGAAGGTCGGAGT-3ʹ and 5ʹ-GATGGCAACAATATCCACTT-3ʹ); GAPDH: (F, 5ʹ-ACCACAGTCCATGCCATCAC-3ʹ, and R, 5ʹ-TCCACCACCCTGTTGCTGTA-3ʹ), and U6: (F, 5ʹ-ATTGGAACGATACAGAGAAGATT-3ʹ, and R, 5ʹ-GGAACGCTTCACGAATTTG-3ʹ).
Cell Culture and Transfection
Two human non-small cell lung cancer cells (H1299, catalog #CBP30016L, and A549, catalog #CBP60084) and human pulmonary bronchial epithelium cell BEAS-2B (catalog #CBP60577) were purchased from Cobioer (Nanjing, China). All cells were cultivated in RPMI‑1640 medium (Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% penicillin/streptomycin (Solarbio) in 5% CO2 incubator at 37°C.
Small interfering RNA (siRNA) against SNHG14 (si-SNHG14, GeneBank accession No. NR_146177; 5ʹ-GCACAAUAUCUUUGAACUA-3ʹ) and its negative control (si-NC), miR-382-5p mimics (miR-382-5p) and its negative control (miR-NC), miR-382-5p antagonist (miR-382-5p inhibitor) and its matched control (inhibitor-NC) were synthesized in GenePharma (Shanghai, China). The sequences of SPIN1 were cloned and inserted into pcDNA (GenePharma) to construct SPIN1 overexpression plasmid (pcDNA-SPIN1). The transfection was performed using Lip2000 Transfection Reagent (Solarbio).
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) Assay
MTT (Solabio) was used to detect the cell viability of H1299 and A549 cells. The H1299 and A549 cells (2×103 per well) were firstly cultivated in 96-well plate for 24 h. Following the transfection, the transfected H1299 and A549 cells were cultured for another 0 h, 24 h, 48 h, and 72 h. After centrifugation, MTT was injected and incubated for another 4 h. Then, dimethyl sulfoxide (DMSO) was added to dissolve the formazan. The absorbance at 490 nm was measured on a Multiscan Spectrum.
Flow Cytometry Analysis of Cell Apoptosis
The apoptotic rate of H1299 and A549 cells was assessed using Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Solarbio). The H1299 and A549 cells (1×105) were resuspended in binding buffer containing Annexin V-FITC and incubated for 10 min at 37°C in the dark. Then, the cell samples were incubated with PI for another 5 min in the dark. The apoptotic rate was evaluated through flow cytometry within 1 h.
Transwell Assay
Transwell chambers (8.0 μm; Solarbio) were used to test the migrated and invaded abilities of H1299 and A549 cells. For cell migration, the lower chamber was injected with RPMI‑1640 medium containing 10% FBS, while the upper one was added H1299 and A549 cells (5×103) into RPMI‑1640 medium without FBS and cultivated for 48 h. The cells on the backside of polycarbonate film were fixed and stained with 4% methanol and 0.1% crystal violet, respectively. The random 8 fields were selected, and the cells were counted under a microscope.
For cell invasion, the protocols were similar to that in cell migration. The difference was the upper chamber was covered with a Matrigel matrix (Solarbio).
Dual-Luciferase Reporter Assay
The wild type and mutant sequences of SNHG14, 3ʹ-untranslated regions (3ʹUTR) of SPIN1 were inserted into pmirGLO vector (Youbio, Changsha, China) to construct luciferase reporters, namely SNHG14-WT, SNHG14-MUT, SPIN1 3ʹUTR-WT, or SPIN1 3ʹUTR-MUT. The luciferase report and miR-NC or miR-382-5p were co-transfected into H1299 and A549 cells using Lip2000 Transfection Reagent (Solarbio). A luciferase reporter detection kit (Solarbio) was utilized to detect the luciferase activity.
RNA Pull-Down Assay
Biotin-labeled miR-382-5p (Bio-miR-382-5p) and negative control (Bio-miR-NC) were obtained from RiBoBio (Guangzhou, China). The Bio-miR-382-5p or Bio-miR-NC was added into the lysate samples of H1299 and A549 cells. The streptavidin magnetic beads were used to recruit Bio-labelled RNA-RNA complex. After elution, the level of SNHG14 was evaluated by qRT-PCR.
Western Blot Assay
The protein in H1299 and A549 cells was extracted using a protein extraction kit (Beyotime, Shanghai, China). Then, the concentration of protein samples was detected using BCA protein assay kit (Beyotime), and the protein samples were separated via sodium dodecyl sulfonate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene fluoride (PVDF; GE Healthcare, Piscataway, NJ, USA) membrane. Subsequently, the membrane was sealed in skim milk for 4 h and then incubated with primary antibody overnight at 4°C. The membrane was incubated with secondary antibody for another 2 h at 37°C. The intensity of bands was measured using eyoECL plus kit (Beyotime). The rabbit primary antibody SPIN1 (1/500; ab196938), β-actin (1/1000; ab8227), and goat anti-rabbit secondary antibody (1/10,000; ab175781) were purchased from Abcam (Cambridge, MA, USA).
Statistical Analysis
GraphPad Prism 7 (GraphPad Inc., La Jolla, CA, USA) was used to process the experimental data which were repeated for at least three times. The Student’s t-test was used to compare the data between the two groups, and one-way analysis of variance (ANOVA) was utilized to analyze the comparison among multiple groups. P-value less than 0.05 was considered as statistically different.
Results
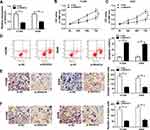
SNHG14 Was Highly Expressed in NSCLC Tissues and Cells
In order to search the characters of SNHG14 in NSCLC, we firstly examined the level of SNHG14 in NSCLC tissues and cells. The level of SNHG14 was increased in fifty NSCLC tissues related to that in corresponding adjacent normal tissues (Figure 1A). Also, SNHG14 was obviously up-regulated in H1299 and A549 cells compared to that in normal lung cell BEAS-2B (Figure 1B). Kaplan-Meier survival analysis revealed that patients with low SNHG14 level had longer overall survival time than those in high SNHG14 level group (Figure 1C). These results indicated that SNHG14 may play a vital role in NSCLC.
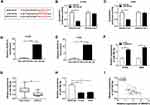
SNHG14 Knockdown Constrained Cell Proliferation, Migration, and Invasion While Induced Apoptosis in NSCLC Cells
Next, we investigated the functions of SNHG14 in NSCLC. Thus, the si-SNHG14 was transfected into H1299 and A549 cells. The knockdown efficiency was confirmed by qRT-PCR, indicated by the apparent decrease of SNHG14 in H1299 and A549 cells transfected with si-SNHG14 (Figure 2A). Following MTT assay exhibited that the transfection of si-SNHG14 resulted in the striking reduction of cell viability in NSCLC cells in comparison with that in si-NC group (Figure 2B and C). While the apoptotic rate showed the opposite trend. Briefly, the apoptotic rate was dramatically elevated in NSCLC cells transfected with si-SNHG14 (Figure 2D). Besides, the migrated and invaded abilities were distinctly declined in si-SNHG14-transfected NSCLC cells (Figure 2E and F). Taken together, SNHG14 silencing repressed cell proliferation, metastasis, but facilitated apoptosis in NSCLC cells.
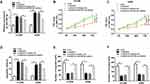
miR-382-5p Was Negatively Interacted with SNHG14 in NSCLC Cells
To illustrate the potential mechanism of SNHG14 in NSCLC, starBase online database (http://starbase.sysu.edu.cn/starbase2/index.php) was used to predict the putative candidates of SNHG14. The results showed that miR-382-5p had complementary binding sites with SNHG14 (Figure 3A). Subsequently, the transfection of miR-382-5p in NSCLC cells contributed to the conspicuous down-regulation of luciferase activity of SNHG14-WT reporter in contrast to that in miR-NC group, while the luciferase activity of SNHG14-MUT reporter had no apparent fluctuation in any group (Figure 3B and C). Besides, Bio-miR-382-5p enriched much more SNHG14 in H1299 and A549 cells compared with that in Bio-miR-NC group (Figure 3D and E). Meanwhile, the level of miR-382-5p was remarkably enhanced in H1299 and A549 cells transfected with si-SNHG14 (Figure 3F). In addition, the level of miR-382-5p was drastically reduced in NSCLC tissues and cells (Figure 3G and H). The scatter plot exhibited that miR-382-5p was negatively linear correlated with SNHG14 (Figure 3I). These data disclosed that SNHG14 sponged miR-382-5p in NSCLC cells.
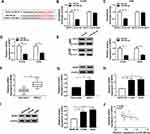
SNHG14 Depletion Regulated Cell Behaviors in NSCLC Cells by Regulating miR-382-5p
To investigate the functions of SNHG14 and miR-382-5p in NSCLC, si-SNHG14 and miR-382-5p inhibitor were co-transfected into NSCLC cells. As presented in Figure 4A, the level of miR-38-5p was notably elevated in NSCLC cells transfected with si-SNHG14, while the introduction of miR-382-5p inhibitor mitigated the level of miR-382-5p in NSCLC cells. Furthermore, the introduction of miR-382-5p inhibitor attenuated the inhibitory impact on cell viability in NSCLC cells repressed by si-SNHG14 (Figure 4B and C). While the apoptotic rate was evidently facilitated in si-SNHG14-transfected H1299 and A549 cells, but weakened by the introduction of miR-382-5p inhibitor (Figure 4D). Besides, the Transwell assay demonstrated that the transfection of miR-382-5p inhibitor rescued the restraint effects on the migrated and invaded abilities in H1299 and A549 cells blocked by si-SNHG14 (Figure 4E and F). These results revealed that miR-382-5p inhibitor counteracted the suppressive effects on cell proliferation, migration, and invasion as well as the promoted effect on apoptosis in NSCLC cells caused by si-SNHG14.
SPIN1 Was a Target of miR-382-5p in NSCLC Cells
To explore the underlying mechanism of miR-382-5p in NSCLC, starBase was utilized to search the putative candidate of miR-382-5p. As exhibited in Figure 5A, SPIN1 3ʹUTR had complementary binding sites with miR-382-5p. Also, the luciferase activity of SPIN1 3ʹUTR-WT reporter was obviously reduced in NSCLC cells transfected with miR-382-5p, while the luciferase activity of SPIN1 3ʹUTR-MUT reporter had no significant change in any group (Figure 5B and C). Meanwhile, the levels of SPIN1 mRNA and protein were notably declined in NSCLC cells transfected with miR-382-5p compared to that in miR-NC group (Figure 5D and E). Besides, the levels of SPIN1 mRNA and protein were effectively up-regulated in NSCLC tissues and cells (Figure 5F–I). The scatter diagram displayed that SPIN1 was negatively linear correlated with miR-382-5p (Figure 5J). To sum, these data indicated that SPIN1 was negatively interacted with miR-382-5p in NSCLC cells.
miR-382-5p Overexpression Retarded Cell Proliferation, Metastasis While Facilitated Apoptosis in NSCLC Cells by Targeting SPIN1
To illustrate the functions of miR-382-5p and SPIN1 in NSCLC, miR-382-5p and pcNDA-SPIN1 were co-transfected into NSCLC cells. Our data showed the successful overexpression transfection efficiency of pcNDA-SPIN1 in H1299 and A549 cells (Supplement Figure 1A and B). As presented in Figure 6A–C, the levels of SPIN1 mRNA and protein were dramatically declined in miR-382-5p-transfected H1299 and A549 cells, while partially regained in miR-382-5p + pcNDA-SPIN1 group. Following MTT assay exhibited that the emergence of pcDNA-SPIN1 receded the constraint impact on cell viability hindered by miR-382-5p mimics (Figure 6D and E). The apoptotic rate was markedly elevated in NSCLC cells transfected with miR-382-5p, while partly abated by the introduction of pcDNA-SPIN1 (Figure 6F). The transfection of miR-382-5p resulted in the striking down-regulation of migrated and invaded abilities in NSCLC cells, while restored in miR-382-5p + pcDNA-SPIN1 group (Figure 6G and H). These data demonstrated that the overexpression of SPIN1 neutralized the repressive effects on cell proliferation, metastasis and the promoted effect on apoptosis in H1299 and A549 cells induced via miR-382-5p.
SNHG14 Silencing Impeded SPIN1 Expression by Sponging miR-382-5p in H1299 and A549 Cells
Based on the above results, SNHG14, SPIN1 were elevated but miR-382-5p was reduced in NSCLC. To elucidate the relationship among SNHG14, miR-382-5p, and SPIN1 in NSCLC, si-SNHG14 and miR-382-5p inhibitor were co-transfected into NSCLC cells. As exhibited in Figure 7A and B, SPIN1 mRNA and protein were strikingly decreased in NSCLC cells transfected with si-SNHG14, but partly regained in NSCLC cells co-transfected with si-SNHG14 and miR-382-5p inhibitor. These data unraveled that the depletion of SNHG14 down-regulated SPIN1 expression by sponging miR-382-5p H1299 and A549 cells.
Discussion
Tumor progression is complex processes in human, and SNHG14 was identified to be involved in tumor progression in diverse types of cancers. In the current study, we mainly focused on the mechanism of SNHG14 in NSCLC. The results revealed that SNHG14 facilitated NSCLC progression through miR-382-5p/SPIN1 axis.
Emerging evidence implied that the ectopic expression of SNHG14 was associated with tumor progression. For example, Ji et al documented that SNHG14 was elevated in cervical cancer, and the down-regulation of SNHG14 curbed cell growth, metastasis but induced apoptosis via miR-206.20 Another study in ovarian cancer manifested that SNHG14 was enhanced in ovarian cancer, and the depletion of SNHG14 inhibited cell growth and metastasis by sponging miR-219a-5p.11 In this research, we validated that SNHG14 was highly expressed in NSCLC. The depletion of SNHG14 blocked cell growth, metastasis in NSCLC cells, as well as induced apoptosis in vitro. The above results of SNHG14 in NSCLC were consistent with the previous report.21 These data disclosed that SNHG14 might play important roles in NSCLC.
Recent reports demonstrated that the dysregulation of miR-382-5p was implicated in tumor progression. For instance, a study in glioma unraveled that miR-382-5p was obviously declined in glioma, and its overexpression impeded cell growth, metastasis of glioma cells.22 In this exploration, miR-382-5p apparently declined in NSCLC, and SNHG14 was verified to sponge miR-382-5p in NSCLC cells. SNHG14 silencing confined cell viability, metastasis, but accelerated apoptosis in NSCLC cells by regulating miR-382-5p. These results were similar to the report of miR-382 in NSCLC.17 These data demonstrated that SNHG14 augmented NSCLC progression by sponging miR-382-5p.
Convincing evidence uncovered that the aberrant expression of SPIN1 was related to tumor progression. A study in breast cancer uncovered that SPIN1 promoted cell growth and metastasis regulated by miR-1271.19 Li et al reported that SPIN1 suppressed cell growth but accelerated apoptosis in glioma mediated by miR-489.18 In the present study, SPIN1 was a target of miR-382-5p and was up-regulated in NSCLC. miR-382-5p overexpression impeded cell growth and metastasis while impelled apoptosis in NSCLC cells by affecting SPIN1. These results of SPIN1 in NSCLC were in line with the previous study.23 Furthermore, SNHG14 positively regulated SPIN1 expression by sponging miR-382-5p in NSCLC cells. These results implicated that SNHG14 positively modulated SPIN1 expression to expedite NSCLC progression by sponging miR-382-5p.
To better elucidate the effects of SNHG14 on NSCLC, the mice model experiment will be performed in the future. Accumulating evidence indicated that PI3K/AKT/mTOR signaling pathway was implicated in many biological processes in tumor progression.24 A reporter in gastric cancer disclosed that SNHG14 boosted gastric cancer development via PI3 K/AKT/mTOR signaling pathway.13 Next, we will explore whether the SNHG14/miR-382-5p/SPIN1-induced tumor progression is mediated by PI3K/AKT/mTOR signaling pathway.
In conclusion, SNHG14 knockdown retarded NSCLC progression by regulating SPIN1 expression via miR-382-5p. This new regulatory network in NSCLC may provide novel therapeutic targets for NSCLC patients.
Highlights
1. SNHG14 sponged miR-382-5p in NSCLC cells;
2. SNHG14 knockdown suppressed cell proliferation, migration, and invasion but triggered apoptosis in NSCLC cells by regulating miR-382-5p;
3. SPIN1 was a direct candidate of miR-382-5p in NSCLC cells;
4. miR-382-5p confined cell proliferation, migration, and invasion while induced apoptosis in NSCLC cells by targeting SPIN1;
5. SNHG14 modulated SPIN1 by sponging miR-382-5p in NSCLC cells.
Funding
There is no funding to report.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. In: Ahmad A, Gadgeel S, eds. Lung Cancer and Personalized Medicine. Advances in Experimental Medicine and Biology, Springer; 2016:1–19.
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332
4. Koudelakova V, Kneblova M, Trojanec R, et al. Non-small cell lung cancer-genetic predictors. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157(2).
5. Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi:10.1016/j.bbagrm.2014.08.012
6. Schmidt LH, Spieker T, Koschmieder S, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6(12):1984–1992. doi:10.1097/JTO.0b013e3182307eac
7. Liu X-H, Liu Z-L, Sun M, et al. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13(1):464. doi:10.1186/1471-2407-13-464
8. Nie F-Q, Sun M, Yang J-S, et al. Long noncoding RNA ANRIL promotes non–small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther. 2015;14(1):268–277. doi:10.1158/1535-7163.MCT-14-0492
9. Nie W, Ge H-J, Yang X-Q, et al. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371(1):99–106. doi:10.1016/j.canlet.2015.11.024
10. Di W, Weinan X, Xin L, et al. Long noncoding RNA SNHG14 facilitates colorectal cancer metastasis through targeting EZH2-regulated EPHA7. Cell Death Dis. 2019;10(7):514. doi:10.1038/s41419-019-1707-x
11. Li L, Zhang R, Li S. Long noncoding RNA SNHG14 promotes ovarian cancer cell proliferation and metastasis via sponging miR-219a-5p. Eur Rev Med Pharmacol Sci. 2019;23(10):4136–4142. doi:10.26355/eurrev_201905_17915
12. Li J, Wang A, Wang S, et al. LncSNHG14 promotes the development and progression of bladder cancer by targeting miRNA-150-5p. Eur Rev Med Pharmacol Sci. 2019;23(3):1022–1029. doi:10.26355/eurrev_201902_16989
13. Liu Z, Yan Y, Cao S, et al. Long non‐coding RNA SNHG14 contributes to gastric cancer development through targeting miR‐145/SOX9 axis. J Cell Biochem. 2018;119(8):6905–6913. doi:10.1002/jcb.26889
14. Huang Y, Shen XJ, Zou Q, et al. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67(1):129–139. doi:10.1007/s13105-010-0050-6
15. Wang R, Wang Z, Yang J, et al. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene. 2011;30(23):2644. doi:10.1038/onc.2010.642
16. Yu T, Li J, Yan M, et al. MicroRNA-193a-3p and-5p suppress the metastasis of human non-small-cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2015;34(4):413. doi:10.1038/onc.2013.574
17. Chen T, Ren H, Thakur A, et al. miR-382 inhibits tumor progression by targeting SETD8 in non-small cell lung cancer. Biomed Pharmacother. 2017;86:248–253. doi:10.1016/j.biopha.2016.12.007
18. Li Y, Ma X, Wang Y, et al. miR-489 inhibits proliferation, cell cycle progression and induces apoptosis of glioma cells via targeting SPIN1-mediated PI3K/AKT pathway. Biomed Pharmacother. 2017;93:435–443. doi:10.1016/j.biopha.2017.06.058
19. Du H, Liu B. MiR-1271 as a tumor suppressor in breast cancer proliferation and progression via targeting SPIN1. Eur Rev Med Pharmacol Sci. 2018;22(9):2697–2706. doi:10.26355/eurrev_201805_14966
20. Ji N, Wang Y, Bao G, et al. LncRNA SNHG14 promotes the progression of cervical cancer by regulating miR-206/YWHAZ. Pathol Res Pract. 2019;215(4):668–675. doi:10.1016/j.prp.2018.12.026
21. Zhang Z, Wang Y, Zhang W, et al. Long non-coding RNA SNHG14 exerts oncogenic functions in non-small cell lung cancer through acting as an miR-340 sponge. Biosci Rep. 2019;39(1):BSR20180941. doi:10.1042/BSR20180941
22. Wang J, Chen C, Yan X, et al. The role of miR-382-5p in glioma cell proliferation, migration and invasion. Onco Targets Ther. 2019;12:4993. doi:10.2147/OTT.S196322
23. Song Q, Ji Q, Xiao J, et al. miR-409 inhibits human non-small-cell lung cancer progression by directly targeting SPIN1. Mol Ther Nucleic Acids. 2018;13:154–163. doi:10.1016/j.omtn.2018.08.020
24. Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4(51). doi:10.3389/fnmol.2011.00051
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.