Back to Journals » Cancer Management and Research » Volume 12
LncRNA RPSAP52 Upregulates TGF-β1 to Increase Cancer Cell Stemness and Predict Postoperative Survival in Glioblastoma
Authors Wang S, Guo X, Lv W, Li Y, Zhang L, Dong C, Zhang J, Cheng G
Received 16 August 2019
Accepted for publication 11 February 2020
Published 15 April 2020 Volume 2020:12 Pages 2541—2547
DOI https://doi.org/10.2147/CMAR.S227496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Shuwei Wang, Xinru Guo, Wenying Lv, Yanteng Li, Leiming Zhang, Chao Dong, Jianning Zhang, Gang Cheng
Department of Neurosurgery, Sixth Medical Center, Chinese PLA General Hospital, Beijing 100048, People’s Republic of China
Correspondence: Jianning Zhang; Gang Cheng
Department of Neurosurgery, Sixth Medical Center, Chinese PLA General Hospital, No. 6 Pucheng Road, Beijing 100048, People’s Republic of China
Tel +86 10-66951351
Email [email protected]; [email protected]
Introduction: Ribosomal protein SA pseudogene 52 (RPSAP52) has been characterized as an oncogenic lncRNA in pituitary tumors. Analysis of TCGA dataset revealed the upregulation of RPSAP52 in glioblastoma (GBM). We, therefore, investigated the roles of RPSAP52 in GBM.
Methods: A total of 50 GBM patients (33 males and 20 females; 54– 75 years; mean age: 61.8± 5.8 years) were selected from the 89 cases of GBM patients. Under the guidance of MRI, brain biopsy was performed to collect GBM tissues from each patient for the diagnosis of GBM. U-373 MG cells were employed and had transient transfections. qRNA, Western blot, and a series of experiments were performed to characterize their associations.
Results: The results showed that RPSAP52 was upregulated in GBM patients, and its high expression levels predicted poor survival. In GBM tissues, expression levels of RPSAP52 were significantly and positively correlated with that of TGF-β 1. In GBM tissues, RPSAP52 positively regulated the expression of TGF-β 1. Cell stemness assay showed that, compared to C and NC groups, overexpression of RPSAP52 and TGF-β 1 led to increased, while silencing of RPSAP52 led to decreased CD133+ cells. Overexpression of TGF-β 1 attenuated the effects of RPSAP52 siRNA silencing.
Conclusion: Therefore, RPSAP52 upregulates TGF-β 1 to increase cancer cell stemness and predict postoperative survival in GBM.
Keywords: glioblastoma, RPSAP52, TGF-β 1, survival, stemness
Introduction
Glioblastoma (GBM) is a rare type of malignant brain tumor that only affects 3.6 out of 100,000 people worldwide.1 However, GBM has been considered as a major cause of cancer deaths due to its highly aggressive nature and the lack of effective therapeutic approaches.1,2 With active treatment, such as surgical resection, radiation therapy and chemotherapy, the median survival time of GBM patients after the initial diagnosis is 15–18 months, and only less than 5% GBM patients can live long than 5 years.3 Targeted therapies are emerging therapeutic approaches for GBM,4,5 However, the development of novel targeted therapies is limited because of the unclear molecular pathogenesis.6
TGF-β signaling plays critical roles in nearly all aspects of cancer development and progression.7 Inhibition of the TGF-β pathway is considered as a promising therapeutic target for the treatment of different types of cancer including GBM.8,9 It has been well established that the TGF-β signaling may interact with long (>200 nt) non-coding RNAs (lncRNAs) to participate in cancer biology.10,11 Ribosomal protein SA pseudogene 52 (RPSAP52) is a recently characterized oncogenic lncRNA in pituitary tumors,12 while its functions in other types of cancer are unknown. Through the analysis of TCGA dataset, we found that RPSAP52 was only upregulated in GBM tissues but not in non-tumor tissues. This observation triggered our interest to investigate the roles of RPSAP52 in GBM. In this study, we found that RPSAP52 may interact with the TGF-β signaling to regulate GBM cell stemness.
Materials and Methods
GBM Patients
This study passed the ethics review board of the Sixth Medical Center, Chinese PLA General hospital, and was carried out according to the Declaration of Helsinki. A total of 50 GBM patients (33 males and 20 females; 54 to 75 years; mean age: 61.8±5.8 years) were selected from the 89 cases of GBM patients admitted to the aforementioned hospital between May 2012 and July 2014. Inclusion criteria: 1) patients confirmed by histopathological exam; 2) newly diagnosed GBM cases. Exclusion criteria: 1) other clinical disorders were diagnosed; 2) therapies were initiated; 3) recurrent GBM. All patients were informed of the details of this project and signed informed consent.
Biopsy
Under the guidance of MRI, brain biopsy was performed to collect GBM tissues from each patient for the diagnosis of GBM. Adjacent (within 3 cm around the tumors) non-tumor tissues were also collected from each patient. All tissue samples were confirmed by performing a histopathological exam.
Treatment and Follow-Up
Based on patients’ conditions, all patients received surgical resection combined with chemotherapy, radiation therapy or targeted drug therapy. From the day of admission, all patients were followed up for 5 years to record patients’ survival conditions. Only the patients who completed the follow-up or died of GBM were included in the survival analysis.
U-373 MG Cells and Transient Transfections
Human GBM cell line U-373 MG (Sigma-Aldrich, USA) was used as the cell model of GBM. Cells were cultivated in a 5% CO2 incubator at 37 ºC with 95% humidity. Cell culture medium was composed of 10% FBS and 90% DMEM. U-373 MG cells were harvested at 75–85% confluence before transfections. RPSAP52 and TGF-β1 expression vectors were constructed using pcDNA3.1 vector (GeneCopoeia, Guangzhou, China) as the backbone. Negative control (NC) siRNA and RPSAP52 siRNA were designed and synthesized by GenePharma (Shanghai, China). U-373 MG cells were counted and 3×106 cells were transfected with 10 nM vector (empty vector as NC group) or 50 nM siRNA (NC siRNA as NC group) through lipofectamine 2000 (GenePharma)-mediated transient transfection. Following experiments were performed using cells harvested at 24 h post-transfection. Untransfected cells were used as Control (C) cells.
RNA Extractions and qPCR
U-373 MG cells were collected at 24 h post-transfection and cell number was counted. Cell pellets containing 3×104 cells were resuspended in 1 mL Ribozol reagent (Sigma-Aldrich, USA) to extract total RNAs. Biopsies were ground in liquid nitrogen, followed by the addition of Ribozol reagent (Sigma-Aldrich, USA) to extract total RNAs. All operations were performed following the instructions from Sigma-Aldrich. All RNA samples were digested with DNase I (Sigma-Aldrich) at 37 ºC for 90 min to remove genomic DNAs. With ploy (T) as a primer, reverse transcriptions (RTs) were performed using SSRT III system (Thermo Fisher Scientific). With cDNA as a template, qPCR mixtures were prepared using Power SYBR® Green PCR Master Mix (Applied Biosystems) to measure the expression levels of RPSAP52 and TGF-β1 mRNA. GAPDH was used as the endogenous control. All qPCR reactions were performed in a triplicate manner and 2−ΔΔCT method was used for data normalization.
Western Blot
Cell pellets containing 3×104 cells were resuspended in 1 mL RIPA solution (GenePharma) to extract total proteins. Protein samples were quantified using BCA assay (GenePharma). Protein samples were first denatured in a 95°C incubator for 10 min, followed by electrophoresis using 10% SDS-PAGE gel. Proteins were transferred to PVDF membranes, followed by blocking in PBS containing 5% fat-free milk at 25°C for 2 h. After that, TGF-β1 (1:1500, ab92486, Abcam) and GAPDH (1:1500, ab37168, Abcam) rabbit polyclonal primary antibodies were used to incubate with the membranes at 4°C for 18 h. Membranes were further incubated with HRP (IgG) secondary antibody (goat anti-rabbit, 1:2000; ab6721; Abcam) at 25°C for 2 h. Signal production was performed using ECL Substrate (Thermo Fisher Scientific). Expression levels of TGF-β1 were normalized to endogenous control GAPDH using Image J v1.48 software.
Cell Stemness Assay
U-373 MG cells were collected at 24 h post-transfection and were counted after trypsinization. After that, 3×105 cells were incubated with immunoglobulin (Ig) G1-PE (cat. no. 130-093-193; Miltenyi Biotec, Bergisch Gladbach, Germany) or phycoerythrin (PE)-conjugated CD133 (cat. no. 566593, Biosciences, USA) antibody at 4°C for 30 min. Flow cytometry was performed to separate CD133+ cells. Flow cytometry data were processed using the Cell Quest software v5.1 (BD Biosciences, San Jose, CA, USA).
Statistical Analysis
All experiments were performed in 3 biological replicates. Mean values of 3 replicates were calculated and used in the following data analysis. Correlations were explored using linear regression. Survival analysis was performed by dividing the 50 GBM patients into high and low RPSAP52 level groups (n = 25), following by using K-M plotter to plot survival curves and log-rank test to compare survival curves. Paired t test was used to explore differences between non-tumor and GBM tissues. ANOVA (one-way) combined with Tukey’s test were used to explore differences among multiple transfection groups. P < 0.05 was statistically significant.
Results
Upregulation of RPSAP52 Predicted Poor Survival of GBM Patients
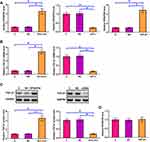
The differential expression of RPSAP52 in GBM was first explored using TCGA dataset. It was observed that RPSAP52 can only be detected in GBM tissues but not in non-tumor tissues (0.1 vs 0, http://gepia.cancer-pku.cn/detail.php?gene=RPSAP52). qPCR was performed to measure the expression levels of RPSAP52 in both GBM and non-tumor tissues from the 50 GBM patients included in this study. Different from TCGA dataset, we detected the expression of RPSAP52 in both non-tumor tissues and GBM tissues. However, expression levels of RPSAP52 were significantly higher in GBM tissues in comparison to non-tumor tissues (Figure 1A, p < 0.0001). Survival curves were plotted and compared using the aforementioned methods. Compared to patients in low RPSAP52 level group, patients in high RPSAP52 level group experienced a significantly lower overall survival rate (Figure 1B).
RPSAP52 Significantly and Positively Correlated with TGF-β1 in GBM Tissues
Expression levels of TGF-β1 in both GBM and non-tumor tissues from the 50 GBM patients included were also measured by qPCR. Data were compared between two types of tissues using paired t test. Compared to non-tumor tissues, expression levels of TGF-β1 were significantly higher in GBM tissues (Figure 2A, p < 0.0001). The correlations between TGF-β1 and RPSAP52 were analyzed by linear regression. It showed that expression levels of TGF-β1 were significantly and positively correlated with the expression levels of RPSAP52 across GBM tissues (Figure 2B) but not non-tumor tissues (Figure 2C).
RPSAP52 Positively Regulated TGF-β1 in U-373 MG Cells
U-373 MG cells were transfected with RPSAP52, TGF-β1 expression vectors, or RPSAP52 siRNA to further investigate the interactions between RPSAP52 and TGF-β1. Compared to C and NC groups, expression levels of RPSAP52 and TGF-β1 were significantly altered at 24 h post-transfection (Figure 3A, p < 0.05), indicating successful transfections. Compared to C and NC groups, overexpression of RPSAP52 led to upregulated TGF-β1 at both mRNA (Figure 3B) and protein (Figure 3C) levels (p < 0.05). In addition, silencing of RPSAP52 led to downregulated TGF-β1 at both mRNA (Figure 3B) and protein (Figure 3C) levels (p < 0.05). In contrast, overexpression of TGF-β1 did not affect the expression of RPSAP52 (Figure 3D).
RPSAP52 Increased U-373 MG Cell Stemness Through TGF-β1
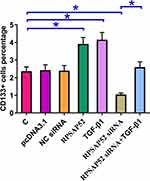
Cell stemness assay was performed to assess the effects of overexpressing RPSAP52 or TGF-β1, or silencing of RPSAP52 on the stemness of U-373 MG cells. Cell stemness assay showed that, compared to C and NC (NC siRNA or empty pcDNA3.1 vector transfection) groups, overexpression of RPSAP52 and TGF-β1 led to increased, while silencing of RPSAP52 led to decreased percentage of CD133+ cells. In addition, overexpression of TGF-β1 attenuated the effects of RPSAP52 siRNA silencing (Figure 4, p < 0.05).
Discussion
This study mainly investigated the expression pattern and functionality of RPSAP52 in GBM. We showed that RPSAP52 was overexpressed in GBM and predicted poor survival of GBM patients. In addition, RPSAP52 may positively regulate TGF-β1 to increase the stemness of GBM cells.
The functions of RPSAP52 have only been investigated in pituitary tumors.12 In pituitary tumors, RPSAP52 is significantly overexpressed and can regulate high mobility group A (HMGA) protein by serving as a miRNA sponge to promote cancer cell proliferation.12 We analyzed TCGA dataset and observed the expression of RPSAP52 in GBM tissues but not in adjacent non-tumor tissues. However, the present study detected the expression of RPSAP52 in non-tumor tissues as well from GBM patients included in this study. This is possibly due to the higher sensitivity of qPCR compared to RNA-seq. Through the analysis of TCGA data, we observed the differential expression of RPSAP52 in most types of cancer, such as colon cancer (0.26 vs 0.07) and thyroid cancer (1.57 vs 0.06). The differential expression may indicate its involvement in these types of malignancies. Future studies are needed to investigate the roles of RPSAP52 in other types of cancer.
TGF-β signaling plays different roles at different stages of cancer development.7,13 For instance, activation of TGF-β inhibits cancer cell proliferation at early stages to play tumor-suppressive roles, while it inhibits cancer cell invasion and migration at advanced cancer stages to promote cancer progression.7,13 Recent studies have also shown that TGF-β is critical for the stemness of cancer cells. For example, the activation of TGF-β has been proved to be critical for the maintenance of glioma-initiating cells.14 In effect, TGF-β is a therapeutic target to decrease cancer cell stemness in high-grade gliomas.15 Consistently, our study also showed the enhancing effects of TGF-β1 in regulating GBM cell stemness. Interestingly, our study showed that RPSAP52 can positively regulate the expression of TGF-β1 to participate in the regulation of the stemness of GBM cells. However, the underlying mechanisms are still unclear. Our preliminary bioinformatics analysis showed that RPSAP52 may sponge multiple miRNAs, such as miR-663, which can target TGF-β1.16 Therefore, RPSAP52 may serve as a miRNA sponge to positively regulate TGF-β1.
In conclusion, RPSAP52 is upregulated in GBM and predicts poor survival. In addition, RPSAP52 can positively regulate TGF-β1 to increase GBM cell stemness.
Acknowledgments
We thank the financial support from National Natural Science Foundation (81572899).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shabihkhani M, Telesca D, Movassaghi M, et al. Incidence, survival, pathology, and genetics of adult Latino Americans with glioblastoma. J Neurooncol. 2017;132(2):351–358. doi:10.1007/s11060-017-2377-0
2. Alexander BM, Cloughesy TF. Adult glioblastoma. J Clin Oncol. 2017;35(21):2402–2409. doi:10.1200/JCO.2017.73.0119
3. Wick W, Osswald M, Wick A, et al. Treatment of glioblastoma in adults. Ther Adv Neurol Disord. 2018;11:1756286418790452. doi:10.1177/1756286418790452
4. Li X, Wu C, Chen N, et al. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7(22):33440–33450. doi:10.18632/oncotarget.7961
5. Tateishi K, Iafrate AJ, Ho Q, et al. Myc-driven glycolysis is a therapeutic target in glioblastoma. Clin Cancer Res. 2016;22(17):4452–4465. doi:10.1158/1078-0432.CCR-15-2274
6. Balça-silva J, Matias D, Do Carmo A, et al. Cellular and molecular mechanisms of glioblastoma malignancy: implications in resistance and therapeutic strategies. Semin Cancer Biol. 2019;58:130–141. doi:10.1016/j.semcancer.2018.09.007
7. Syed V. TGF‐β Signaling in Cancer. J Cell Biochem. 2016;117(6):1279–1287. doi:10.1002/jcb.v117.6
8. Colak S, Ten Dijke P. Targeting TGF-β signaling in cancer. Trends Cancer. 2017;3(1):56–71. doi:10.1016/j.trecan.2016.11.008
9. Xiao A, Brenneman B, Floyd D, et al. Statins affect human glioblastoma and other cancers through TGF-β inhibition. Oncotarget. 2019;10(18):1716–1728. doi:10.18632/oncotarget.26733
10. Li W, Kang Y. A new Lnc in metastasis: long noncoding RNA mediates the prometastatic functions of TGF-β. Cancer Cell. 2014;25(5):557–559. doi:10.1016/j.ccr.2014.04.014
11. Mondal T, Subhash S, Vaid R, et al. MEG3 long noncoding RNA regulates the TGF-β pathway genes through formation of RNA–DNA triplex structures. Nat Commun. 2015;6:7743. doi:10.1038/ncomms8743
12. D’angelo D, Mussnich P, Sepe R, et al. RPSAP52 lncRNA is overexpressed in pituitary tumors and promotes cell proliferation by acting as miRNA sponge for HMGA proteins. J Mol Med (Berl). 2019;97:1–14.
13. Zhao M, Mishra L, Deng CX. The role of TGF-β/SMAD4 signaling in cancer. Int J Biol Sci. 2018;14(2):111–123.
14. Ikushima H, Todo T, Ino Y, et al. Autocrine TGF-β signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5(5):504–514. doi:10.1016/j.stem.2009.08.018
15. Joseph JV, Balasubramaniyan V, Walenkamp A, et al. TGF-β as a therapeutic target in high grade gliomas–promises and challenges. Biochem Pharmacol. 2013;85(4):478–485. doi:10.1016/j.bcp.2012.11.005
16. Li Q, Cheng Q, Chen Z, et al. MicroRNA-663 inhibits the proliferation, migration and invasion of glioblastoma cells via targeting TGF-β1. Oncol Rep. 2016;35(2):1125–1134. doi:10.3892/or.2015.4432
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.