Back to Journals » OncoTargets and Therapy » Volume 12
LncRNA MIR100HG promotes cancer cell proliferation, migration and invasion in laryngeal squamous cell carcinoma through the downregulation of miR-204-5p
Authors Huang Y, Zhang C, Zhou Y
Received 22 January 2019
Accepted for publication 7 March 2019
Published 17 April 2019 Volume 2019:12 Pages 2967—2973
DOI https://doi.org/10.2147/OTT.S202528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Yongyang Huang, Chao Zhang, Yanhui Zhou
Department of ENT, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, Henan 471000, People’s Republic of China
Purpose: LncRNA MIR100HG promotes several types of malignancies, while its involvement in other human diseases is unknown.
Patients and methods: Our study included 70 patients with LSCC who were diagnosed and treated in the First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology from January 2016 to July 2018. qRT-PCR, cell transfection, in vitro cell proliferation assay, cell migration and invasion assay were applied for the research.
Results: In the present study we found that MIR100HG was upregulated, while miR-204-5p was downregulated in tumor tissues than in adjacent healthy tissues of laryngeal squamous cell carcinoma (LSCC) patients. Expression of MIR100HG was significantly affected by AJCC stage. A significant and inverse correlation between MIR100HG and miR-204-5p was found in tumor tissues but not in adjacent healthy tissues of LSCC patients. Overexpression of MIR100HG resulted in the downregulation of miR-204-5p in LSCC cells, while miR-204-5p overexpression failed to significantly affect MIR100HG expression. Overexpression of MIR100HG led to promoted, while miR-204-5p, overexpression led to inhibited proliferation, migration and invasion of LSCC cells. In addition, miR-204-5p overexpression attenuated the enhancing effects of MIR100HG overexpression on cancer cell proliferation, migration and invasion.
Conclusion: Therefore, lncRNA MIR100HG promoted cancer cell proliferation, migration and invasion in LSCC possibly through the downregulation of miR-204-5p.
Keywords: laryngeal squamous cell carcinoma, lncRNA MIR100HG, miR-204-5p
Introduction
Laryngeal squamous cell carcinoma (LSCC) as the most common type of laryngeal cancer is a rare type of human malignancy but causes unacceptable higher mortality rate due to it highly aggressive nature.1 Although human papillomavirus (HPV) infection has been proved as a risk factor of LSCC, HPV positive patients only accounts for a small portion of LSCC cases and early screening of HPV infection failed to significantly reduce the incidence of LSCC.2–4 At the present, the treatment of LSCC is still challenged by the high prevalence of cancer metastasis by the time of first diagnosis.5
The human genome transcribes both messenger RNAs (mRNAs) and non-coding RNAs (ncRNAs) to regulate biological processes.6 Different from the roles of mRNAs as mediators between DNA and protein products, ncRNAs directly participate in both physiological and pathological processes in the form of RNA.7 Long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) with different lengths are two major subgroups of ncRNAs. A growing body of literatures has shown that lncRNAs and miRNAs may interact with each other to participate in human cancers.8–10 LncRNA MIR100HG promotes breast cancer,11 while its involvement in other human diseases is unknown. Our preliminary RNA-seq data suggested the upregulated expression of lncRNA MIR100HG and its inverse correlation with miR-204-5p. In the present study we showed that lncRNA MIR100HG promoted cancer cell proliferation, migration and invasion in LSCC possibly through the downregulation of miR-204-5p, which has been characterized as a tumor suppressive miRNA in LSCC.12
Materials and methods
Research subjects
Our study included 70 patients with LSCC who were diagnosed and treated in the First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology from January 2016 to July 2018. Inclusion criteria: 1) patients diagnosed through pathological biopsies; 2) patients with complete medical record; 3) patients willing to participate and signed informed consent. Exclusion criteria:1) Patients who were diagnosed with multiple diseases; 2) patients who had been treated before admission. The patients included 37 males and 33 females, and age ranged from 22 to 66 years, with a mean age of 44.8±5.7 years. According to AJCC staging, there were 12 cases at stage I, 18 at stage II, 25 at stage III and 15 at stage IV. Based on the location of tumors, there were 40 cases of glottic carcinoma, 22 cases of supraglottic carcinoma, and 8 cases of subglottic carcinoma. This study was approved by the Ethics Committee of the First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology before the admission of patients.
Specimens and cell line
All patients received biopsies and tumor and adjacent (within 2 cm around tumors) healthy tissues were collected. All tissue specimens were confirmed by 3 experienced pathologists. UM-SCC-17A Human LSCC cell line was used to performed all in vitro cell experiments. Cells of this cell line were purchased from MilliporeSigma (Burlington, MA, USA). Cells were cultivated under conditions recommended by MilliporeSigma.
Real-time quantitative reverse transcription PCR (qRT-PCR)
To detect the expression of lncRNA MIR100HG, total RNA was extracted using Trizol reagent (Invitrogen, USA), Applied Biosystems™ High-Capacity cDNA Reverse Transcription Kit was used to perform in vitro transcription, and PCR reaction systems were prepared using SYBR® Green Real-Time PCR Master Mixes (Thermo Fisher Scientific, USA). To detect the expression of miR-204-5p, miRNAs were extracted using miRNeasy Mini Kit (QIAGEN), reverse transcription and performed using qScript microRNA cDNA Synthesis Kit (Quantabio) and PCR reaction systems were prepared using miScript SYBR Green PCR Kit (QIAGEN). Primers of lncRNA MIR100HG, miR-204-5p, as well as endogenous controls GAPDH and U6 were designed and synthesized by Sangon (Shanghai, China). Data normalizations were performed using 2−ΔΔCT method. Expression of lncRNA MIR100HG was normalized to GAPDH and expression of miR-204-5p, was normalized to U6. It is worth noting that we detected mature miRNA after adding poly A. The forward primer was: 5ʹ-TCGTCCCTTTGCCTTCCCAGC-5ʹ (reverse complementary sequence of miR-204-5p) and reverse primer was poly T.
Cell transfection
Vectors expressing lncRNA MIR100HG were constructed by Sangon (Shanghai, China). miR-204-5p miRNA Mimic and negative control miRNA were purchased from abm (Richmond, BC, Canada). Cells were cultivated overnight to reach 70–80% confluence. Cells transfections were performed using Lipofectamine 2,000 reagent (11,668-019, Invitrogen, Carlsbad, USA). Doses of vectors and miRNAs were 10 and 50 nM, respectively. Cells treated with Lipofectamine 2,000 reagent were control cells. Cells transfected with empty vectors or negative control miRNA were negative control cells.
In vitro cell proliferation assay
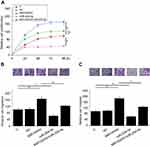
Overexpression of lncRNA MIR100HG and miR-204-5p were confirmed by RT-qPCR, and cell proliferation assay was carried out only in cases of overexpression rates of both lncRNA MIR100HG and miR-204-5p were higher than 200%. Cell suspensions with a cell density of 3×104 cells/ml were prepared. Cell suspensions were transferred to a 96-well plate with 0.1 ml per well. Cells were cultivated at 37 °C with 5% CO2, followed by the addition of CCK-8 solution (10 ul, Sigma-Aldrich) 24, 48, 72 and 96 h later. After that, cells were cultivated from additional 4 h and OD values at 450 nm were measured.
Cell migration and invasion assay
Overexpression of lncRNA MIR100HG and miR-204-5p were confirmed by RT-qPCR, and cell migration and invasion assays were carried out only in cases of overexpression rates of both lncRNA MIR100HG and miR-204-5p were higher than 200%. Cell suspensions with a cell density of 3×104 cells/ml were prepared using serum-free cell culture medium. Cell suspension was transferred to the upper chamber with 0.1 ml per well, while the lower chamber was filled with cell culture medium containing 20% FCS (Sigma-Aldrich, USA), which is the chemical attractant. Cells were cultivated for at 37 °C with 5% CO2, followed by the staining of upper chamber membranes with 0.5% crystal violet (Sigma-Aldrich) for 20 min at room temperature. Stained cells were observed and counted under an optical microscope. Migration and invasion assays were performed in the same way except that the upper chamber membrane was pre-coated with Matrigel (356,234, Millipore, Billerica, USA) before invasion assay.
Statistical analysis
All experiments were repeated 3 times and data were recorded and mean±standard dev. Comparisons of expression levels of lncRNA MIR100HG and miR-204-5p between tumor tissues and adjacent healthy tissues were performed by paired t test. Comparisons among multiple groups were performed by one-way ANOVA and Tukey test. Correlations between expression levels of lncRNA MIR100HG and miR-204-5p were analyzed by Pearson’s correlation coefficient. p<0.05 indicated a difference with statistical significance.
Results
LncRNA MIR100HG and miR-204-5p showed opposite expression pattern in LSCC
Expression of lncRNA MIR100HG and miR-204-5p in tumor tissues and adjacent healthy tissues of 70 LSCC patients was detected by RT-qPCR. Compared with adjacent healthy tissues, MIR100HG was significantly upregulated (Figure 1A), while miR-204-5p was significantly downregulated (Figure 1B) in tumor tissues (p<0.05).
Expression of MIR100HG and miR-204-5p in tumor tissues was significantly affected by AJCC stage
According to AJCC staging, there were 12 cases at stage I, 18 at stage II, 25 at stage III and 15 at stage IV. As shown in Figure 2A, expression levels of MIR100HG were significantly increased with the increase of AJCC stages (p>0.05). In contrast, expression levels of miR-204-5p were significantly decreased with the increase of AJCC stages (Figure 2B, p<0.05).
Expression levels of MIR100HG and miR-204-5p were significantly and inversely correlated in tumor tissues
Correlations between expression levels of lncRNA MIR100HG and miR-204-5p were analyzed by Pearson’s correlation coefficient. As shown in Figure 3, a significant and inverse correlation between MIR100HG and miR-204-5p levels was found in tumor tissues. However, the correlation between expression levels of MIR100HG and miR-204-5p was not significant in adjacent healthy tissues of LSCC patients.
Overexpression of MIR100HG mediated the downregulation of miR-204-5p
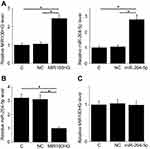
Overexpression of MIR100HG and miR-204-5p was reached in cells of UM-SCC-17A cell line at 24h after transfection (Figure 4A, p<0.05). Compared with control (C) and negative control (NC) groups, overexpression of MIR100HG led to the downregulation of miR-204-5p in LSCC cells (Figure 4B, p<0.05), while miR-204-5p, overexpression failed to significantly affect MIR100HG expression (Figure 4C).
LncRNA MIR100HG promoted cancer cell proliferation, migration and invasion through miR-204-5p
Compared with control (C) and negative control (NC) groups, overexpression of MIR100HG led to promoted, while miR-204-5p, overexpression led to inhibited proliferation (Figure 5A), migration (Figure 5B) and invasion (Figure 5C) of cells of UM-SCC-17A cell line (p<0.05). In addition, miR-204-5p, overexpression attenuated the enhancing effects of MIR100HG overexpression on cancer cell proliferation, migration and invasion (p<0.05).
Discussion
LncRNA MIR100HG has been proven to be an oncogenic lncRNA in breast cancer. Based on our knowledge, the involvement of lncRNA MIR100HG in other human diseases is unknown. The key finding of the present study is that lncRNA MIR100HG promoted LSCC through the downregulation of miR-204-5p.
miR-204-5p is recognized as a tumor suppression miRNA in most types of cancers, such as colorectal cancer13 and papillary thyroid carcinoma.14 However, a recent study reported that miR-204-5p contribute to development of BRAF inhibitor resistance, which is a big challenge in the treatment of melanoma, indicating the oncogenic role of miR-204-5p in this disease.15 Gao et al in a recent study reported that miR-204-5p was downregulated in LSCC and upregulated expression of miR-204-5p led to inhibited cancer cell invasion.12 Consistent with previous studies, our study also showed the downregulated expression of miR-204-5p in LSCC. Besides cell migration and invasion, our also showed that miR-204-5p overexpression inhibited the proliferation of cancer cells of a LSCC cell line. Our findings enriched our understanding on the functionality of miR-204-5p in LSCC.
It has been reported that the expression of miR-204-5p in cancer development can be regulated by certain lncRNAs, such as lncRNA UCA116 and lncRNA TUG1.17 In the present study we first reported the upregulated expression of lncRNA MIR100HG in LSCC and its functionality as an oncogene. Interesting, we proved that lncRNA MIR100HG is likely an upstream negative regulator of miR-204-5p, and the downregulation of miR-204-5p by lncRNA MIR100HG participated in the regulation of proliferation, migration and invasion of LSCC cells. Our date provided new insights to the pathogenesis of LSCC.
It is worth noting that the expression levels of miR-204-5p and lncRNA MIR100HG were significantly and inversely correlated only in LSCC tissues but not in adjacent healthy tissues, indicating the exiting of pathological mediators between miR-204-5p and lncRNA MIR100HG in LSCC. In addition, overexpression of miR-204-5p only partially attenuated the enhancing effects of lncRNA MIR100HG overexpression on cancer cell proliferation, migration and invasion, indicating the existing of multiple downstream effectors of lncRNA MIR100HG in LSCC.
Conclusion
LncRNA MIR100HG overexpression promotes LSCC by downregulating miR-204-5p.
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics Committee of the First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients and controls after we explained the nature and possible consequences of the study.
Acknowledgment
No funding was received for this study.
Author contributions
YYH: manuscript writing, literature search and data analysis; YHZ: data analysis and statistical analysis. CZ: research design and data analysis. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schorn VJ, Miles BA. Laryngeal squamous cell carcinoma. In: Lin F, Patel Z, editors. ENT Board Prep. New York: Springer; 2014:227–233.
2. Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–1131. doi:10.1056/NEJM200104123441503
3. Gama RR, Carvalho AL, Longatto Filho A, et al. Detection of human papillomavirus in laryngeal squamous cell carcinoma: systematic review and meta-analysis. Laryngoscope. 2016;126(4):885–893. doi:10.1002/lary.25738
4. Bishop JA, Lewis JS
5. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–396. doi:10.1016/j.mayocp.2015.12.017
6. Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol. 2010;220(2):126–139. doi:10.1002/path.2638
7. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. doi:10.1038/nrg3074
8. Jalali S, Bhartiya D, Lalwani MK, Sivasubbu S, Scaria V, Zhang L. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One. 2013;8(2):e53823. doi:10.1371/journal.pone.0053823
9. Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Seminars in Cell & Developmental Biology. 2014;34:9–14. doi:10.1016/j.semcdb.2014.05.015
10. Ye S, Yang L, Zhao X, Song W, Wang W, Zheng S. Bioinformatics method to predict two regulation mechanism: TF-miRNA-mRNA and lncRNA-miRNA-mRNA in pancreatic cancer. Cell Biochem Biophys. 2014;70(3):1849–1858. doi:10.1007/s12013-014-0142-y
11. Wang S, Ke H, Zhang H, et al. LncRNA MIR100HG promotes cell proliferation in triple-negative breast cancer through triplex formation with p27 loci. Cell Death Dis. 2018;9(8):805. doi:10.1038/s41419-018-1111-y
12. Gao W, Wu Y, He X, et al. MicroRNA-204-5p inhibits invasion and metastasis of laryngeal squamous cell carcinoma by suppressing forkhead box C1. J Cancer. 2017;8(12):2356–2368. doi:10.7150/jca.19470
13. Yin Y, Zhang B, Wang W, et al. miR-204-5p inhibits proliferation and invasion and enhances chemotherapeutic sensitivity of colorectal cancer cells by downregulating RAB22A. Clin Cancer Res. 2014;20(23):6187–6199. doi:10.1158/1078-0432.CCR-14-1030
14. Liu L, Wang J, Li X, et al. MiR-204-5p suppresses cell proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma. Biochem Biophys Res Commun. 2015;457(4):621–626. doi:10.1016/j.bbrc.2015.01.037
15. Diaz-Martinez M, Benito-Jardon L, Alonso L, Koetz-Ploch L, Hernando E, Teixido J. miR-204-5p and miR-211-5p contribute to BRAF inhibitor resistance in melanoma. Cancer Res. 2018;78(4):1017–1030. doi:10.1158/0008-5472.CAN-17-1318
16. Bian Z, Jin L, Zhang J, et al. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep. 2016;6:23892. doi:10.1038/srep23892
17. Yu C, Li L, Xie F, et al. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc Res. 2018;114(1):168–179. doi:10.1093/cvr/cvx180
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.