Back to Journals » Cancer Management and Research » Volume 12
LncRNA MALAT1 Regulates the Cell Proliferation and Cisplatin Resistance in Gastric Cancer via PI3K/AKT Pathway
Received 26 December 2019
Accepted for publication 2 March 2020
Published 13 March 2020 Volume 2020:12 Pages 1929—1939
DOI https://doi.org/10.2147/CMAR.S243796
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Qingqiang Dai, Tianqi Zhang, Chen Li
Department of Surgery, Shanghai Key Laboratory of Gastric Neoplasms, Shanghai Institute of Digestive Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, People’s Republic of China
Correspondence: Chen Li
Department of Surgery, Shanghai Key Laboratory of Gastric Neoplasms, Shanghai Institute of Digestive Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, People’s Republic of China
Tel +86-21-64670644
Fax +86-21-64393909
Email [email protected]
Background: Many studies showed that long non-coding RNA MALAT1 is served as an oncogene. However, the specific role of MALAT1 in gastric cancer is not fully elucidated. The aim of this study is to elucidate the regulatory effects of MALAT1 on tumor development and cisplatin resistance in gastric cancer.
Methods: TCGA database was applied to investigate the expression levels of MALAT1 in GC tissues and normal gastric tissues and its correlation with GC patients’ survival. Univariate and multivariate analysis were performed to investigate whether MALAT1 expression is an independent risk for overall survival of gastric cancer patients. The expression of MALAT1 was detected by Quantitative real-time PCR. After knockdown or overexpression of MALAT1, the cellular functions of GC cells were detected by cell-proliferation, flow cytometry, transwell assay and colony formation assays, respectively. Western blot analysis was performed to detect the protein levels of Bcl-2 and key genes in the PI3K/AKT pathway in GC cells. Finally, CCK-8 assay was performed to explore the effect of MALAT1 on cisplatin resistance of GC cells.
Results: Higher expression of MALAT1 was detected in GC tissues than that of adjacent normal tissues, high MALAT1 expression is an independent risk for overall survival of gastric cancer patients. Knockdown of MALAT1 inhibited proliferation, migration and invasion of GC cells, while overexpression of MALAT1 Overexpression of MALAT1 yielded opposite results. Western blot results showed that protein expressions of p-PI3K, p-AKT and p-STAT3 were downregulated after MALAT1 knockdown in GC cells, while these proteins were upregulated after MALAT1 overexpression. Additionally, the IC50 in MGC803/CDDP cells transfected with si-MALAT1 was lower than in those transfected with si-NC. The apoptotic rate in MGC803 cells transfected with pcDNA-MALAT1 was remarkably lower than those transfected with NC.
Conclusion: We demonstrated that MALAT1 is highly expressed in GC, high MALAT1 expression is an independent risk factor for OS among GC patients. Moreover, MALAT1 promotes malignant progression of GC and contributes to cisplatin resistance of GC cells, indicating MALAT1 may serve as a biological hallmark for predicting the prognosis of GC.
Keywords: gastric cancer, MALAT1, proliferation, migration and invasion, cisplatin resistance
Introduction
Gastric cancer (GC) is one of the most commonly diagnosed cancer worldwide which has led to tremendous burdens throughout the world especially in China.1–3 Despite the implementation of perioperative chemotherapy and radiotherapy, the prognosis of GC patients still remains poor.4,5 The comprehensive understanding of the molecular mechanism of GC contributes to develop effective therapeutic approaches and to improve the clinical outcomes of GC patients. Currently, surgical resection and chemotherapy are the preferred options for treating GC, which can greatly prevent recurrence and metastasis of GC.6,7 Besides, platinum is recommended by the main clinical practice guidelines for postoperative GC patient’s chemotherapy treatment. However, chemotherapy resistance is the main cause of chemotherapy failure, remarkably limiting its clinical application. It is believed that GC is the result of genetic and environmental factors. Alterations in certain oncogenes and tumor-suppressor genes finally lead to disordered cell proliferation, migration and invasion.8,9
Long non-coding RNAs (lncRNAs), RNA molecules longer than 200 nucleotides, have an important role in gene regulation and cell function.10 Major attention was attracted to long noncoding RNAs, thanks to its vital role involved in many molecular events, which usually contribute to the cancer progression. Accumulating evidences have shown that lncRNAs are closely associate with the occurrence and progression of malignancies and tumor drug resistance, which are served as tumor hallmarks.11–13 For instance, lncRNA metastasis-associated lung adenocarcinoma transcript 1(MALAT1) has been reported to promote proliferation and metastasis in epithelial ovarian cancer,14 hepatocellular carcinoma15 and colorectal cancer.16 Through literature review, we concluded that MALAT1 is involved in regulating proliferation, apoptosis, differentiation and metastasis of tumor cells.14–16 The specific role of MALAT1 in GC still needs to be further investigated.
In this study, we showed that MALAT1 is highly expressed in GC. Besides, high MALAT1 expression is an independent risk factor for OS among GC patients. Moreover, MALAT1 promotes malignant progression of GC and contributes to cisplatin resistance of GC cells, which indicating that MALAT1 may serve as a biological hallmark for predicting the prognosis of GC.
Materials and Methods
TCGA Database Analysis
GC gene expression data (mRNA, normalized RNAseqV2 RSEM) was download from TCGA database, containing 30 normal gastric samples and 343 gastric cancer samples. Kaplan-Meier Plotter online (http://kmplot.com/analysis/) was used to analyze the correlation between the MALAT1 expression with overall survival (OS) of GC.
Patient Samples
A total of 30 pairs of gastric cancer tissues and paired nontumor tissues were collected at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine from 2018 to 2019. The study was approved by the Human Research Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine and all patients provided written informed consent. Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C until use. All the trials were conducted in accordance with the Declaration of Helsinki.
Cell Culture
The human GC cell lines MKN45, MKN28, MGC-803, HGC-27, NCI-N87, AGS and GES-1 were purchased from American Type Culture Collection (ATCC). Cisplatin-resistance MGC803 cells (MGC803/CDDP) were obtained from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Cells were cultured in RPMI 1640 medium (Gibco, BRL, San Francisco, CA, USA) supplemented with 5μg/mL penicillin/streptomycin and 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) in a humidified incubator at 37°C with 5% CO2.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted with TRIzol reagent (Invitrogen, Austin, TX, USA), and real-time PCR analysis was conducted according to the manufacturer’s instructions (Life Technologies, USA). The mRNA level was measured using the SYBR Green PCR Master Mix (Applied Biosystems, Waltham, MA, USA) and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences used are listed in Table 1.
 |
Table 1 Sequences of siRNAs and Primers |
RNA Interference and Plasmid
Small interfering RNAs (siRNAs) that specifically target human MALAT1 were purchased from Ribobio Technology (Guangzhou, China). The siRNA (100nM siMALAT1) was transfected into cells using the RNAi-MAX reagent (Life Technologies, CA, USA) according to the manufacturer’s instructions. The plasmids
pcDNA-MALAT1 was a kind gift from Prof. Huating Wang (The Chinese University of Hong Kong, China). Plasmid (4 mg/mL) was transfected into cells using Lipofectamine 2000 (Life Technologies, CA, USA). The RNA interference sequences are listed in Table 1.
Cell-Proliferation and Cell Cycle Analysis
Cell viability was measured using Cell Counting Kit-8 (Dojindo, Japan). Cell cycle was analyzed by flow cytometry on a FACScan (Beckman Instruments, Fullerton, CA, USA). The cell proliferation assays and cell cycle analysis were performed as described previously.17
Cell Counting Kit-8 (CCK-8) Assay
Transfected GC cells in logarithmic growth phase were collected and seeded in 96-well plates at a density of 3×104/well. After cell culture for 24 h, 100 μL of cisplatin (Selleck, Houston, USA) with different concentrations of 0, 1, 2, 4, 8, 16, 32, 64, 128 and 256 µM were added to each well, respectively. The blank wells were set up for calibration. There were 6 replicates for each group. After incubation for another 24 h, a total of 10 µL of CCK-8 (Dojindo, Kumamoto, Japan) reagent were added to the wells and the cells were allowed for 2 h of reaction, OD value was measured at 450 nm by spectrophotometry (BioTek, Vermont, USA). The IC50 was finally calculated.
Flow Cytometry Analysis of Cell Apoptosis
Transfected GC cells were treated with 10 µM cisplatin. After 24 hrs of culture, both attached and the floating cells were harvested, washed twice with ice-cold PBS, and suspended in 100 µL binding buffer. The cells were stained with 3 µL Annexin V-FITC and 5 µL propidium-iodide (PI) and incubated at room temperature for 15 min in the dark. Finally, 300 µL 1× binding buffer was added to each sample of cells. Apoptosis was analyzed by flow cytometry using the FACSCalibur system (BD Biosciences, USA).
Transwell Assay
Cell migration and invasion assays were conducted in 24-well plates with 8-mm Transwell inserts (Corning Life Science, Acton, MA, USA). For the migration assays,
GC cells were suspended in 200 mL serum-free RPMI 1640 medium (4 x104 cells) and were cultured in the upper chamber. Fetal bovine serum-conditioned medium (10%) (700 mL) was added to the lower chambers of the 24-well plates. For the invasion assays, the inserts were coated with Matrigel (50 mL/well) (BD Biosciences) before the addition of GC cells. After 24 h of culture, GC cells that remained on the upper side of the inserts were removed with cotton swabs. GC cells that had migrated to the lower side of the inserts were fixed in methanol and stained with 0.5% crystal violet for 20 min. Migrated cells were photographed using Nikon Digital Sight DS-U2 (Nikon, Tokyo, Japan) and Olympus BX50 microscopes (Olympus Optical Co. Ltd., Tokyo, Japan). Six visual fields were randomly selected to calculate the number of cells that had migrated.
Colony Formation Assays
Cells were plated in six-well culture dishes (BD) at a density of 300 cells/well. Two weeks later, Cells were stained with crystal violet on the plates and counted. Experiments were repeated three times.
Western Blot
Cells were lysed in RIPA buffer containing complete protease and phosphatase inhibitor cocktail (Sigma, USA). The protein concentration of the cell lysates was quantified by a BCA Protein Assay Kit (Pierce, Rockford). The same amount of protein samples was resolved onto 10% SDS-PAGE and then transferred onto PVDF membranes. After blocking with 5% non-fat milk at 37°C for 2 hours, the membranes were incubated in the primary antibodies diluted by TBST buffer at 4°C for overnight, following by incubation with the HRP-conjugated secondary antibody for 2 hrs at room temperature.
GAPDH antibody was used to verify equal protein loading. The protein bands images were captured and analyzed by a Tanon detection system with ECL reagent (Thermo) and the antigen-antibody reaction was visualized by enhanced chemiluminescence (ECL, Thermo, USA). The antibodies used in this study were obtained from Cell Signaling Technology.
Statistical Methods
Student’s t-test or one-way ANOVA was used for statistical analysis when appropriate. All statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). A two-tailed value of P<0.05 was considered statistically significant.
Results
MALAT1 Is Highly Expressed in GC Tissues and Cell Lines and Correlates with Poor Survival
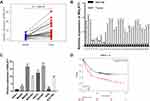
To testify the biological functions of MALAT1 in GC progression, we investigated the expression levels of MALAT1 in GC tissues using TCGA database and found MALAT1 significantly overexpressed in GC tissues compared to adjacent normal tissues (Figure 1A). To confirm this result, we detected the expression level of MALAT1 in 30 pairs of GC tissues and adjacent normal tissues by qRT-PCR. The results showed higher expression of MALAT1 in GC tissues than that of adjacent normal tissues (Figure 1B). Similarly, MALAT1 was highly expressed in GC cell lines compared with that of GES-1 (Figure 1C). Besides, the expression levels of MALAT1 in GC tissues significantly correlated with the poor survival of GC patients (p<0.001, Figure 1D), indicating MALAT1 might participate in the development of GC.
High MALAT1 Expression Is an Independent Risk Factor for OS Among GC Patients
To investigate whether high MALAT1 expression is an independent risk for OS of GC patients, we performed univariate and multivariate analysis. Univariate and multivariate Cox analyses showed that MALAT1 expression was an independent risk factor for OS among GC patients (HR = 1.26, 95% CI: 1.06–1.51, P = 0.010, Table 2). Besides, Kaplan-Meier results indicated that higher expression of MALAT1 is significantly associated with poor prognosis of GC patients (p<0.001, Figure 1D). These results suggested that MALAT1 may serve as a biological hallmark for predicting the prognosis of GC.
 |
Table 2 Univariate Analysis and Multivariate Analysis of the Correlation of MALAT1 Expression with OS Among Gastric Cancer Patients Based on TCGA |
MALAT1 Promotes GC Cell Migration and Invasion
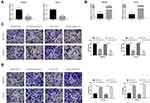
To explore the biological function of MALAT1 with respect to the migration and invasion in GC, we established MALAT1 knockdown cells, MGC803/siMALAT1 and HGC27/siMALAT1 cells, by transfecting MALAT1 siRNA into MGC803 and HGC27 cells. The efficiency of si-UCA1 was validated by qRT-PCR (Figure 2A). We also established MALAT1 overexpression cells, MGC803/MALAT1 and HGC27/MALAT1 cells, via the transfection of MGC803 and HGC27 cells with pcDNA-MALAT1. The overexpression efficiency of pcDNA-MALAT1 was confirmed by qRT-PCR (Figure 2B). To explore the effect of MALAT1 on the migration and invasion ability of GC cells, transwell assays were performed. The knockdown of MALAT1 significantly decreased the migration and invasion ability of MGC803 and HGC27 cells (Figure 2C). In contrast, the overexpression of MALAT1 remarkably increased the migration and invasion ability of MGC803 and HGC27 cells (Figure 2D). Taken together, these results suggested that MALAT1 promotes the migration and invasion of GC cells.
Promotion of MALAT1 on GC Cell Proliferation and Cell Cycle
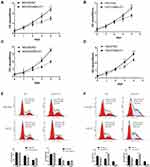
To detect the regulatory effect of MALAT1 on the proliferation of GC cells, we performed cell-proliferation assay. Cell-proliferation assay showed that cell proliferation of MGC803/siMALAT1 and HGC27/siMALAT1 cells were significantly decreased (Figure 3A and B). However, MGC803/MALAT1 and HGC27/MALAT1 cells were notably increased growth and proliferation of GC cells compared with their control cells (Figure 3C and D). Consistent with cell-proliferation assay results, the number of cell colony formation was markedly increased in the MGC803/MALAT1 and HGC/MALAT1 cells and fewer cell colony formation was detected in the MGC803/siMALAT1 and HGC27/siMALAT1 cells (Figure S1, *p<0.05). Given that upregulated MALAT1 increased the GC cell growth, we sought to determine its cell cycle progression via flow cytometric analysis. Knockdown of MALAT1 inhibits MGC803 and HGC27 cell cycle progression and the population of cells in the S phase was significantly decreased (Figure 3E). In contrast, increased S phase was detected in MGC803/MALAT1 and HGC27/MALAT1 cells compared with that of control cells (Figure 3F). These results indicated that MALAT1 could promote GC proliferation and affect cycle progression by regulating G1/S transition.
MALAT1 Regulates PI3K/AKT Pathway
PI3K/AKT pathway has been confirmed to regulate cellular functions, including cell migration and invasion.14,18,19 Therefore, we hypothesized whether MALAT1 contributes to GC development through PI3K/AKT pathway. Western blot analysis demonstrated that protein levels of p-PI3K, p-AKT and p-STAT3 were downregulated after MALAT1 knockdown in GC cells (Figure 4A). Besides, the protein expressions of p-PI3K, p-AKT and p-STAT3 were upregulated after MALAT1 overexpression in GC cells (Figure 4B), indicating MALAT1 may contribute to the migration and invasion in GC via PI3K/AKT pathway.
MALAT1 Contributes to Cisplatin Resistance in GC Cells
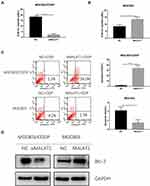
To further investigate the role of MALAT1 in cisplatin resistance of GC cells, we performed CCK-8 assay to detect cell viability with cisplatin treatment. The IC50 in MGC803/CDDP cells transfected with si-MALAT1 after cisplatin treatment was significantly decreased compared to that of control group (Figure 5A). On the contrary, the IC50 in MGC803 cells transfected with pcDNA-MALAT1 was higher than those transfected with NC (Figure 5B). The result showed that MALAT1 was associated with the cisplatin resistance of GC cells. Furthermore, flow cytometry was performed to detected cell apoptosis after GC cells treated with 10μM cisplatin. The apoptotic rate in MGC/CDDP cells transfected with siMALAT1 was remarkably higher than those transfected with si-NC (Figure 5C). However, MGC803 cells transfected with pcDNA-MALAT1 demonstrated a lower apoptotic rate than those transfected with NC (Figure 5C). Western blot analysis showed that Bcl-2 was upregulated after MALAT1 overexpression; moreover, Bcl-2 was downregulated after knockdown of MALAT1 (Figure 5D). Hence, these results suggested that MALAT1 could promote the cisplatin resistance of GC cells.
Discussion
GC is one of the most common malignant tumor with a high morbidity.1 Currently, platinum is recommended by the main clinical practice guidelines for postoperative GC patient’s chemotherapy treatment; however, there is still a large part of patients benefitting little from it due to the chemoresistance, which requires for effective approaches to overcome the chemotherapy limitations. At present, besides surgical resection, chemotherapy is the major approach in treating GC patients, and it markedly decreases the incidence of postoperative recurrence and metastasis.6,7 It is noteworthy that chemotherapy resistance during the postoperative treatment remarkably restricts its clinical application. In recent years, many researches have shown that lncRNAs demonstrated their capacities in regulating drug resistance of tumor treatment. Lin et al20 reported that lncRNA HOXA-AS3 confers cisplatin resistance by interacting with HOXA3 in non-small-cell lung carcinoma cells. Li et al21 showed that lncRNA UCA1 mediates resistance to cisplatin by regulating the miR-143/FOSL2-signaling pathway in ovarian cancer. Yuan et al22 demonstrated that lncRNA LINC-PINT regulates laryngeal carcinoma cell stemness and chemoresistance through miR-425-5p/PTCH1/SHH axis.
Previous studies have proved the regulatory effects of lncRNAs on the occurrence and progression of tumors.23,24 Hu et al23 reported that lncRNA-HGBC stabilized by HuR promotes gallbladder cancer progression by regulating miR-502-3p/SET/AKT axis. Yao et al24 showed that JHDM1D-AS1 interacts with DHX15 to promote non-small-cell lung cancer growth and metastasis. In our study, we detected MALAT1 expression in GC tissues and adjacent normal tissues. The expression of MALAT1 in GC tissues significantly correlated with the poor survival of GC patients. Besides, high MALAT1 expression is an independent risk factor for OS among GC patients. Furthermore, in vitro experiments demonstrated the role of MALAT1 in regulating proliferation, migration and invasion of GC cells.
PI3K/AKT pathway is an important signaling pathway involved in tumorigenesis, which demonstrates its anti-apoptotic effect mainly by affecting multiple effector molecules.14,18,19 In this research, we found that the expression levels of p-PI3K, p-AKT and p-STAT3 were remarkably downregulated after MALAT1 knockdown in GC cells, while the protein levels of p-PI3K, p-AKT and p-STAT3 were significantly upregulated after MALAT1 overexpression in GC cells, indicating that MALAT1 contributes to proliferation and metastasis in GC via the PI3K/AKT pathway. Furthermore, we hypothesized the underlying role of MALAT1 in chemotherapy resistance of GC. MALAT1 expression was upregulated in MGC803/CDDP cells than that of MGC803 cells. The cisplatin resistance of MGC803 cells was markedly elevated after MALAT1 overexpression, indicating that MALAT1 regulates cisplatin resistance in GC. Besides, we found MALAT1 could regulate the expression level of Bcl-2, which may serve as an effector molecule in MALAT1-induced cisplatin resistance of GC. However, the specific mechanism of MALAT1 in regulating cisplatin resistance of GC still needs to be further investigated.
Conclusion
In conclusion, we demonstrated that MALAT1 is highly expressed in GC, high MALAT1 expression is an independent risk factor for OS among GC patients. Moreover, MALAT1 promotes malignant progression of GC and contributes to cisplatin resistance of GC cells, indicating MALAT1 may serve as a biological hallmark for predicting the prognosis of GC.
Abbreviations
GC, gastric cancer; OS, overall survival; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; LncRNAs, long non-coding RNAs.
Data Sharing Statement
All data and materials are available.
Consent for Publication
All the patients have provided written informed consent before enrollment and in compliance with the Declaration of Helsinki. The study was approved by the Human Research Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine. All the patients and authors consent for publication.
Acknowledgments
Thanks to Prof. Hua-ting Wang (The Chinese University of Hong Kong) for providing Human MALAT1 expression vector as a kind gift.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by The Cross-Institutes Research Fund of Shanghai Jiao Tong University (No. YG2017MS58).
Disclosure
The authors have declared that no competing interest exists regarding this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J Gastroenterol. 2015;21(24):7343–7348. doi:10.3748/wjg.v21.i24.7343
5. Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol. 2016;22(8):2403–2414. doi:10.3748/wjg.v22.i8.2403
6. Eto K, Hiki N, Kumagai K, et al. Prophylactic effect of neoadjuvant chemotherapy in gastric cancer patients with postoperative complications. Gastric Cancer. 2018;21(4):703–709. doi:10.1007/s10120-017-0781-y
7. Warschkow R, Baechtold M, Leung K, et al. Selective survival advantage associated with primary tumor resection for metastatic gastric cancer in a Western population. Gastric Cancer. 2018;21(2):324–337. doi:10.1007/s10120-017-0742-5
8. Wang ZQ, Cai Q, Hu L, et al. Long noncoding RNA UCA1 induced by SP1 promotes cell proliferation via recruiting EZH2 and activating AKT pathway in gastric cancer. Cell Death Dis. 2017;8(6):e2839. doi:10.1038/cddis.2017.143
9. Wang ZQ, He CY, Hu L, et al. Long noncoding RNA UCA1 promotes tumour metastasis by inducing GRK2 degradation in gastric cancer. Cancer Lett. 2017;408:10–21. doi:10.1016/j.canlet.2017.08.013
10. Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339(2):159–166. doi:10.1016/j.canlet.2013.06.013
11. Peng WX, Koirala P, Zhang W, et al. lncRNA RMST enhances DNMT3 expression through interaction with HuR. Mol Ther. 2019;28(1).
12. Wang A, Bao Y, Wu Z, et al. Long noncoding RNA EGFR-AS1 promotes cell growth and metastasis via affecting HuR mediated mRNA stability of EGFR in renal cancer. Cell Death Dis. 2019;10(3):154. doi:10.1038/s41419-019-1331-9
13. Chang ZW, Jia YX, Zhang WJ, et al. LncRNA-TUSC7/miR-224 affected chemotherapy resistance of esophageal squamous cell carcinoma by competitively regulating DESC1. J Exp Clin Cancer Res. 2018;37(1):56.
14. Jin Y, Feng SJ, Qiu S, Shao N, Zheng JH. LncRNA MALAT1 promotes proliferation and metastasis in epithelial ovarian cancer via the PI3K-AKT pathway. Eur Rev Med Pharmacol Sci. 2017;21(14):3176–3184.
15. Liu S, Qiu J, He G, et al. LncRNA MALAT1 acts as a miR-125a-3p sponge to regulate FOXM1 expression and promote hepatocellular carcinoma progression. J Cancer. 2019;10(26):6649–6659. doi:10.7150/jca.29213
16. Si Y, Yang Z, Ge Q, et al. Long non-coding RNA Malat1 activated autophagy, hence promoting cell proliferation and inhibiting apoptosis by sponging miR-101 in colorectal cancer. Cell Mol Biol Lett. 2019;24:50. doi:10.1186/s11658-019-0175-8
17. Wang Z, Ma X, Cai Q, et al. MiR-199a-3p promotes gastric cancer progression by targeting ZHX1. FEBS Lett. 2014;588(23):4504–4512. doi:10.1016/j.febslet.2014.09.047
18. Wang B, Cao C, Liu X, et al. BRCA1-associated protein inhibits glioma cell proliferation and migration and glioma stem cell self-renewal via the TGF-beta/PI3K/AKT/mTOR signalling pathway. Cell Oncol (Dordr). 2019. doi:10.1007/s13402-019-00482-8
19. Yang JH, Yu K, Si XK, et al. Liensinine inhibited gastric cancer cell growth through ROS generation and the PI3K/AKT pathway. J Cancer. 2019;10(25):6431–6438. doi:10.7150/jca.32691
20. Lin S, Zhang R, An X, et al. LncRNA HOXA-AS3 confers cisplatin resistance by interacting with HOXA3 in non-small-cell lung carcinoma cells. Oncogenesis. 2019;8(11):60. doi:10.1038/s41389-019-0170-y
21. Li Z, Niu H, Qin Q, et al. lncRNA UCA1 mediates resistance to cisplatin by regulating the miR-143/FOSL2-signaling pathway in ovarian cancer. Mol Ther Nucleic Acids. 2019;17:92–101. doi:10.1016/j.omtn.2019.05.007
22. Yuan Z, Xiu C, Liu D, et al. Long noncoding RNA LINC-PINT regulates laryngeal carcinoma cell stemness and chemoresistance through miR-425-5p/PTCH1/SHH axis. J Cell Physiol. 2019;234(12):23111–23122. doi:10.1002/jcp.v234.12
23. Hu YP, Jin YP, Wu XS, et al. LncRNA-HGBC stabilized by HuR promotes gallbladder cancer progression by regulating miR-502-3p/SET/AKT axis. Mol Cancer. 2019;18(1):167. doi:10.1186/s12943-019-1097-9
24. Yao G, Chen K, Qin Y, et al. Long non-coding RNA JHDM1D-AS1 interacts with DHX15 protein to enhance non-small-cell lung cancer growth and metastasis. Mol Ther Nucleic Acids. 2019;18:831–840. doi:10.1016/j.omtn.2019.09.028
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.