Back to Journals » OncoTargets and Therapy » Volume 12
Long non-coding RNA homeobox A11 antisense RNA (HOXA11-AS) promotes retinoblastoma progression via sponging miR-506-3p
Authors Han N, Zuo L , Chen H, Zhang C, He P, Yan H
Received 21 November 2018
Accepted for publication 16 March 2019
Published 7 May 2019 Volume 2019:12 Pages 3509—3517
DOI https://doi.org/10.2147/OTT.S195404
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Ning Han, Ling Zuo, Han Chen, Chunxia Zhang, Pei He, Hongtao Yan
Department of Ophthalmology, The Second Hospital of Jilin University, Changchun, Jilin 130041, People’s Republic of China
Background: Long non-coding RNA homeobox A11 antisense RNA (HOXA11-AS) has been reported to be involved in initiation and development of multiple cancers. However, the detailed biological roles and underlying molecular mechanism of HOXA11-AS remain unclear in retinoblastoma (RB). Herein, the goals of this study were to explore the biological function and the potential mechanism of HOXA11-AS in RB.
Materials and methods: The expression of HOXA11-AS in RB tissues and cell lines was detected using real-time PCR (qRT-PCR). Cell proliferation, cycle arrest and apoptosis were measured using a cell counting kit 8 and flow cytometry. The target miRNAs of HOXA11-AS was predicted by Starbase2.0 software and was confirmed using a dual-luciferase reporter assay.
Result: We found that HOXA11-AS expression was markedly elevated in RB tissues and cell lines compared to normal retina tissues and human retinal epithelial cells, respectively. Functional analysis showed that knockdown of HOXA11-AS in RB cells significantly suppressed cell proliferation, and induced cell cycle arrest at G1/G0 phase and promoted cell apoptosis. We also found that HOXA11-AS could serve as a competing endogenous RNA (ceRNA) that inhibited miR-506-3p expression, which regulated its downstream target NIMA-related kinase 6 (NEK6) in RB. In addition, miR-506-3p inhibitors partially reversed the effect of HOXA11-AS depletion on proliferation, cycle arrest and apoptosis in RB cells.
Conclusion: Taken together, these findings demonstrated that HOXA11-AS could promote RB progression by sponging miR-506-3p, suggesting that HOXA-11-AS might be a potential therapeutic target for RB.
Keywords: retinoblastoma, LncRNA, miR-506-3p, proliferation, apoptosis
Introduction
The retinoblastoma (RB) is the most common intraocular malignant tumor usually occurring during childhood.1 Despite great improvement of diagnostic and surgical techniques, the prognosis of patients with RB is still unsatisfactory.2,3 Therefore, exploring the molecular mechanisms underlying RB tumorigenesis and progression is urgently needed to find the effective diagnostic and therapeutic targets for the treatment of this disease.
Long noncoding RNAs (lncRNAs) are a class of newly identified regulatory RNA member with more than 200 nucleotides in length and without the protein-coding ability.4 Accumulating evidence has suggested that lncRNAs implicated in a wide range of biological processes, including cell proliferation, apoptosis and invasion.5,6 Number of cancer-associated lncRNAs has been identified to be involved in occurrence and development of various cancers.7,8 Besides, several lncRNAs have been reported to be correlated with RB progression functioning as oncogene and tumor suppressor in RB.9,10
Homeobox A11 antisense RNA (HOXA11-AS), a highly conserved lncRNA located in the HOXA gene cluster on chromosome 7p15.2,11 is primordially discovered in a mouse embryonic cDNA library.12 HOXA11-AS has been reported to be involved in tumor progression in several types of cancer.13–15 However, the expression status, detailed biological function and the possible molecular mechanisms of HOXA11-AS involved in RB progression remain unknown. Therefore, the aims of this study were to explore the biological function and underlying molecular mechanism of HOXA11-AS in RB.
Materials and methods
Human tissue samples
A total of 30 RB tissue samples were collected from RB patients treated with surgical resection without preoperative chemotherapy or radiotherapy at the Second Hospital of Jilin University. 10 normal retina tissue samples were obtained from patients with ruptured globes. All samples were extracted from enucleated eyes and immediately stored in liquid nitrogen for further study. All experiments about using human tissues have been conducted in accordance with the World Medical Association Declaration of Helsinki, and that all subjects provided written informed consent. This study was approved by the Clinical Research Ethics Committee of the Second Hospital of Jilin University (Changchun, China).
Cell culture and transfection
The human retinal epithelial cells ARPE-19 and four human RB cell lines Y79, Weri-Rb1, SO-Rb50, and HXO-RB44 were bought from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin-streptomycin (Invitrogen; Carlsbad, CA, USA) at 37°C in a humidified atmosphere with 5% CO2.
For HOXA11-AS down-regulation, a small interference RNA (siRNA) targeting human HOXA11-AS (si-HOXA11-AS) and its negative control nonsensical sequence (si-NC) were constructed and synthesized by GenePhara (Shanghai, China). The miR-506-3p mimic, the appropriate negative control mimic (miR-NC) and miR-506-3p inhibitor (miR-506-3p in) were purchased from RiboBio (Guangzhou, China). Y79 cells were transiently transfected with one of the aforementioned siRNAs, mimics and inhibitor using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Transfection efficiency was determined in every experiment at 48 hrs post-transfection.
RNA isolation and qRT-PCR
Total RNA was extracted from tissue samples and cultured cells using TRIzol® reagent (Invitrogen) following the manufacturer’s instructions. Reverse transcription was performed for quantification using the TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) or a Prime Script First Strand cDNA Synthesis kit (Takara, Dalian, China) according to the manufacturer’s protocol. The cDNAs were amplified using TaqMan miRNA kit (Applied Biosystems) or SYBR Premix Ex Taq (Takara) using the ABI 7900 Fast System (Applied Biosystems). The primers are listed in Table 1. Relative gene expression was calculated according to the 2–ΔΔCt method after normalization to U6 for miR-506-3p or GAPDH for HOXA11-AS or NEK6.
 | Table 1 Real-time PCR primers used for mRNA expression analysis |
Cell proliferation assay
Cell proliferation was measured using Cell Counting Kit-8 (CCK8; Beyotime, Beijing) according to the manufacturer’s guide. Briefly, transfected cells in each group were plated into 96-well plates at density of 2,000 cells/well. In indicated time, the CCK8 regent (10 μL) was added to each well for another 4 hrs at 37°C with 5% CO2. The absorbance at the wavelength of 450 nm was finally measured using a microplate reader.
Flow cytometry for cell cycle and apoptosis analysis
Flow cytometry was used for cell cycle arrest and apoptosis analysis. Cells were harvested at 48 hrs post-transfection. For cell cycle arrest, transfected cells were seeded in six-well and were fixed with 75% ethanol overnight at 4°C. Then, cells stained with propidium iodide (PI) supplemented with RNaseA (Sigma) at room temperature in the dark for 15 mins. Cell cycle arrest was determined with a FACScan flow cytometer (BD Biosciences) and analyzed with the ModFit software (BD Biosciences).
The Annexin V/Dead Cell Apoptosis Kit (Invitrogen) was used to determine cell apoptosis according to the manufacturer’s instructions. The cell apoptosis was analyzed using the FACScan flow cytometer equipped with CellQuest Software (BD Biosciences).
Luciferase reporter assays
HOXA11-AS fragment containing the predicated miR-506-3p binding site (wild-type) or mutant putative sequences of the binding sites (mutant-type) were synthesized and subcloned into a pmirGLO Dual-luciferase vector (Promega, USA) to form the reporter vector pmiRGLO-HOXA11-wild type (HOXA11-AS-WT) or pmiRGLO-HOXA11-AS-mutant (HOXA11-AS-MT). Y79 cells were co-transfected with reporter vector HOXA11-AS-WT or HOXA11-MT and miR-506-3p mimic or miR-NC by using Lipofectamine 2000. The relative luciferase activities were determined using a dual-luciferase reporter assay system (Promega) at 48 hrs after transfection according to the manufacturer’s guide.
Statistical analyses
All data are presented as as mean ± SD from at least three separate experiments. Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago) using Student’s t-test and one way ANOVA. Correlations between HOXA11-AS expression and miR-506-3p or NEK6 expression were evaluated by Pearson’s correlation analysis. Significance level was set as P<0.05.
Results
HOXA11-AS expression was upregulated in RB cells and tissues
We initially detected the expression of HOXA11-AS in 30 RB tissues and 10 normal retina tissues by qRT-PCR. The results showed that the expression of HOXA11-AS was significantly increased in RB tissues compared to the normal retina tissues (Figure 1A). Subsequently, we analyzed the expression of HOXA11-AS in four human RB cell lines (Y79, Weri-Rb1, SO-Rb50 and HXO-RB44) and human retinal epithelial cells ARPE-19. Consistent with the results in RB tissues, HOXA11-AS expression was also dramatically increased in four RB cell lines as compared to ARPE-19 cells (Figure 1B), especially in Y79 cells. Thus, Y79 cells were chosen for the following experiments.
Knockdown of HOXA11-AS suppressed proliferation and facilitated apoptosis in RB cells
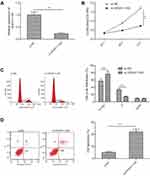
To explore the biological function of HOXA11-AS in RB cells, siRNA against HOXA11-AS (si-HOXA11-AS) were transfected into Y79 cells to decrease the expression of HOXA11-AS (Figure 2A). CCK-8 assay was performed to investigate the effect of HOXA11-AS on cell proliferation. The results demonstrated that downregulation of HOXA11-AS significantly inhibited cell proliferation of Y79 cells in comparison to negative control (si-NC) (Figure 2B). To determine whether the effect of HOXA11-AS on RB cells proliferation reflected cell cycle arrest, cell cycle progression was determined by flow cytometry analysis. The results revealed that knockdown of HOXA11-AS significantly increased cell cycle arrest at G1/G0 phase, and decreased arrest at S phase (Figure 2C). Moreover, we investigate the effect of HOXA11-AS on apoptosis of RB cells. We found that knockdown of HOXA11-AS significantly induced cell apoptosis of Y79 cells (Figure 2D).
HOXA11-AS acted as a molecular sponge for miR-506-3p in RB cells
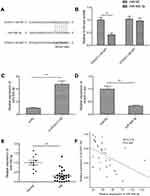
Accumulating evidence suggested that lncRNAs implicated in tumor progression by functioning as competing endogenous RNAs (ceRNAs) to specific miRNAs.16 Here, a bioinformatics prediction program (StarBase 2.0,
miR-506-3p inhibition restored the si-HOXA11-AS induced effects on cell proliferation, cycle arrest and apoptosis in RB cells
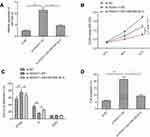
To determine whether HOXA11-AS exerts biological roles in RB cells via regulating miR-506-3p, we carried out the rescue experiments by transfecting si-HOXA11-AS plus miR-506-3p inhibitor into Y79 cells. As shown in Figure 4A, result from qRT-PCR assay revealed that miR-506-3p inhibitor could decrease HOXA11-AS expression in Y79 cells transfected with si-HOXA11-AS (Figure 4A). In addition, CCK8 assay revealed that the inhibitory effect of HOXA11-AS knockdown on RB cell proliferation could be partially restored by miR-506-3p inhibitor (miR-506-3p-in) (Figure 4B). Flow cytometry assay revealed that repression of miR-506-3p largely rescued the effect of cell cycle arrest and apoptosis induced by si-HOXD-AS1 in Y79 cells (Figure 4C and D). These results implied that HOXA11-AS exerted its role in RB by targeting miR-506-3p.
HOXA11-AS regulated the expression of NEK6 through miR-506-3p in RB cells
Growing evidences suggested that lncRNAs exerted its function through acting as molecular sponges or ceRNAs to regulate specific miRNAs and their targets of miRNAs.17 A previous study showed that miR-506-3p inhibited RB progression by targeting mitosis Gene A (NIMA)-related kinase 6 (NEK6).18 Thus, we determine whether HOXA11-AS can regulate NEK6 expression which is mediated by miR-506-3p by a rescue experiment. As shown in Figure 5A, knockdown of HOXA11-AS in Y79 cells significantly decreased the mRNA expression levels of NEK6, while inhibition of miR-506-3p could reverse this trend. In addition, we also examined the expression of NEK6 expression in 30 RB tissue and 10 normal retina tissue samples by qRT-PCR. We found that the NEK6 mRNA expression was upregulated in RB tissues compared to normal tissue samples (Figure 5B). Pearson’s correlation analysis revealed that NEK6 expression was negatively correlated with miR-506-3p expression in RB tissues (Figure 5C), and positively correlated with HOXA11-AS expression in RB tissues (Figure 5D). These data suggest that HOXA11-AS could regulate NEK6 expression through miR-506-3p in RB.
Discussion
Recently, number of lncRNAs have been reported to play a significant regulatory role in RB initiation and development by functioning as a tumor suppressor or oncogene.8,9 For example, Hu et al reported that XIST promoted RB progression partially by modulating the miR-124/STAT3 axis.19 Li et al demonstrated that knockdown of LINC00152 significantly inhibited RB cell growth in vitro and in vivo.20 Zhang et al found that H19 inhibited RB progression via counteracting the roles of miR-17–92 cluster.21 Wang et al revealed that DANCR acted as ceRNA for miR-34c and miR-613 to regulate progression and metastasis in RB oncogenesis via targeting MMP-9.22 In this study, we found that HOXA11-AS expression was upregulated in RB tissues and cell lines. Knockdown of HOXA11-AS significantly inhibited RB cell proliferation, and induced cell arrest at G1/G0 phase and cell apoptosis. Mechanic analysis demonstrated that HOXA11-AS contributed to RB progression by modulating miR-506-3p/NEK6 axis.
HOXA11-AS1 (also known as NCRNA00076), a member of the homeobox (HOX) family gene,10 has been reported to be upregulated in multiple types of cancer, including renal cancer,23 hepatocellular carcinoma,24 laryngeal squamous cell carcinoma,25 non-small cell lung cancer,26 glioma,27 breast cancer,28 osteosarcoma29 and gastric cancer,30 suggesting that HOXA11-AS1 played an oncogenic role in these types of cancers. On the contrary, in ovarian cancer31 and colorectal cancer,32 HOXA11-AS1 expression was downregulated and functioned as a tumor suppressor. These inconsistent results suggested that HOXA11-AS1 played different roles in tumor progression depending on details of cancer types. However, until now, no systematic study was performed on the expression and role of HOXA11-AS in RB. Our present data revealed that HOXA11-AS expression was upregulated in RB tissues and cell lines, suggesting that HOXA11-AS might be involved in RB progression. Furthermore, we identified the effects of HOXA11-AS on the biological behaviors of RB, showing that knockdown of HOXA11-AS significantly inhibited RB cell proliferation, and induced cell arrest at G1/G0 phase and cell apoptosis. These results implied that HOXA11-AS functioned as an oncogene in RB.
It was well known that lncRNAs exert their biological function by functioning as ceRNAs sponging for miRNAs, thus affecting the expression levels of miRNAs target genes.16,33 To investigate the possible molecular mechanism of HOXA11-AS involved in RB progression, we used starbase 2.0 algorithm to predict miRNAs that targeted HOXA11-AS. Among miRNAs, miR-506 was chosen to act as an object based on its biological roles in RB. miR-506-3p has been reported to be downregulated and functioned as a tumor suppressor in RB.18 Through luciferase reporter assay and qRT-PCR, we confirmed that miR-506-3p was a direct target of HOXA11-AS in RB. We also found that miR-506-3p expression was negatively correlated with HOXA11-AS expression in RB tissues. Importantly, miR-506-3p inhibition restored the si-HOXA11-AS induced effects on cell proliferation, cycle arrest and apoptosis in RB cells. Those data indicated that HOXA11-AS might function as a sponge for miR-506-3p in RB.
LncRNA could function as ceRNAs to regulate their targets of miRNAs.17 NEK6 has been proven to be a target of miR-506-3p in RB.18 Growing evidence showed that NEK6 played crucial roles in tumorigenesis.34,35 Here, we determine whether HOXA11-AS can regulate NEK6 expression. We found that knockdown of HOXA11-AS in Y79 cells significantly decreased the mRNA expression levels of NEK6, while inhibition of miR-506-3p could reverse this trend. In addition, we also found that the mRNA expression levels of NEK6 were upregulated in RB tissues compared to normal tissue samples, which was consistent with the previous result.18 Pearson’s correlation analysis revealed that NEK6 expression was negatively correlated with miR-506-3p expression in RB tissues, and positively correlated with HOXA11-AS expression in RB tissues. These data suggest that HOXA11-AS could regulate NEK6 expression in RB cells through miR-506-3p.
In conclusion, to our knowledge, the present study first provides evidence that HOXA11-AS was upregulated in RB tissues and cell lines, and that HOXA11-AS played an oncogenic role in RB tumorigenesis through sponging miR-506-3p at least partially. These data suggested that HOXA11-AS might serve as a potential therapeutic target for the RB treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Dimaras H, Corson TW. Retinoblastoma, the visible CNS tumor: a review. J Neurosci Res. 2019;97(1):29–44.
3. Fabian ID, Onadim Z, Karaa E, et al. The management of retinoblastoma. Oncogene. 2018;37(12):1551–1560. doi:10.1038/s41388-017-0050-x
4. Schaukowitch K, Kim TK. Emerging epigenetic mechanisms of long non-coding RNAs. Neuroscience. 2014;264:25–38. doi:10.1016/j.neuroscience.2013.12.009
5. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi:10.1038/nrg2521
6. Sun M, Kraus WL. From discovery to function: the expanding roles of long noncoding RNAs in physiology and disease. Endocr Rev. 2015;36(1):25–64. doi:10.1210/er.2014-1034
7. Chen L, Dzakah EE, Shan G. Targetable long non-coding RNAs in cancer treatments. Cancer Lett. 2018;418:119–124. doi:10.1016/j.canlet.2018.01.042
8. Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi:10.1186/1476-4598-10-93
9. Huang J, Yang Y, Fang F, Liu K. MALAT1 modulates the autophagy of retinoblastoma cell through miR-124-mediated stx17 regulation. J Cell Biochem. 2018;119(5):3853–3863. doi:10.1002/jcb.26464
10. Yang Y, Peng XW. The silencing of long non-coding RNA ANRIL suppresses invasion, and promotes apoptosis of retinoblastoma cells through ATM-E2F1 signaling pathway. Biosci Rep. 2018;38:BSR20180558. doi:10.1042/BSR20180558
11. Yu H, Lindsay J, Feng ZP, et al. Evolution of coding and non-coding genes in HOX clusters of a marsupial. BMC Genomics. 2012;13:251. doi:10.1186/1471-2164-13-251
12. Potter SS, Branford WW. Evolutionary conservation and tissue-specific processing of Hoxa 11 antisense transcripts. Mamm Genome. 1998;9(10):799–806. doi:10.1007/s003359900870
13. Xue JY, Huang C, Wang W, Li HB, Sun M, Xie M. HOXA11-AS: a novel regulator in human cancer proliferation and metastasis. Onco Targets Ther. 2018;11:4387–4393. doi:10.2147/OTT.S166961
14. Lu S, Jiang X, Su Z, Cui Z, Fu W, Tai S. The role of the long non-coding RNA HOXA11-AS in promoting proliferation and metastasis of malignant tumors. Cell Biol Int. 2018;42:1596–1601. doi:10.1002/cbin.v42.12
15. Lu CW, Zhou DD, Xie T, et al. HOXA11 antisense long noncoding RNA (HOXA11-AS): a promising lncRNA in human cancers. Cancer Med. 2018;7(8):3792–3799. doi:10.1002/cam4.1571
16. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi:10.1016/j.cell.2011.07.014
17. Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–369. doi:10.1016/j.cell.2011.09.028
18. Wu L, Chen Z, Xing Y. MiR-506-3p-3p inhibits cell proliferation, induces cell cycle arrest and apoptosis in retinoblastoma by directly targeting NEK6. Cell Biol Int. 2018. doi:10.1002/cbin.11041
19. Hu C, Liu S, Han M, Wang Y, Xu C. Knockdown of lncRNA XIST inhibits retinoblastoma progression by modulating the miR-124/STAT3 axis. Biomed Pharmacother. 2018;107:547–554. doi:10.1016/j.biopha.2018.08.020
20. Li S, Wen D, Che S, et al. Knockdown of long noncoding RNA 00152 (LINC00152) inhibits human retinoblastoma progression. Onco Targets Ther. 2018;11:3215–3223. doi:10.2147/OTT.S160428
21. Zhang A, Shang W, Nie Q, Li T, Li S. Long non-coding RNA H19 suppresses retinoblastoma progression via counteracting miR-17-92 cluster. J Cell Biochem. 2018;119(4):3497–3509. doi:10.1002/jcb.26521
22. Wang JX, Yang Y, Li K. Long noncoding RNA DANCR aggravates retinoblastoma through miR-34c and miR-613 by targeting MMP-9. J Cell Physiol. 2018;233(10):6986–6995. doi:10.1002/jcp.26621
23. Yang FQ, Zhang JQ, Jin JJ, et al. HOXA11-AS promotes the growth and invasion of renal cancer by sponging miR-146b-5p to upregulate MMP16 expression. J Cell Physiol. 2018;233(12):9611–9619. doi:10.1002/jcp.26864
24. Zhan M, He K, Xiao J, et al. LncRNA HOXA11-AS promotes hepatocellular carcinoma progression by repressing miR-214-3p. J Cell Mol Med. 2018;22:3758–3767. doi:10.1111/jcmm.2018.22.issue-8
25. Qu L, Jin M, Yang L, et al. Expression of long non-coding RNA HOXA11-AS is correlated with progression of laryngeal squamous cell carcinoma. Am J Transl Res. 2018;10(2):573–580.
26. Yu W, Peng W, Jiang H, Sha H, Li J. LncRNA HOXA11-AS promotes proliferation and invasion by targeting miR-124 in human non-small cell lung cancer cells. Tumour Biol. 2017;39(10):1010428317721440. doi:10.1177/1010428317721440
27. Xu C, He T, Li Z, Liu H, Ding B. Regulation of HOXA11-AS/miR-214-3p/EZH2 axis on the growth, migration and invasion of glioma cells. Biomed Pharmacother. 2017;95:1504–1513. doi:10.1016/j.biopha.2017.08.097
28. Li W, Jia G, Qu Y, Du Q, Liu B, Liu B. Long non-coding RNA (LncRNA) HOXA11-AS promotes breast cancer invasion and metastasis by regulating epithelial-mesenchymal transition. Med sci monit. 2017;23:3393–3403.
29. Cui M, Wang J, Li Q, Zhang J, Jia J, Zhan X. Long non-coding RNA HOXA11-AS functions as a competing endogenous RNA to regulate ROCK1 expression by sponging miR-124-3p in osteosarcoma. Biomed Pharmacother. 2017;92:437–444. doi:10.1016/j.biopha.2017.05.081
30. Liu Z, Chen Z, Fan R, et al. Over-expressed long noncoding RNA HOXA11-AS promotes cell cycle progression and metastasis in gastric cancer. Mol Cancer. 2017;16(1):82. doi:10.1186/s12943-017-0651-6
31. Richards EJ, Permuth-Wey J, Li Y, et al. A functional variant in HOXA11-AS, a novel long non-coding RNA, inhibits the oncogenic phenotype of epithelial ovarian cancer. Oncotarget. 2015;6(33):34745–34757. doi:10.18632/oncotarget.5784
32. Li T, Xu C, Cai B, et al. Expression and clinicopathological significance of the lncRNA HOXA11-AS in colorectal cancer. Oncol Lett. 2016;12(5):4155–4160. doi:10.3892/ol.2016.5129
33. Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi:10.1038/nature11993
34. De Donato M, Righino B, Filippetti F, et al. Identification and antitumor activity of a novel inhibitor of the NIMA-related kinase NEK6. Sci Rep. 2018;8(1):16047. doi:10.1038/s41598-018-34471-y
35. He Z, Ni X, Xia L, Shao Z. Overexpression of NIMA-related kinase 6 (NEK6) contributes to malignant growth and dismal prognosis in human breast cancer. Pathol Res Pract. 2018;214(10):1648–1654. doi:10.1016/j.prp.2018.07.030
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.