Back to Journals » OncoTargets and Therapy » Volume 12
LncRNA GATA6-AS inhibits cancer cell migration and invasion in gallbladder cancer by downregulating miR-421
Received 14 April 2019
Accepted for publication 18 July 2019
Published 1 October 2019 Volume 2019:12 Pages 8047—8053
DOI https://doi.org/10.2147/OTT.S212231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Kezhi Li, Jianwei Tang, Yong Hou
Department of General Surgery, The First People’s Hospital of Qujing, Qujing City, Yunnan Province 655000, People’s Republic of China
Correspondence: Yong Hou
Department of General Surgery, The First People’s Hospital of Qujing, No.1 Yuanlin Road of Qilin District, Qujing City, Yunnan Province 655000, People’s Republic of China
Tel +86 87 4331 1977
Fax +86 87 4331 1977
Email [email protected]
Background: Long non-coding RNS (lncRNA) GATA6-AS regulates endothelial cell growth, which is involved in many types of human diseases.
Purpose: Our study was carried out to investigate the involvement of GATA6-AS in gallbladder cancer (GBC).
Patients and methods: Sixty-two patients with GBC were recruited in this study. PCR analysis was performed to determine the expression of GATA6-AS, miR-421 and TIMP-2 in tissues or cell lines. Transwell migration and invasion assay was applied.
Results: We found that GATA6-AS was downregulated in tumor tissues than in adjacent healthy tissues of GBC patients, and GATA6-AS expression levels in tumor tissues decreased with the increase of clinical stages. MiR-421 was upregulated in tumor tissues than in adjacent healthy tissues of GBC patients and was inversely correlated with the expression levels of GATA6-AS. MiR-421 overexpression failed to significantly affect GATA6-AS in GBC cells, while GATA6-AS overexpression resulted in inhibited miR-421 expression. GATA6-AS overexpression led to decreased migration and invasion rates of GBC cells. MiR-421 overexpression led to increased migration and invasion rates of GBC. Rescue experiments (co-transfection) showed that miR-421 overexpression led to attenuated effects of GATA6-AS overexpression.
Conclusion: GATA6-AS may inhibit cancer cell migration and invasion in GBC by downregulating miR-421.
Keywords: gallbladder cancer, lncRNAGATA6-AS, miR-421, migration, invasion, cell growth
Introduction
Gallbladder cancer (GBC) is one of the most common types of gastrointestinal malignancies and is characterized by the high prevalence of lymph node and distant metastases by the time of initial diagnosis.1 In effect, only less than 10% of GBC patients are candidates for surgical resection, which is the only radical treatment.2 In addition, recurrence rate of GBC is high even after surgical resection, leading to the unacceptable low overall 5-year survival rate (as low as 5%).3 In spite of the efforts made on understanding molecular pathways involved in GBC, pathogenesis of this disease is still unclear, which is the major difficulty in clinical prevention and treatment.4
Studies on genetic alterations involved in GBC have shown that genetic factors are the key players in GBC,5–7 while the limited number of oncogenes and tumors suppressors characterized so far may not be able to fully explain the complex pathogenesis of this disease. Long non-coding RNAs (>200 nt, lncRNAs) are emerging classes of critical determinant in diverse biological and pathological processes, such as tumorigenesis.8,9 LncRNAs participate in cancer biology by regulating the expression of oncogenes or tumor suppressors.9,10 Therefore, investigation of the function of lncRNAs in cancer biology is of great clinical significances. LncRNA GATA6-AS regulates endothelial cell growth, which is involved in many types of human diseases.10 Our study was therefore carried out to investigate the role of GATA6-AS in GBC.
Materials and methods
Subjects
The present study included 62 patients with GBC (22 males and 40 females, 60 to 82 years,71.1±6.1 years) to serve as research subjects. All those patients were admitted by the First People’s Hospital of Qujing between January 2015 and January 2018. Inclusion criteria were as follows: 1) patients who were diagnosed by histopathological examinations for the first time and no therapies were received and 2) patients signed informed consent. Exclusion criteria were as follows: 1) patients complicated with other clinical disorders and 2) patients with a history of malignancies. Based on the staging criteria proposed by AJCC, there were 12, 17, 20 and 13 cases at stages I, II, III and IV, respectively. This study was approved by the Ethics Committee of the First People’s Hospital of Qujing before the admission of patients.
Specimen and cell lines
Cancer (GBC) tissues and adjacent non-cancer tissues were collected from each patient before any therapies through biopsy. iopsy was performed using a fine needle under the guidance of MRI. Tissue specimens were stored in liquid nitrogen before use.
Two GBC cell lines SGC-996 and GBC-SD were used in this study to perform all in vitro cell experiments. Cells of these two cell lines were bought from the Cell Bank of the Chinese Academy of Science (Shanghai, China). Cells were cultivated according to manufacturer’s instructions.
RT-qPCR
Trizol reagent (Invitrogen, USA) was used to extract total RNAs from GBC tissues, adjacent noncancer tissues and cells of both SGC-996 and GBC-SD cell lines. Total RNA samples were subjected to reverse transcriptions using AMV reverse transcriptase (GIBCO, USA) to obtain cDNA. After that, PCR systems were prepared using Applied Biosystems™ PowerUp™ SYBR™ Green Master Mix with 18S rRNA as an endogenous control to detect the expression of GATA6-AS and TIMP-2 mRNA.
mirVana miRNA Isolation kit (Thermo Fisher Scientific, Inc.) was used to extract miRNAs from GBC tissues, adjacent noncancer tissues and cells of both SGC-996 and GBC-SD cell lines. miRNA samples were subjected to reverse transcription using TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific), followed by preparation of PCR systems using MystiCq® microRNA® SYBR® Green qPCR ReadyMix™ (Sigma-Aldrich, USA) with U6 as an endogenous control to detect miR-421 expression.
All PCRs were repeated 3 times and data were processed using 2−ΔΔCq method.
Transient cell transfection
miR-421 mimic and negative control miRNA were bought from Sigma-Aldrich (USA). GATA6-AS expression pcDNA3.1 vectors were constructed by Sangon (Shanghai, China). All cell transient transfections were performed using Lipofectamine 2000 (Invitrogen, USA) with 10 nM vectors and 40 nM miRNAs. Two controls, including control (cells without transfection) and negative control (cells transfected with empty vectors or negative control miRNA), were used. Subsequent experiments were performed at 24 hrs after transfection.
Transwell migration and invasion assay
Cells of SGC-996 and GBC-SD cell lines were harvested at 24 hrs after transfection to prepare single-cell suspensions using serum-free cell culture medium with a cell density of 3×104 cells per mL. Single-cell suspensions were transferred to the upper Transwell chamber, while the lower Transwell chamber was filled with cell culture medium containing 20% FBS to induce cell migration and invasion. To mimic in vivo cell invading barriers, the upper chamber was coated with Matrigel (356,234, Millipore, USA) overnight at room temperature before invasion assay. Cells invasion and migration were allowed for 2 hrs. After that, membranes of the upper chamber were collected, cleaned and stained with 0.5% crystal violet (Sigma-Aldrich, USA) for 15 mins at room temperature. An optical microscope was used to observe the migrating and invading cells, and 5 visual fields were randomly selected to count cells.
Statistical analysis
All experiments were repeated 3 times. Differences between GBC and noncancer tissues were analyzed by paired t-test. Differences among different clinical stages and different cell treatment groups were analyzed by one-way ANOVA and Tukey test. Linear regression was used to analyze the correlation between expression levels of GATA6-AS and miR-421. The statistical significant cutoff value was p<0.05.
Results
GATA6-AS was downregulated in GBC tissues and affected by clinical stages
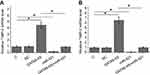
RT-qPCR was carried out to analyze the expression of GATA6-AS in GBC and noncancer tissues of GBC patients. The data were compared by paired t-test. It was observed that compared to adjacent noncancer tissues, expression levels of GATA6-AS were significantly lower (about 1.97-fold) in GBC tissues (Figure 1A, p<0.05). Comparisons of GATA6-AS expression levels in GBC tissues through one-way ANOVA and Tukey test showed that GATA6-AS expression levels decreased significantly with the increase of clinical stages (Figure 1B, p<0.05).
Mir-421 was upregulated in GBC tissues and inversely correlated with GATA6-AS
RT-qPCR was carried out to analyze the expression of miR-421 in GBC and noncancer tissues of GBC patients. The data were compared by paired t-test. It was observed that compared to adjacent noncancer tissues, expression levels of miR-421 were significantly higher (about 2.05-fold) in GBC tissues (Figure 2A, p<0.05). Linear regression was used to analyze the correlation between expression levels of GATA6-AS and miR-421. It was observed thatGATA6-AS and miR-421 were inversely and significantly correlated in GBC tissues (Figure 2B), but not in adjacent noncancer tissues (Figure 2C).
GATA6-AS overexpression resulted in the downregulation of mir-421
To further investigate the interactions between GATA6-AS and miR-421, GATA6-AS expression vectors and miR-421 mimics were transfected into cells of GBC cell lines SGC-996 and GBC-SD. Compared to two controls (control C and negative control NC), expression levels of GATA6-AS and miR-421 were significantly increased at 24 hrs after transfection in cells of both cell lines (more than 2.5-fold change, Figure 3A, p<0.05). In addition, miR-421 overexpression failed to significantly affect GATA6-AS in GBC cells (Figure 3B), while GATA6-ASoverexpression resulted in inhibited miR-421 expression (more than 3-fold change, Figure 3C, p<0.05).
GATA6-AS overexpression resulted in inhibited migration and invasion of GBC cells through mir-421
Transwell migration and invasion assay showed that compared to two controls (control C and negative control NC), GATA6-AS overexpression led to decreased migration (Figure 4A) and invasion (Figure 4B) rates of GBC cells (p<0.05). MiR-421 overexpression led to increased migration and invasion rates of GBC cells and attenuated the effects of GATA6-AS overexpression (p<0.05).
GATA6-AS overexpression led to upregulated TIMP-2 mRNA through mir-421
TIMP-2 as a key regulator in cancer cell invasion and migration is regulated by miR-421. qPCR was performed to analyze the effects of GATA6-AS and miR-421 overexpression on TIMP-2 expression. Compared to NC and C groups, GATA6-AS overexpression led to the upregulation of TIMP-2 mRNA in both SGC-996 (Figure 5A, p<0.05) and GBC-SD (Figure 5B, p<0.05) cells. Moreover, miR-421 overexpression played an opposite role and attenuated the effects of GATA6-AS overexpression (p<0.05).
Discussion
GATA6-AS as an important regulator in endothelial cell growth is involved in many types of human diseases. However, the role of GATA6-AS human diseases is unknown. The present study first reported the downregulated expression of GATA6-AS in GBC and proved that GATA6-AS overexpression may inhibit GBC cell migration and invasion by downregulating miR-421, which is an oncogenic miRNA.11
Endothelial cells are cells in the interior surface of lymphatic or blood vessels. Abnormal growth and proliferation of endothelial cells are involved in many types of human diseases including different types of human cancer.12 In effect, endothelial cell angiogenesis is a critical step in cancer development and progression.13 GATA6-AS has been characterized as a regulator of endothelial cell growth, suggestive of a potential role in cancer development. The present study showed that GATA6-AS was significantly downregulated in GBC tissues, and overexpression of GATA6-AS resulted in inhibited cancer cell migration and invasion. Therefore, besides cell growth, GATA6-AS can also regulate other cell behaviors to participate in cancer biology.
miR-421 has been characterized as oncogenic miRNA in several types of cancer.14,15 miR-421 promotes the progression of cancer mainly by targeting tumor suppressors to regulate cancer cell behaviors. However, to our best knowledge, the involvement of miR-421 in GBC is unclear. Our study showed the upregulated expression pattern of miR-421 in GBC and also proved that miR-421 overexpression can promote GBC cell migration and invasion. Our data suggested the oncogenic role of miR-421 in GBC.
Our study showed that GATA6-AS is likely an upstream inhibitor of miR-421 in GBC cells. It has been reported that the expression of miR-421 during cancer development can be regulated by certain lncRNAs.16 In a recent study, Zhang et al and Li et al showed that MEG3 can inhibit miR-421 by serving as it sponge.16,17 However, in this study, no promising binding site of miR-421 was found on GATA6-AS. Therefore, other mechanisms may exist to mediate the interaction between these two factors. It is known that miR-421 can target TIMP-2 to suppress the invasion and migration of cancer cells in glioblastoma.11 In this study, we proved that miR-421 can also target TIMP-2 in GBC cells, and GATA6-AS attenuated the effects of miR-421 overexpression on TIMP-2 expression. We, therefore, identified a novel GATA6-AS/miR-421/TIMP-2 pathway in GBC.
Wnt/β-catenin signaling pathway is also a major downstream effector of miR-421.18 However, our preliminary data showed that GATA6-AS1 overexpression only led to slightly increased expression level of β-catenin. Therefore, functions of GATA6-AS1 in GBC are unlikely mediated by Wnt/β-catenin pathway.
This study is limited by the small sample size and the lack of animal model in vivo experiments. Our future studies will include more patients and establish animal models to further verify our conclusions.
Conclusion
GATA6-AS was downregulated and miR-421 was upregulated in GBC. GATA6-AS overexpression may inhibit GBC cell migration and invasion by downregulating miR-421.
Ethical approval and informed consent
The protocol of the present study was approved by the Ethics Review Committee of the First People’s Hospital of Qujing (Qujing, China). All participants signed informed consent.
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. Kezhi Li, Jianwei Tang and Yong Hou have made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data. Kezhi Li was involved in drafting the manuscript or revising it critically for important intellectual content. Kezhi Li, Jianwei Tang and Yong Hou gave final approval of the version to be published. Each author agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15:168–181. doi:10.1634/theoncologist.2009-0302
2. Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–176.
3. Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi:10.1038/sj.onc.1206928
4. Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi:10.1038/ng.2813
5. Ooi A, Suzuki S, Nakazawa K, et al. Gene amplification of Myc and its coamplification with ERBB2 and EGFR in gallbladder adenocarcinoma. Anticancer Res. 2009;29:19–26.
6. Shibata T, Kokubu A, Gotoh M, et al. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358–1368. doi:10.1053/j.gastro.2008.06.082
7. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi:10.2147/CLEP.S37357
8. Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi:10.4161/rna.20481
9. Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26:155–165. doi:10.1038/modpathol.2012.160
10. Neumann P, Jaé N, Knau A, et al. The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat Commun. 2018;9:237. doi:10.1038/s41467-017-02431-1
11. Lu W, Zhang N, An J, et al. MicroRNA-421 enhances cell migration and invasion by down-regulating TIMP-2 in glioblastoma. Int J Clin Exp Med. 2016;9(8):15876–15883.
12. Fox SB, Gatter KC, Bicknell R, et al. Relationship of endothelial cell proliferation to tumor vascularity in human breast cancer. Cancer Res. 1993;53:4161–4163.
13. Chen HH, Zhou HJ, Fang X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res. 2003;48:231–236.
14. Li Y, Li W, Zhang JG, Li HY, Li YM. Downregulation of tumor suppressor menin by miR-421 promotes proliferation and migration of neuroblastoma. Tumuor Biol. 2014;35:10011–10017. doi:10.1007/s13277-014-1921-1
15. Wu JH, Yao YL, Gu T, et al. MiR-421 regulates apoptosis of BGC-823 gastric cancer cells by targeting caspase-3. Asian Pac J Cancer Prev. 2014;15:5463–5468. doi:10.7314/apjcp.2014.15.13.5463
16. Zhang W, Shi S, Jiang J, Li X, Lu H, Ren F. LncRNA MEG3 inhibits cell epithelial-mesenchymal transition by sponging miR-421 targeting E-cadherin in breast cancer. Biomed Pharmacother. 2017;91:312–319. doi:10.1016/j.biopha.2017.04.085
17. Li G, Song H, Chen L, Yang W, Nan K, Lu P. TUG1 promotes lens epithelial cell apoptosis by regulating miR-421/caspase-3 axis in age-related cataract. Exp Cell Res. 2017;356:20–27. doi:10.1016/j.yexcr.2017.04.002
18. Liang H, Wang C, Gao K, et al. ΜicroRNA‑421 promotes the progression of non‑small cell lung cancer by targeting HOPX and regulating the Wnt/β‑catenin signaling pathway. Mol Med Rep. 2019;20(1):151–161. doi:10.3892/mmr.2019.10226
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.