Back to Journals » Cancer Management and Research » Volume 12
LncRNA PVT1 Acts as a Tumor Promoter in Thyroid Cancer and Promotes Tumor Progression by Mediating miR-423-5p-PAK3
Authors Lin QY, Qi QL, Hou S, Chen Z, Zhang L, Zhao HG, Lin CH
Received 22 September 2020
Accepted for publication 5 November 2020
Published 30 December 2020 Volume 2020:12 Pages 13403—13413
DOI https://doi.org/10.2147/CMAR.S283443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Qiu-Yu Lin,1,* Qian-Le Qi,1,* Sen Hou,1 Zhen Chen,2 Laney Zhang,3 Hong-Guang Zhao,1 Cheng-He Lin1
1Nuclear Medicine Department, The First Hospital of Jilin University, Changchun City 130000, Jilin Province, People’s Republic of China; 2Chengdu Xinke Pharmaceutical Co., LTD, Chengdu City 610000, Sichuan Province, People’s Republic of China; 3Biological Sciences at Cornell University (2022), Ithaca, NY, USA
*These authors contributed equally to this work
Correspondence: Cheng-He Lin
Nuclear Medicine Department, The First Hospital of Jilin University, No. 71 Xinmin Street, Changchun City 130000, Jilin Province, People’s Republic of China
Tel +86 431-88782766
Email [email protected]
Introduction: Thyroid cancer (TC) is an endocrine tumor whose risk of onset has been rising, so the deep understanding of its molecular mechanism helps formulate new treatment strategies.
Methods: This paper was aimed at exploring the regulatory mechanism of long non-coding RNA (LncRNA) plasmacytoma variant translocation 1 (PVT1) in TC. The expression of PVT1, miR-423-5p and p21-activated kinase 3 (PAK3) in TC tissues and cell lines was detected by real-time PCR. PAK3 levels were detected by Western blot. Regulatory relationships between target genes and the proliferation, invasion and apoptosis of cells and genes were analyzed.
Results: PVT1 and PAK3 upregulated while miR-423-5p downregulated in the tissues and cell lines. PVT1 downregulation inhibited TC cells from malignantly proliferating and invading, and promoted their apoptosis. PVT1 specifically regulated miR-423-5p, and its overexpression could weaken the anti-tumor effect of this miR on TC cells. In addition, miR-423-5p directly targeted PAK3, and knocking down its expression could weaken the inhibitory effect of PAK3 downregulation on TC progression. Besides, PVT1 acted as a competitive endogenous RNA to sponge this miR and thus regulate PAK3 expression.
Discussion: In conclusion, PVT1 can mediate the molecular mechanism of the miR-423-5p-PAK3 axis regulatory network on regulating TC, so it is a new direction of treating the disease.
Keywords: PVT1, thyroid cancer, miR-423-5p, PAK3
Introduction
Thyroid cancer (TC) is an endocrine tumor that is more common among females, and its occurrence is affected by hormones, reproductive factors and other factors.1,2 The disease has been gradually valued due to its increasing incidence.3 Its typical histological classification is differentiated TC that consists of papillary and follicular TC, accounting for at least 90% of all TC.4 Currently, ultrasonography and cytopathological examinations are two diagnostic methods for TC, but they are not suitable for all patients with the disease. The former is effective for only invasive and metastatic papillary TC, whereas the latter is inconclusive for 20–30% of the patients and may cause over diagnosis to them.5 Patients with TC possibly suffer from metastasis and recurrence. The possibility of lymph node metastasis is 59% and the recurrence rate of the disease is over 25%, which is the reason for the poor prognosis of the patients.6,7 Despite the continuous optimization of therapeutic methods such as surgery, chemotherapy, radioactive iodine therapy and targeted therapy, the cure and the prognosis of patients with advanced TC are unsatisfactory.8,9 Therefore, we believe that new methods for screening out and treating patients with TC still need to be explored.
Long non-coding RNAs (LncRNAs), a kind of molecular regulators that attract much attention, can regulate the information conduction of downstream target genes and target proteins via splicing, adenylation, transport, stability and translation, thereby regulating gene expression and protein functions.10–12 For instance, LncRNA- metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) increases the levels of tumor-associated macrophage fibroblast growth factor-2 (FGF2) and then promotes the angiogenesis of TC.13 LncRNA-taurine-upregulated gene 1 (TUG1) acts as a molecular sponge for miR-145 to regulate the progression and metastasis of TC.14 LncRNA-n340790 that is an endogenous RNA of miR-1254 competitively binds to this miR to downregulate its expression, so as to promote the malignant progression of the disease.15 Therefore, the roles of LncRNAs in the survival mechanism of TC need to be further explored. As a member of the LncRNA family, plasmacytoma variant translocation 1 (PVT1) is located in cancer-related region 8q24 and acts as a tumor promoter in various cancers.16 As reported by Xu and other researchers, PVT1 and forkhead box M1 (FOXM1) form a positive feedback loop to promote the growth and invasion of gastric cancer.17 According to Gao et al, PVT1 can promote the progression of cervical cancer through the negative regulation of miR-424.18 Moreover, this LncRNA is overexpressed in the tissues and saliva of patients with pancreatic cancer, so it has a certain clinical diagnostic value for this cancer.19 It has been found that miR-423-5p abnormally reduces in more aggressive TC phenotypes, which indicates that this miR may have a potential effect on inhibiting tumors.20 As a member of the p21-activated kinase (PAK) family, PAK3 is a carcinogenic factor that regulates crucial cell behaviors and participates in the occurrence and progression of tumors such as TC.21
In this paper, we observed highly expressed PVT1 in the tissues, serum and cells of patients with TC. Through the analysis of cell functions, we found that PVT1 could promote TC cells to proliferate and invade and reduce their apoptosis in vitro. Therefore, bioinformatics analysis and biological experiments were carried out in this study, to verify the potential mechanism of PVT1 on playing its malignant role. In short, PVT1 acts as a competitive endogenous RNA (ceRNA) to sponge miR-423-5p, and then regulates PAK3 in TC, which indicates that this LncRNA may become a new direction of diagnosing and treating the disease.
Materials and Methods
Sample Collection
Forty-seven patients with TC, who were admitted to The First Hospital of Jilin University from January 2018 to January 2020, were enrolled as a TC group. All patients did not receive any treatment before operation. After their consent, their cancer and adjacent tissues were obtained during the operation. All tissue samples were identified and confirmed by three pathologists, and then stored in a liquid nitrogen container for later use. Further 40 health controls that underwent physical examinations during the same period were enrolled as an HC group. Serum was collected from the two groups, and all samples were stored at −80°C for later use. This study has been approved by The First Hospital of Jilin University Ethics Committee, and the experiment processes were conducted in strict accordance with the Declaration of Helsinki. This study has obtained the informed consent of all included subjects, who were informed of its purpose.
Cell Culture
Human TC cell lines (HTh-7, ACT-1, C643, TTA1; Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd., China; ZQ0314, ZQ0310, ZQ0321, ZQ0281) and normal thyroid cells (Htori-3; CMBIO, Shanghai, China; CM-2116S) were purchased. They were placed in a Dulbecco’s modification of Eagle’s medium (DMEM; Biolab Science and Technology Co., Ltd., Beijing, China; GS0187-XFS) containing 10% phosphate buffer solution (PBS), 100 μg/mL of penicillin and 100 μg/mL of streptomycin (Beijing Xiangsheng Xingye Technology Co., Ltd., China; 15140122) for culture at 37°C and 5% CO2.
Cell Transfection
Recombinant plasmids pEGFP-PVT1/PAK3 and pSilencer-PVT1/PAK3 were constructed to upregulate and downregulate expression, respectively. Using Lipofectamine™ 2000 kits (Thermo Fisher Scientific, Shanghai, China; 11668019), PVT1 low expression plasmid pSilencer-PVT1 (si-PVT1), PVT1 high expression plasmid pEGFP-PVT1 (PVT1), PAK3 low expression plasmid pSilencer-PAK3 (si-PAK3), PAK3 high expression plasmid pEGFP-PAK3 (PAK3), negative control RNA (si-NC), miR-423-5p-mimics (miR-423-5p) and miR negative control (miR-NC) were respectively transfected into the cells. The operating steps were strictly carried out based on the kit instruction.
Real-Time Quantitative PCR
Trizol reagents (Biolab Science and Technology Co., Ltd., Beijing, China; QN2070-ZOG) were adopted for extracting total RNA from the tissues, serum and cells. The reverse transcription of the extracted RNA was performed with reverse transcription kits (Biolab Science and Technology Co., Ltd., Beijing, China; QN0931-MOD), and the synthesized complementary DNA (cDNA) was amplified after the transcription. β-Actin and U6 were the internal references of the mRNA and the miRNA, respectively, with 2−ΔΔct used for data analysis.
Western Blot
Radio-immunoprecipitation assay (RIPA) lysis buffer (G-CLONE, Beijing, China; EX-6020-100mL) was conducted to extract the total protein from the tissues and the cultured TC cells, with the protein concentration detected by bicinchoninic acid (BCA) protein assay kits (Beijing Dingguochangsheng Biotechnology Co., Ltd., China; BCA-01). Next, the protein was separated by electrophoresis and then transferred to the membrane, which was sealed in 5% skimmed milk (NobleRyder, Beijing, China; 1706404) at room temperature for 60 min and then incubated with primary antibodies all night at 4°C. The primary antibodies consisted of PAK3 and β-Actin, with a dilution ratio of 1: 1000. The membrane was incubated with a secondary antibody (Taize Jiaye Technology Development Co., Ltd., Beijing, China; A21202) for 60 min. The ChemiScope 6000 ECL system (Clinx Science Instruments, Shanghai, China) was used to analyze protein bands.
Cell Proliferation Assay
The cells were inoculated on a 96-well plate at 4*103 cells/well, and then incubated at 37°C for 3 days. They were added with 20 μL of 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (Biolab Science and Technology Co., Ltd., Beijing, China; GL0247-SBJ) once every 24 hours, and then continuously cultured at 37°C for 4 hours. Dimethyl sulfoxide (200 μL) was added to each well. Then, the optical density (OD) values of the cells in each group were measured at 450 mm using a Multiskan FC microplate reader (Beijing Neobio Science & Technology Co., Ltd., China).
Cell Invasion Assay
Transwell chambers were used for cell invasion assay. The transfected TC cells (2×104 cells/well) were suspended in a serum-free medium (200 µL) and then inoculated on the upper chamber (containing Matrigel). Subsequently, a complete medium (500 µL) acting as a chemical attractant was added to the lower chamber. After 24-hour incubation in the incubator, the cells on the upper membrane surface were removed with cotton swabs, while those penetrating the lower surface were stained with crystal violet.
Determination of Apoptosis
Apoptosis detection kits (G-CLONE, Beijing, China; CC2210-20T) were used for detection. The cells were prepared into a suspension (1*106 cells/mL), digested with 0.25% trypsin, cleaned with PBS for twice, and then mixed with binding buffer (100 μL). After that, AnnexinV-FITC and Propidium Iodide (PI; 10 μL each) were added to the suspension, which was incubated at room temperature in the dark for 5 min. The DxFLEX flow cytometer (Morey Biosciences, Inc., Shanghai, China) was used for detection. Three repeated experiments were conducted to obtain the average value.
Bioinformatics Analysis
The online prediction tool StarBase (http://starbase.sysu.edu.cn/) was used for the targeted prediction between LncRNA-miRNAs, whereas Targetscan (http://www.targetscan.org/vert_72/) was used for the targeted prediction between miRNA-mRNAs.
Dual Luciferase Reporter Gene Assay (DLRGA)
PVT1 wild-type (PVT1-Wt), PVT1 mutant (PVT1-Mut) and the complementary DNA fragments of miR-423-5p fragments were subcloned to the downstream of luciferase genes in luciferase reporter vectors. miR-423-5p was co-transfected with PAK3 wild-type (Wt) or PAK3-mutant (Mut) for 48 hours. After that, firefly and renin luciferase activities in cell lysates were continuously determined using DLRGA kits (Biolab Science and Technology Co., Ltd., Beijing, China; KFS303-LBV).
RNA Immunocoprecipitation (RIP)
Experiments were carried out using EZMagna RIP kits (GuYan Biotech Co., Ltd., Shanghai, China; GOY-E5944). The cells were cleaved, incubated with protein A magnetic beads, and then conjugated with antibodies at 4°C. After 6 hours, the beads were cleaned and then incubated with protease K (0.1%SDS/0.5mg/mL) at 55°C for 30 min. Finally, the immunoprecipitated RNA was detected and analyzed with specific primers to confirm the presence of PVT1 and miR-423-5p.
RNA Pull-Down Assay
TC cells were transfected with biotinylated miR-423-5p-Wt, miR-423-5p-Mut and negative control Bio-NC, respectively. Forty-eight hours later, the cell lysates were incubated with M-280 streptomyces magnetic beads, and then PVT1 levels in the RNA complex that bound to the beads were detected.
Statistical Methods
Statistical analysis and figure plotting were carried out using GraphPad 6. The data were expressed as mean ± standard deviation. All experiments were conducted independently for at least 3 times. Difference comparisons between groups were performed through independent samples t test, one-way analysis of variance (ANOVA), LSD-t test, repeated measures ANOVA and Bonferroni. P<0.05 indicated that the difference was statistically significant. The receiver operating characteristic (ROC) curve was plotted to evaluate the area under the curve (AUC) for diagnosing TC, and Pearson test was used for correlation analysis.
Results
PVT1 Overexpression in Tissues and Serum
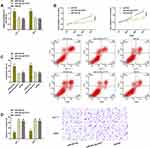
PVT1 upregulated in the tissues and serum of patients with TC (P<0.05). Its AUC, sensitivity, specificity and optimal cut-off value for diagnosing TC in serum samples were 0.914, 85.11%, 85.00% and 0.65, respectively (Figure 1).
PVT1 Expression Beneficial to Promote TC Cell Survival
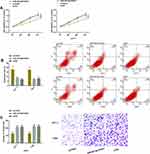
PVT1 expression was higher in TC cell lines and was the highest in ACT-1 and C643 cells, so the last two cells were chosen for follow-up experimental analysis. After transfected with si-PVT1, the two cells showed lower proliferation and invasion and higher apoptotic rates, while the above results were remarkably reversed after transfection with PVT1 (P<0.05) (Figure 2).
PVT1 Used as a ceRNA to Regulate miR-423-5p
We verified the relationship between PVT1 and miR-423-5p via DLRGA, RIP, and RNA pull-down assay, respectively. The results showed that miR-423-5p upregulation only reduced PVT1-Wt (instead of PVT1-Mut) luciferase activities. PVT1 and miR-423-5p in Ago2 antibody precipitation were remarkably higher than those in IgG. PVT1 was pulled down only by biotin-labeled miR-423-5p-Wt. Then, after PVT1 downregulation/upregulation, TC cells had higher/lower miR-423-5p levels. For verifying whether miR-423-5p had an effect on TC, we carried out relevant clinical research on this miR. The results showed that this miR had low levels in the cancer tissues of TC patients, and was negatively correlated with PVT1 (r=−0.658, P<0.001). These findings had statistical significance (P<0.05) (Figure 3).
PVT1 Affected Survival of TC Cells by Regulating miR-423-5p
We transfected miR-423-5p and miR-423-5p+PVT1 into TC cells, respectively, to verify whether the effects of this miR on the cell survival were regulated by PVT1. Cell function experiments showed that compared with those in the miR-NC group, the proliferation and invasion of the cells were inhibited, while their apoptosis was induced in the miR-423-5p group. However, the co-transfection of miR-423-5p+PVT1 reversed the effects of this miR on the above survivability, with an increase in the proliferation and invasion levels and a sharp decrease in apoptosis. These findings had statistical significance (P<0.05) (Figure 4).
PVT1 Targeted miR-423-5p to Mediate PAK3 Expression
We screened out the downstream target genes of miR-423-5p through the miRNA target gene online prediction website and found potential binding sites between PAK3 and this miR. DLRGA was conducted for determining the relationship between the two genes. The results showed that they had a targeted regulatory relationship and that miR-423-5p overexpression inhibited luciferase activities in PAK3-Wt, which could be eliminated by the co-transfection of PVT1. The effects of PVT1/miR-423-5p on PAK3 on TC cells were further analyzed. The results showed that the transcription and protein levels of PAK3 remarkably reduced after miR-423-5p transfection, which could be also eliminated by the co-transfection of PVT1. Moreover, we found through clinical studies that PAK3 was highly expressed in the cancer tissues of TC patients. Correlation analysis showed that PAK3 was positively correlated with PVT1 (r=0.709, P<0.001), but negatively correlated with miR-423-5p (r=−0.619, P<0.001) (Figure 5).
PVT1 Targeted miR-423-5p to Mediate Effects of PAK3 on Survival of TC Cells
For confirming the effects of PAK3 on TC, we carried out cell experiments. The results showed that the proliferation and invasion of TC cells were inhibited and their apoptotic rate rose after knocking out PAK3, while the phenomenon was eliminated after the co-transfection of miR-423-5p and PVT1 (P<0.05) (Figure 6).
Discussion
LncRNAs are involved in the pathogenesis of tumor cells and exert a regulatory function by mediating the cycles, proliferation, invasion, apoptosis, drug resistance and other processes of cells.22 Their abnormal expression in cancer patients may cause the normal physiological process of cells to get out of control and eventually lead to the development and progression of tumors.23 This study explored TC, which is a common malignant endocrine tumor,24 so we analyzed the potential regulatory value of LncRNAs through clinical samples and cell functional experiments in vitro.
PVT1 has been confirmed to function as a tumor promoter in TC, but its molecular mechanism remains unclear.25 Therefore, we further studied its potential regulatory mechanism on the malignant survival of TC cells. Consistent with previous studies, the results showed that PVT1 was significantly higher in TC tissues, serum and cell lines than that in corresponding normal tissues, serum and thyroid cells. In addition, the AUC, sensitivity and specificity of PVT1 for diagnosing TC in serum samples were >0.900, 85.11% and 85.00%, respectively, which indicates that PVT1 has high diagnostic potential for the disease. Next, we performed cell function assays for ACT-1 and C643 cells, and the results showed that consuming PVT1 could inhibit the proliferation and invasion of the cells in vitro and induce their apoptosis. Nowadays, more researchers have discussed the potential value of PVT1 in TC. For example, Zhou and others have revealed that PVT1 can promote the proliferation of TC cells and their cycle progression by recruiting enhancer of zeste homolog 2 (EZH2).26 According to Feng et al, it can also specifically inhibit miR-30a and positively regulate downstream target protein insulin-like growth factor 1 receptor, thus promoting the survival or metastasis behaviors (such as proliferation, invasion and migration) of papillary TC.27 In general, the data have shown that PVT1 has an effect on the survival of TC cells.
miRNA molecular sponge is the most classical target gene regulation theory of LncRNAs, which are used as specific absorbents of miRs to reduce the expression and transcription of miRNAs.28,29 There are many previous reports on PVT1 acting as a molecular sponge to regulate miRNAs. For instance, Li and other researchers have found that PVT1 promotes the malignant progression of esophageal squamous cell carcinoma through sponging miR-203.30 According to Wang et al, it also acts as a molecular sponge for miR-199a-5p and plays a linkage role in non-small cell lung cancer.31 The above studies all demonstrate that PVT1 as a miRNA sponge has a specific regulatory effect. The analysis of the online prediction tool showed target sites between miR-423-5p and PVT1, and their targeted regulatory relationship was confirmed through DLRGA, RIP, and RNA pull-down assay. We also found that miR-423-5p was highly expressed in cancer tissues, and the correlation analysis confirmed the negative correlation of PVT1 with this miR. In previous studies, both PVT1 and miR-423-5p have regulatory effects on some malignant tumors such as ovarian cancer, breast cancer and nasopharyngeal cancer, which suggests that there may be a close relationship between the two genes.32–34 In our study, we transfected miR-423-5p into TC cells for determining whether miR-423-5p could affect the growth of TC via PVT1 regulation. The results showed that miR-423-5p upregulation could effectively inhibit the cells from proliferating and invading and promote their apoptosis, but the above results were reversed after the co-transfection of miR-423-5p and PVT1, which suggests that PVT1 can regulate miR-423-5p and then affect the survival of TC cells.
miRNAs function mainly through regulating downstream target genes.35 Belonging to the effector protein kinase family of small Rho GTPases, PAK3 can serve as a crucial effector for the malignant phenotype of tumor cells.36,37 According to Ma et al, its over-activation has been manifested in various malignant tumors,38 but there are currently few reports about this protein in TC. In this study, we found targeted binding sites between PAK3 and miR-423-5p through the miRNA online target gene prediction, and confirmed their regulatory relationship through DLRGA. Besides, PAK3 expression remarkably reduced after the transfection of single miR-423-5p, whereas both the transcription levels and the protein levels of PAK3 remarkably rose after the co-transfection of miR-423-5p and PVT1. After si-PAK3 transfection, the proliferation and invasion of the cells were obviously inhibited, but their apoptotic rate increased. As reported by Stiuso and researchers, miR-423-5p has a negatively regulatory effect on PAK3 levels in HuH7 liver cancer cells,39 which is similar to our research results. Moreover, the correlation analysis showed that PAK3 had a positive correlation with PVT1 and a negative correlation with miR-423-5p. The above findings suggest that PVT1 regulates the miR-423-5p-PAK3 pathway and then affects the survival of TC cells.
This study has confirmed that PVT1 interferes with the survival of TC cells by competitively binding to miR-423-5p and positively regulating PAK3 expression, but it still needs improvement. First of all, we can add the research on the diagnostic value of PVT1 for the molecular phenotypes of TC. Secondly, we can analyze the relationship between PVT1 and the pathological parameters of patients with TC. Besides, we can study the effects of PVT1 on the epithelial–mesenchymal transition of TC cells, so as to further supplement its potential regulatory effects.
Conclusion
In summary, PVT1 acts as a tumor promoter in TC and promotes tumor survival by mediating the miR-423-5p-PAK3 pathway, so it may provide a new clinical direction for diagnosing and treating patients with TC.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Project Number: 81901774). Qiu-Yu Lin and Qian-Le Qi are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liotti F, De Pizzol M, Allegretti M, Prevete N, Melillo RM. Multiple anti-tumor effects of reparixin on thyroid cancer. Oncotarget. 2017;8(22):35946–35961. doi:10.18632/oncotarget.16412
2. Moleti M, Sturniolo G, Di Mauro M, Russo M, Vermiglio F. Female reproductive factors and differentiated thyroid cancer. Front Endocrinol (Lausanne). 2017;8:111. doi:10.3389/fendo.2017.00111
3. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653.
4. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
5. Fagin JA, Wells SA
6. Zhuang X, Tong H, Ding Y, et al. Long noncoding RNA ABHD11-AS1 functions as a competing endogenous RNA to regulate papillary thyroid cancer progression by miR-199a-5p/SLC1A5 axis. Cell Death Dis. 2019;10(8):620. doi:10.1038/s41419-019-1850-4
7. Tan J, Qian X, Song B, et al. Integrated bioinformatics analysis reveals that the expression of cathepsin S is associated with lymph node metastasis and poor prognosis in papillary thyroid cancer. Oncol Rep. 2018;40(1):111–122.
8. Valerio L, Pieruzzi L, Giani C, et al. Targeted therapy in thyroid cancer: state of the art. Clin Oncol (R Coll Radiol). 2017;29(5):316–324. doi:10.1016/j.clon.2017.02.009
9. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi:10.1016/S0140-6736(16)30172-6
10. Engreitz JM, Haines JE, Perez EM, et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539(7629):452–455.
11. Ferre F, Colantoni A, Helmer-Citterich M. Revealing protein-lncRNA interaction. Brief Bioinform. 2016;17(1):106–116. doi:10.1093/bib/bbv031
12. Paralkar VR, Taborda CC, Huang P, et al. Unlinking an lncRNA from its associated cis element. Mol Cell. 2016;62(1):104–110. doi:10.1016/j.molcel.2016.02.029
13. Huang JK, Ma L, Song WH, et al. LncRNA-MALAT1 promotes angiogenesis of thyroid cancer by modulating tumor-associated macrophage FGF2 protein secretion. J Cell Biochem. 2017;118(12):4821–4830. doi:10.1002/jcb.26153
14. Lei H, Gao Y, Xu X. LncRNA TUG1 influences papillary thyroid cancer cell proliferation, migration and EMT formation through targeting miR-145. Acta Biochim Biophys Sin (Shanghai). 2017;49(7):588–597. doi:10.1093/abbs/gmx047
15. Li Q, Shen W, Li X, Zhang L, Jin X. The lncRNA n340790 accelerates carcinogenesis of thyroid cancer by regulating miR-1254. Am J Transl Res. 2017;9(5):2181–2194.
16. Cui M, You L, Ren X, Zhao W, Liao Q, Zhao Y. Long non-coding RNA PVT1 and cancer. Biochem Biophys Res Commun. 2016;471(1):10–14. doi:10.1016/j.bbrc.2015.12.101
17. Xu MD, Wang Y, Weng W, et al. A positive feedback loop of lncRNA-PVT1 and FOXM1 facilitates gastric cancer growth and invasion. Clin Cancer Res. 2017;23(8):2071–2080. doi:10.1158/1078-0432.CCR-16-0742
18. Gao YL, Zhao ZS, Zhang MY, Han LJ, Dong YJ, Xu B. Long noncoding RNA PVT1 facilitates cervical cancer progression via negative regulating of miR-424. Oncol Res. 2017;25(8):1391–1398. doi:10.3727/096504017X14881559833562
19. Xie Z, Chen X, Li J, et al. Salivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancer. Oncotarget. 2016;7(18):25408–25419. doi:10.18632/oncotarget.8323
20. Yang C, Liu Z, Chang X, et al. NR2F1-AS1 regulated miR-423-5p/SOX12 to promote proliferation and invasion of papillary thyroid carcinoma. J Cell Biochem. 2020;121(2):2009–2018. doi:10.1002/jcb.29435
21. Bautista L, Knippler CM, Ringel MD. p21-Activated kinases in thyroid cancer. Endocrinology. 2020;161(8). doi:10.1210/endocr/bqaa105
22. Liao T, Qu N, Shi RL, et al. BRAF-activated LncRNA functions as a tumor suppressor in papillary thyroid cancer. Oncotarget. 2017;8(1):238–247. doi:10.18632/oncotarget.10825
23. Wang W, Zhou R, Wu Y, et al. PVT1 promotes cancer progression via MicroRNAs. Front Oncol. 2019;9:609. doi:10.3389/fonc.2019.00609
24. Luo JZ, Qin L, Zhang LJ. Expression and function of long non-coding RNA LINC01420 in thyroid cancer. Oncol Lett. 2020;19(1):399–405.
25. Derderian C, Orunmuyi AT, Olapade-Olaopa EO, Ogunwobi OO. PVT1 signaling is a mediator of cancer progression. Front Oncol. 2019;9:502.
26. Zhou Q, Chen J, Feng J, Wang J. Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR). Tumour Biol. 2016;37(3):3105–3113. doi:10.1007/s13277-015-4149-9
27. Feng K, Liu Y, Xu LJ, Zhao LF, Jia CW, Xu MY. Long noncoding RNA PVT1 enhances the viability and invasion of papillary thyroid carcinoma cells by functioning as ceRNA of microRNA-30a through mediating expression of insulin like growth factor 1 receptor. Biomed Pharmacother. 2018;104:686–698. doi:10.1016/j.biopha.2018.05.078
28. Militello G, Weirick T, John D, Doring C, Dimmeler S, Uchida S. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief Bioinform. 2017;18(5):780–788.
29. Gaiti F, Fernandez-Valverde SL, Nakanishi N, et al. Dynamic and widespread lncRNA expression in a sponge and the origin of animal complexity. Mol Biol Evol. 2015;32(9):2367–2382. doi:10.1093/molbev/msv117
30. Li PD, Hu JL, Ma C, et al. Upregulation of the long non-coding RNA PVT1 promotes esophageal squamous cell carcinoma progression by acting as a molecular sponge of miR-203 and LASP1. Oncotarget. 2017;8(21):34164–34176. doi:10.18632/oncotarget.15878
31. Wang C, Han C, Zhang Y, Liu F. LncRNA PVT1 regulate expression of HIF1alpha via functioning as ceRNA for miR199a5p in nonsmall cell lung cancer under hypoxia. Mol Med Rep. 2018;17(1):1105–1110.
32. Du W, Feng Z, Sun Q. LncRNA LINC00319 accelerates ovarian cancer progression through miR-423-5p/NACC1 pathway. Biochem Biophys Res Commun. 2018;507(1–4):198–202. doi:10.1016/j.bbrc.2018.11.006
33. Zhang Y, Li Y, Wang Q, et al. Identification of an lncRNAmiRNAmRNA interaction mechanism in breast cancer based on bioinformatic analysis. Mol Med Rep. 2017;16(4):5113–5120. doi:10.3892/mmr.2017.7304
34. Jia X, Niu P, Xie C, Liu H. Long noncoding RNA PXN-AS1-L promotes the malignancy of nasopharyngeal carcinoma cells via upregulation of SAPCD2. Cancer Med. 2019;8(9):4278–4291. doi:10.1002/cam4.2227
35. Zhao H, Yuan H, Hu J, et al. Optimizing prognosis-related key miRNA-target interactions responsible for cancer metastasis. Oncotarget. 2017;8(65):109522–109535. doi:10.18632/oncotarget.22724
36. Boda B, Jourdain L, Muller D. Distinct, but compensatory roles of PAK1 and PAK3 in spine morphogenesis. Hippocampus. 2008;18(9):857–861. doi:10.1002/hipo.20451
37. Wu HY, Yang MC, Ding LY, Chen CS, Chu PC. p21-Activated kinase 3 promotes cancer stem cell phenotypes through activating the Akt-GSK3beta-beta-catenin signaling pathway in pancreatic cancer cells. Cancer Lett. 2019;456:13–22. doi:10.1016/j.canlet.2019.04.026
38. Ma Y, McCarty SK, Kapuriya NP, et al. Development of p21 activated kinase-targeted multikinase inhibitors that inhibit thyroid cancer cell migration. J Clin Endocrinol Metab. 2013;98(8):E1314–E1322. doi:10.1210/jc.2012-3937
39. Stiuso P, Potenza N, Lombardi A, et al. MicroRNA-423-5p promotes autophagy in cancer cells and is increased in serum from hepatocarcinoma patients treated with sorafenib. Mol Ther Nucleic Acids. 2015;4:e233. doi:10.1038/mtna.2015.8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.