Back to Journals » International Journal of General Medicine » Volume 15
LncRNA FENDRR Servers as a Possible Marker of Essential Hypertension and Regulates Human Umbilical Vein Endothelial Cells Dysfunction via miR-423-5p/Nox4 Axis
Authors Zhao X, Wang C, Liu M, Meng F, Liu K
Received 7 September 2021
Accepted for publication 5 January 2022
Published 5 March 2022 Volume 2022:15 Pages 2529—2540
DOI https://doi.org/10.2147/IJGM.S338147
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Xiaojian Zhao,1 Chen Wang,2 Min Liu,1 Fansen Meng,1 Kai Liu1
1Department of Hypertension, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Correspondence: Kai Liu, Department of Hypertension, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, No. 7 Weiwu Road, Zhengzhou, Henan, 450003, People’s Republic of China, Tel/Fax +86-371-65964376, Email [email protected]
Purpose: Essential hypertension (EH) is an intricate non-communicable infirmity and lncRNAs are validated as essential mediators in EH. The study aimed to propose the expression pattern of FENDRR and miR-423-5p, substantiate the potential mechanism of FENDRR/miR-423-5p/Nox4 axis in EH.
Patients and Methods: The expression of FENDRR and miR-423-5p was evaluated by qRT-PCR and the clinical significance was explored by the ROC curve. Pearson correlation indicated the relationship between FENDRR and miR-423-5p. The function of FENDRR and miR-423-5p on HUVECs was clarified by CCK-8 assay, Transwell assay, and flow cytometry. Western blot was used to assess the relative protein expression of Nox4.
Results: FENDRR was highly expressed and miR-423-5p was lowly expressed in EH patients and a negative correlation between them was determined. FENDRR might serve as a predictive diagnosis in differentiating EH patients. Knockdown of FENDRR or overexpression of miR-423-5p showed expansionary effects in cell proliferation, cell migration, and inhibiting cell apoptosis. Meanwhile, miR-423-5p was determined as a target of FENDRR and mediated the function of FENDRR on HUVECs. Moreover, Nox4 is a down-streaming target gene of miR-423-5p. The protein expression of Nox4 was regulated by the alternation of miR-423-5p expression.
Conclusion: FENDRR played an energetic role in EH and contributed to HUVECs dysfunction by restricting cell proliferation, suppressing cell migration, and accelerating cell apoptosis by manipulating the miR-423-5p/Nox4 axis.
Keywords: FENDRR/miR-423-5p/Nox4 axis, essential hypertension, diagnosis, human umbilical vein endothelial cells, proliferation, apoptosis
Introduction
Hypertension is one commonplace cause leading to disability and death and essential hypertension (EH) is a common type.1–3 The change of lifestyle and the combination treatment of drugs are the basis of controlling essential hypertension.4 The intervention of EH is one of the most critical measures to prevent the occurrence and death of cardiovascular dysfunction.5 Although the management of hypertension has improved over time, a considerable number of hypertensive patients have poor blood pressure control.6 Thus, a sensitive diagnostic tool and the therapeutic target are indispensable and urgent to be applied.
LncRNA is a type of RNA with a length greater than 200 nt that has regulatory roles in gene expression at the transcriptional and posttranscriptional levels.7 LncRNAs can widely regulate the expression of adjacent genes with significant tissue specificity through various mechanisms.8 lncRNAs play key roles in development, gene programming, and gene regulation in vascular diseases.9 LncRNAs (ITGB7 and AGAP2-AS1) are found differently expressed in patients with hypertension and may be associated with the risks of hypertension.10 LncRNA FOXF1 adjacent non-coding developmental regulatory RNA (FENDRR) is found as a critical gene in the development of vascular or malignant diseases. For instance, the expression of FENDRR is elevated in the cell models of hypertensive intracerebral hemorrhage and it regulates the apoptotic cell by targeting miR-126.11 He et al provide another example by indicating that FENDRR controls cell proliferation and regulates cell apoptosis via the miR-214-3p/ ten-eleven-translocation pathway.12 However, the expression and mediate mechanism of FENDRR in the EH patients were indistinct.
MicroRNAs (miRNAs) are short RNA molecules 19 to 25 nucleotides in size that that function as guide molecules in RNA silencing.13 MiRNA is another fundamental factor influencing nearly all biological activities.14 LncRNA serves as a competitive endogenous RNA (ceRNA) of a specific miRNA and inhibits the expression of this target miRNA.15,16 The evidence can be clearly seen in the publication of Perez-Hernandez et al, which enumerates that miR-146a is relative to albuminuria in patients with EH.17 Luo et al illustrate this view evidently by the documentation that the expression of miR-10a-5p and miR-497-5p is lessened in 98 patients with EH.18 MiR-423-5p is one of the hottest miRNAs in the field of hypertension worldwide. In a dissertation about hypertension, the expression of miR-423-5p was decreased in 53 hypertension patients when compared to the normal population.19 Besides, the correlation between miR-423-5p and FENDRR is revealed in hepatocellular carcinoma.20 However, the mechanism of miR-423-5p underlying EH remains unclear.
Accordingly, the study focused on the levels of FENDRR and miR-423-5p in patients with EH and their relationship. The diagnostic effect of FENDRR for differentiating EH patients in clinical practice was determined. What’s more, the functions of FENDRR and miR-423-5p on human umbilical vein endothelial cells (HUVECs) were proposed. The target gene of miR-423-5p was predicted and identified in this publication.
Materials and Methods
Study Patients
A total of 67 patients with EH and 74 healthy controls were recruited from Henan Provincial People’s Hospital and all participants provided written informed consent prior to the collection of blood specimens. The diagnosis of hypertension was based on the guideline for prevention and treatment of hypertension in China. In short, patients with three measurements of systolic blood pressure (SBP) above 140 mmHg or/and diastolic blood pressure (DBP) above 90 mmHg were identified as hypertension. The patients with secondary hypertension, serious diseases of blood system, endocrine diseases were excluded. All patients were diagnosed with hypertension for the first time and had not taken any medicine to control blood pressure. The healthy controls were individuals who take a regular physical examination and were diagnosed without EH. The measurements of controls were lower than 120/80 mmHg. Besides, the persons with a malignant tumor, systematic dysfunction, chronic inflammation, or pregnancy were excluded in this experiment. The venous blood specimens were extracted from all individuals and centrifuged to obtain a plasma sample. All specimens were stored at −80°C for follow-up experiments. The design and protocol of our study were approved by the Ethics Committee of Henan Provincial People’s Hospital. This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Cell Culture and Transfection
HUVECs were purchased from Biobw Biotechnology Co. (Beijing, China) and cultured in DMEM medium plus 10% FBS and 1% mixed antibiotics. The humidity of incubation was maintained at 70–80% and 5% CO2 at 37°C.
The si-FENDRR and its negative control (si-NC) together with miR-423-5p mimics, miR-423-5p inhibitors, and their negative controls (mimic-NC and inhibitor-NC) were purchased from Sangon Biotech (Shanghai, China). All these sequences used in the transfection were provided in Table 1. The transfection experiments were put into practice using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA).
 |
Table 1 Sequences Used for Transfection |
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
TRIzol reagent was purchased and used for isolation of total RNA according to its recommendation. The amount and quality of abstracted RNA specimens were accessed by Qubit 3.0 and NanoDrop 2000 (all from ThermoFisher, Waltham, MA, USA). Reverse transcription was performed on RNA to get cDNA via FastKing One-Step RT-PCR Kit (TIANGEN, Beijing, China) and TaqMan microRNA cDNA Synthesis Kit (biolab, Beijing, China) based on the separate manufacturer’s protocol.
qRT-PCR was conducted on the ABI 7500 real-time system to identify the relative expression of FENDRR and miR-423-5p as well as GAPDH and U6. qPCR SYBR® Green Master Mix was mixed with cDNA sample in conformity to recommended suggestions. The primers were as follows: FENDRR (forward, 5’-TAAAATTGCAGATCCTCCG-3’, and reverse, 5’-AACGTTCGCATTGGTTTA GC-3’), miR-423-5p (forward, 5’-GGGGTGAGGGGCAGAGAG, -3’, and reverse, 5’-TGCGTGTCGTGGAGTC-3’), GAPDH (forward, 5’-GGGCATCTTGGGCTACAC-3’, and reverse, 5’-GGTCCAGGGTTTCTTACTCC-3’, and U6 (forward, 5ʹCTCGCTTCGGCAGCACA–3’, and reverse, 5’-AACGCTTCACGAATTTGCGT-3’). The relative expression was calculated as per the 2−ΔΔCt method.
Cell Proliferation Assay
CCK-8 reagent was from Beyotime (Shanghai, China) and was used to detect the proliferation of HUVECs. 100 μL cell solution was seeded into wells of a 96-well plate and the concentration of cells was maintained 3×103 cells/well. The plate was incubated at 37°C for 0 h,24 h, 48 h, and 72 h and 10 μL CCK-8 reagent was mixed into each well. After further 2-hour incubation, the absorbance data were measured.
Cell Migration Assay
Transwell chambers were applied to measure migratory cells. The upper chambers were added about 1×104 transfected cells and serum-free DMEM medium. DMEM culture supplemented with 10% FBS was filled into the lower chambers. After 24 hours, the cells in the lower chamber were fixed with paraformaldehyde and dyed. Then, five random areas were selected and the number of cells was observed under a microscope.
Cell Apoptosis Assay
The transfected cells were collected after being centrifuged and washed. A binding buffer was used to resuspend cells. Annexin V-EGFP and propidium iodide were added into the suspended cell solution and the tube was put away from light at room temperature for 20 min. The apoptosis was detected with flow cytometry.
Luciferase Activity Assay
The possible targets of FENDRR and miR-423-5p were predicted by ENCORI and TargetScan. The correlation between the sequences of FENDRR and miR-423-5p and the relationship of miR-423-5p and Nox4 were validated with a dual-luciferase reporter assay. The partial sequence of FENDRR containing binding sites or FENDRR mutation were co-transfected into HUVECs with miR-423-5p synthetic sequences separately. Consistently, the mutation and wide Nox4 sequences were cloned into pmirGLO (FENGHUI, Changsha, China). Then the pmirGLO with different cloned sequences as well as separate obtained miR-423-5p sequences were co-transfected into cells using Lipofectamine 3000. The luciferase results of different groups were evaluated with a reporter gene kit (Biolab, Beijing, China).
Western Blot Analysis
The protein samples were isolated from cells via a total protein extraction kit (Bohu, Shanghai, China). The BCA protein quantification kit (Yeason, Shanghai, China) was used to confirm the concentration of each protein specimen. Each channel of SDS/PAGE electrophoresis was added 50 µg of protein sample. The gel with target gene was transferred to the PVDF membrane and blocked with 5% skim milk. Then, the membrane was incubated with rabbit polyclonal anti-NOX 4 (1:2000) overnight followed by goat anti-rabbit for two hours. The specific proteins were visually detected using a chemiluminescence reagent. ImageJ software was applied to quantify protein expression.
Statistical Analysis
The differences between the two groups on baseline characteristics were analyzed by Student’s t-test and chi-square test. ROC was used for the identification of the diagnostic value of FENDRR for EH patients. Comparisons among three or more groups were calculated by one-way ANOVA. Pearson correlation was carried out to determine the correlation between levels of miR-423-5p and the relative expression of FENDRR. All data were calculated with SPSS 20.0 and GraphPad 7.0 and expressed as mean ± SD.
Results
Baseline Characteristics of the Study Cohort
We stated the demographics and clinical indexes and all statistical information was exhibited in Table 2. 67 EH patients with mean age of 47.36 ± 6.34 years old and 74 control persons (mean age 47.91 ± 8.88) were volunteered in our experiment. There are no dramatic differences in the demographic features including sex, age, and BMI between the healthy group and EH group (Table 2, P > 0.05). Likewise, the observation indicated some clinicopathological parameters showed no obvious difference between the control individuals and patients with EH, such as LDL-C, HDL-C, TG, and TC (Table 2, P > 0.05). However, the levels of SBP and DBP in the EH group were significantly higher than those in the control group (Table 2, P < 0.001).
 |
Table 2 Baseline Features and Clinical Characters of the Study Population |
Plasma Expression of FENDRR in Patients with EH
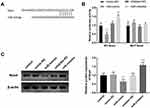
The expression of FENDRR was measured among all participants to identify the landscape of FENDRR expression in patients with EH. Noticeably, the expression of FENDRR in the EH cohort was enriched in EH patients in comparison with controls (Figure 1A, P < 0.001). The anomalous change of FENDRR levels reported a potential link between FENDRR and the pathology of EH.
Diagnostic Accuracy of FENDRR for EH Patients
Taking the aberrant amount of FENDRR into consideration, the diagnostic effect of FENDRR was determined by the ROC curve. The data showed a high possibility of FENDRR as a predictive item with the document that the area under the curve was 0.881 (Figure 1B). Interestingly, the appliance of FENDRR in distinguishing EH patients indicated prominent sensitivity (0.806) and satisfactory specificity (0.824).
Silenced FENDRR Exerts Expansionary Effects on HUVECs in vitro
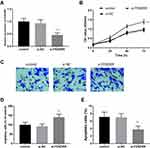
The expression of FENDRR was artificially regulated to detect the effect of the abnormal expression on cell proliferation. As expected, the loss of FENDRR was measured in the HUVECs treated with the si-FENDRR group when compared with the control group (Figure 2A, P < 0.001).
Furthermore, the CCK-8 was performed to evaluate the proliferation of HUVECs on diverse time points. The findings pinpointed that the inhibition of FENDRR improved cell proliferation when compared to control HUVECs (Figure 2B, P < 0.01). Likewise, the Transwell assay also provided the motivating influence of reduced FENDRR in HUVECs. This viewpoint was supported by the result that the loss of FENDRR facilitated migratory cells in the lower chamber in the si-FENDRR group (Figure 2C and D, P < 0.01). Considering the effective roles of FENDRR on cell proliferation and cell migration, the function of FENDRR on the percentage of apoptotic cells was deduced. As demonstrated in Figure 2E, compared to the control HUVECs, the downregulation of FENDRR ameliorated cell apoptosis which inferred FENDRR might play potential roles on HUVECs (P < 0.001).
FENDRR is a ceRNA of miR-423-5p
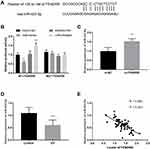
To uncover the further mechanism of FENDRR, the downstream target was forecasted and corroborated in this subject. Figure 3A showed the binding sites between FENDRR and miR-423-5p, unveiling miR-423-5p was a promising ceRNA of FENDRR. Along with this speculation, a certain confirmation was established by the luciferase reporter assay. When referenced to mimic-NC cells, the relative luciferase activity in the HUVECs transfected using miR-mimic was significantly reduced in the WT-FENDRR group (Figure 3B, P < 0.01) while the luciferase activity was elevated in the cells treated with miR-inhibitor. In addition, the silenced FENDRR promoted the relative levels of miR-423-5p in HUVECs, which adequately substantiated the former results (Figure 3C, P < 0.01).
What is more, the relative expression of miR-423-5p was disclosed in EH patients by qRT-PCR, and the data were expressed in Figure 3D. The relative expression of miR-423-5p is remarkably decreased in EH patients compared to control volunteers (P < 0.001). Importantly, the Pearson correlation was implanted to state the relationship between FENDRR and miR-423-5p. As an exhibition, the levels of FENDRR were negatively correlated with the relative expression of miR-423-5p (Figure 3E, R = 0.682, P < 0.001).
The Function of miR-423-5p on HUVECs in vitro
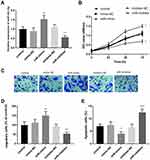
To test the function of miR-423-5p, miR-423-5p mimics were transfected to regulate the expression level of miR-423-5p in HUVECs. Compared with the control group, the expression level of miR-423-5p in HUVECs treated with miR-423-5p mimics increased significantly, however, the transfection of miR-inhibitor decreased the levels of miR-423-5p (Figure 4A, P < 0.01).
Besides, CCK-8 results showed that the enrichment of miR-423-5p enhanced cell proliferation, but the knockdown of miR-423-5p suppressed cell proliferation when compared with the control HUVECs (Figure 4B, P < 0.05). Similarly, this view was supported by the following results, namely the overexpression of miR-423-5p promoted the percentage of migrating cells, nevertheless, the silenced miR-423-5p repressed cell migration (Figure 4C and D, P < 0.01). Meanwhile, upregulation of miR-423-5p inhibited the apoptosis of HUVECs, whereas inhibition of miR-423-5p led to the increase of apoptotic cells (Figure 4E, P < 0.05).
miR-423-5p Mediates the Function of FENDRR on HUVECs
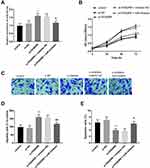
To reveal the regulatory effect of miR-423-5p, miR-423-5p inhibitors and si-FENDRR were co-transfected. As speculated, transfection of miR-423-5p inhibitors reversed the increase of miR-423-5p expression induced by si-FENDRR (Figure 5A, P < 0.05).
More importantly, the deletion of miR-423-5p inhibited the improvement of cell proliferation caused by the decrease of FENDRR (Figure 5B, P < 0.05). Consistently, the silenced miR-423-5p inversed elevated cell migration induced by inhibition of FENDRR (Figure 5C and D, P < 0.01). Figure 5E pinpointed the inhibitor-NC attenuated the declined apoptosis of HUVECs transfected with si-FENDRR alone (P < 0.05).
Nox4 Serves as a Target Gene of miR-423-5p
In order to further reveal the mechanism of miR-423-5p, we predicted and substantiated the target of miR-423-5p. Given the results of ENCORI database and the reported impacts of Nox4 on hypertension, we focused on Nox4. Figure 6A shows the binding bases between Nox4 and miR-423-5p. The implementation of luciferase reporter gene analysis verified this hypothesis. In the WT-Nox4 group, the relative luciferase activity decreased significantly in HUVECs transfected with miR-mimic and miR-inhibitor contributed to raised luciferase activity (Figure 6B, P < 0.01). Further detection of protein expression of Nox4 was used to elucidate the relationship between miR-423-5p and Nox4. As shown in Figure 6C, the relative protein expression of Nox4 was reduced in the miR-mimic group and enhanced in the miR-inhibitor group (P < 0.01).
Discussion
EH is a prevalent preventable chronic disorder with abstruse pathogenesis and complex clinicopathological characteristics.21 Considering that the lncRNA-miRNA axis is one key new regulator of cardiovascular factors and cell function, it is an important candidate for diagnosis and drug-targeted therapy.22 In a current subject, a preternatural abundant lncRNA AK098656 is found in hypertensive patients, which is proved to regulate VSMCs function and blood pressure.23 Another illustration is reported by Li et al, namely, the viability and migration of cell models are repressing by lncRNA MIAT/ miR-29a-5p axis, indicating its influence in pulmonary hypertension.24 Collectively, much attention has been focused on the values of lncRNA/miRNA on the pathologic changes of EH.
In the outstanding achievements of previous studies, one suggested that FENDRR was highly expressed in hypertensive cases, suggesting that FENDRR was abnormally expressed in high blood pressure.25 This dysregulation of FENDRR is also found in other disorders or maladies, like lung malignant tumor,26 acute pancreatitis,27 and pulmonary fibrosis.28 In research involving 70 patients with lung cancer, FENDRR is found aberrantly expressed in tumor tissues and it might be a predictive biomarker in clinical practice.29 In a similar case published in 2019, the expression of FENDRR is raised in retinopathy patients caused by high blood sugar.30 In the present experiment, the expression pattern of FENDRR was proposed and we showed an increased enrichment of FENDRR in the plasma specimens of EH patients, which is in accordance with the preceding findings. The data indicated the progression of EH might be modulated by the change of FENDRR levels. Additionally, FENDRR showed a theoretical possibility as a diagnostic marker in discriminating between EH patients and healthy subjects with preeminent specificity and excellent sensitivity. Latterly, the enormous work on the influence of lncRNAs on HUVECs has been reported in the past few years.31 Incidentally, an illustration about patients with Kawasaki disease pinpoints the knockdown of lncRNA SOCS2-AS1 encourages the apoptotic HUVECs and accommodates the proliferation of HUVECs by controlling the miR-423-5p/ CUEDC2 axis.32 The overexpression of FENDRR appearance expansionary effect on apoptosis of microvascular endothelial cells.11 Hypertension is closely related to reduced endothelial homeostasis.33 Endothelial cell is a main component of vasculature and is able to regulate vascular homeostasis by modulating processes of vascular dilation, blood pressure regulation, and chemical cytokines and chemical mediators.34 In addition, Tian et al use the HUVECs to investigate the function of miR-199a-5p in EH.35 Thus, we used HUVECs to analyze the vital roles of FENDRR in EH. Our investigation manifested the knockdown of FENDRR improved the proliferative and migratory HUVECs and attenuated apoptotic HUVECs, insisting on a standpoint that the expression of FENDRR might take an essential part in EH by managing HUVECs activities.
Beyond this, the expression of miR-423-5p was identified to be lower in the EH patients than in the control cohort, emphasizing the protective roles of miR-423-5p in EH. An experiment written by Zhang et al determines the low expression of miR-423-5p in hypertensive patients, which further underlines our consequence.19 The function of miR-423-5p on HUVECs was described in our research, which ascertained the upregulation of miR-423-5p facilitated cell proliferation and migration and held back cell apoptosis. The emerging effort on the influence of miRNAs on HUVECs has been exhibited in recent decades, like miR-15536 and miR-126.37 In a work of Yao et al, the miR-297/STAT3 axis serves as a protective indicator by restraining apoptosis of HUVECs.38 Taken as another example of miR-6086, the regulation of miR-6086 is substantiated in the proliferation and apoptosis of HUVECs.39 Collectively, miR-423-5p might act as a favorite target in the EH by protecting cell activities.
More importantly, specific regulatory networks are recorded in quite a few publications, such as lncRNA TUG1/miR-145-5p/FGF1040 and lncRNA MALAT1/ miR-539-3p.41 Interestingly, the ceRNA of FENDRR was verified to be miR-423-5p and the alteration of FENDRR expression reversely modulated the expression of miR-423-5p, indicating FENDRR might impact the biological transformation of HUVECs by sequestering miR-423-5p. A negative correlation between FENDRR and miR-423-5p was restated in the former relationship using the Pearson coefficient. This pathway is determined in the outcome of Yu et al published in 2019.20 Additionally, the experiments about co-transfection of si-FENDRR and miR-inhibitor confirmed the regulatory mechanism between FENDRR and miR-423-5p by the results that the knockdown of miR-423-5p reversed the impacts of FENDRR on cell proliferation, migration, together with apoptosis. In diabetic nephropathy, the miR-423-5p protects podocyte injury caused by high glucose by directly targeting Nox4.42 Several investigations report the impacts of Nox4 in hypertension. Blocking Nox4 inhibits the pulse pressure increases caused by angiotensin II.43 According to a previous investigation, Nox4 contributes to hypertension caused by chronic intermittent hypoxia.44 Another publication pinpoints the relative expression of Nox 4 is raised in the hypertensive rats caused by high salt.45 Also, the complementary sites between Nox4 and miR-423-5p were expressed in this study and the target ship of Nox4 and miR-423-5p was corroborated by luciferase activity assay. Besides, the finding of western blot analysis indicated that the change of miR-423-5p regulated the relative levels of Nox4. Combined with the previous research discovery, we inferred that the miR-423-5p might exert its influence on HUVECs through binding Nox4. However, lack of RNA-binding protein immunoprecipitation or pull down assays and lack of internal reference screening experiment are limitations of this study.
Conclusion
In brief, this research drew a conclusion that the FENDRR was highly expressed in patients with EH, and alteration of FENDRR might be a predictor in discriminating EH patients among healthy people. Also, miR-423-5p was proved as a ceRNA of FENDRR and the expression of miR-423-5p was reduced in EH patients. The inhibition of FENDRR contributed to the enhancement of miR-423-5p in EH. We assumed the role of FENDRR and miR-423-5p on cell viability, migration, and apoptosis. The miR-423-5p mediated the function of FENDRR on HUVECs. Additionally, FENDRR/miR-423-5p/Nox4 axis was turned out to be a mediator in process of EH and this proved a particular perspective in the pathogenesis of EH.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Banegas JR, Gijón-Conde T. [Epidemiology of hypertension]. Hipertens Riesgo Vasc. 2017;34(Suppl 2):2–4. Spanish. doi:10.1016/S1889-1837(18)30066-7
2. O’Shea PM, Griffin TP, Fitzgibbon M. Hypertension: the role of biochemistry in the diagnosis and management. Clin Chim Acta. 2017;465:131–143. doi:10.1016/j.cca.2016.12.014
3. Chen J, Chen X, Fu L, Chen J, Chen Y, Liu F. LncRNA GACAT1 targeting miRNA-149 regulates the molecular mechanism of proliferation, apoptosis and autophagy of oral squamous cell carcinoma cells. Aging. 2021;13(16):20359–20371. doi:10.18632/aging.203416
4. Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35(2):231–246. doi:10.1016/j.ccl.2016.12.005
5. Stelcar A, Homsak E, Marcun Varda N. Assessment of early cardiovascular risk in children and adolescents with essential hypertension. Klin Padiatr. 2017;229(5):286–292. doi:10.1055/s-0043-104220
6. Pont L, Alhawassi T. Challenges in the management of hypertension in older populations. Adv Exp Med Biol. 2017;956:167–180.
7. Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41(9):761–772. doi:10.1016/j.tibs.2016.07.003
8. Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19(3):143–157. doi:10.1038/nrm.2017.104
9. Poller W, Dimmeler S, Heymans S, et al. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J. 2018;39(29):2704–2716. doi:10.1093/eurheartj/ehx165
10. Chen R, Chen S, Zhang T, et al. Relationships among long noncoding RNA, environmental factors and hypertension. Wei Sheng Yan Jiu = J Hygiene Res. 2017;46(6):905–912.
11. Dong B, Zhou B, Sun Z, et al. LncRNA-FENDRR mediates VEGFA to promote the apoptosis of brain microvascular endothelial cells via regulating miR-126 in mice with hypertensive intracerebral hemorrhage. Microcirculation. 2018;25(8):e12499. doi:10.1111/micc.12499
12. He Z, Wang X, Huang C, et al. The FENDRR/miR-214-3P/TET2 axis affects cell malignant activity via RASSF1A methylation in gastric cancer. Am J Transl Res. 2018;10(10):3211–3223.
13. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202–1207. doi:10.1016/j.jaci.2017.08.034
14. Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524. doi:10.1038/nrm3838
15. Hu H, Chen W, Zhang S, Xue Y, He Y, Gu Y. NEAT1/miR-101-dependent up-regulation of DNA-PKcs enhances malignant behaviors of pancreatic ductal adenocarcinoma cells. J Cancer. 2021;12(18):5622–5632. doi:10.7150/jca.58824
16. Wang P, Bai C, Shen S, Jiang C, Deng J, Han D. MALAT1 promotes malignant pleural mesothelioma by sponging miR-141-3p. Open Med. 2021;16(1):1653–1667. doi:10.1515/med-2021-0383
17. Perez-Hernandez J, Olivares D, Forner MJ, et al. Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. J Transl Med. 2018;16(1):228. doi:10.1186/s12967-018-1604-6
18. Luo Y, Bao X, Zheng S, et al. A potential risk factor of essential hypertension in case-control study: microRNAs miR-10a-5p. Clin Exp Hypertens. 2020;42(1):36–42. doi:10.1080/10641963.2019.1571597
19. Zhang X, Wang X, Wu J, et al. The diagnostic values of circulating miRNAs for hypertension and bioinformatics analysis. Biosci Rep. 2018;38(4). doi:10.1042/BSR20180525
20. Yu Z, Zhao H, Feng X, et al. Long non-coding RNA FENDRR acts as a miR-423-5p sponge to suppress the Treg-mediated immune escape of hepatocellular carcinoma cells. Mol Ther Nucleic Acids. 2019;17:516–529. doi:10.1016/j.omtn.2019.05.027
21. Zhu Y, You J, Xu C, Gu X. Associations of mitochondrial DNA 3777-4679 region mutations with maternally inherited essential hypertensive subjects in China. BMC Med Genet. 2020;21(1):105. doi:10.1186/s12881-020-01045-7
22. Li M, Duan L, Li Y, Liu B. Long noncoding RNA/circular noncoding RNA-miRNA-mRNA axes in cardiovascular diseases. Life Sci. 2019;233:116440. doi:10.1016/j.lfs.2019.04.066
23. Jin L, Lin X, Yang L, et al. AK098656, a novel vascular smooth muscle cell-dominant long noncoding RNA, promotes hypertension. Hypertension. 2018;71(2):262–272. doi:10.1161/HYPERTENSIONAHA.117.09651
24. Li WW, Cao AH, Sun FY. LncRNA MIAT stimulates oxidative stress in the hypoxic pulmonary hypertension model by sponging miR-29a-5p and inhibiting Nrf2 pathway. Eur Rev Med Pharmacol Sci. 2020;24(17):9022–9029. doi:10.26355/eurrev_202009_22845
25. Kontaraki JE, Marketou ME, Kochiadakis GE, et al. The long non-coding RNAs MHRT, FENDRR and CARMEN, their expression levels in peripheral blood mononuclear cells in patients with essential hypertension and their relation to heart hypertrophy. Clin Exp Pharmacol Physiol. 2018;45(11):1213–1217. doi:10.1111/1440-1681.12997
26. Chen R, Li WX, Sun Y, et al. Comprehensive analysis of lncRNA and mRNA expression profiles in lung cancer. Clin Lab. 2017;63(2):313–320. doi:10.7754/Clin.Lab.2016.160812
27. Zhao D, Ge H, Ma B, et al. The interaction between ANXA2 and lncRNA Fendrr promotes cell apoptosis in caerulein-induced acute pancreatitis. J Cell Biochem. 2019;120:8160–8168.
28. Huang C, Liang Y, Zeng X, et al. Long noncoding RNA FENDRR exhibits antifibrotic activity in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2020;62(4):440–453. doi:10.1165/rcmb.2018-0293OC
29. Herrera-Merchan A, Cuadros M, Rodriguez MI, et al. The value of lncRNA FENDRR and FOXF1 as a prognostic factor for survival of lung adenocarcinoma. Oncotarget. 2020;11(13):1172–1185. doi:10.18632/oncotarget.22154
30. Shi Y, Chen C, Xu Y, Liu Y, Zhang H, Liu Y. LncRNA FENDRR promotes high-glucose-induced proliferation and angiogenesis of human retinal endothelial cells. Biosci Biotechnol Biochem. 2019;83(5):869–875. doi:10.1080/09168451.2019.1569499
31. Wang K, Yang C, Shi J, Gao T. Ox-LDL-induced lncRNA MALAT1 promotes autophagy in human umbilical vein endothelial cells by sponging miR-216a-5p and regulating Beclin-1 expression. Eur J Pharmacol. 2019;858:172338. doi:10.1016/j.ejphar.2019.04.019
32. Zhao J, Chen D. Kawasaki disease: SOCS2-AS1/miR-324-5p/CUEDC2 axis regulates the progression of human umbilical vein endothelial cells. Pediatr Res. 2020. doi:10.1038/s41390-020-1029-9
33. Balduino Mendes AB, Giollo-Junior LT, de Andrade DO, Gregório ML, Yugar-Toledo JC, Vilela-Martin JF. How to investigate the vascular changes in resistant hypertension. Curr Hypertens Rev. 2016;12(2):139–147. doi:10.2174/1573402111666150812143349
34. Ciesielski O, Biesiekierska M, Panthu B, Vialichka V, Pirola L, Balcerczyk A. The epigenetic profile of tumor endothelial cells. effects of combined therapy with antiangiogenic and epigenetic drugs on cancer progression. Int J Mol Sci. 2020;21(7):2606. doi:10.3390/ijms21072606
35. Tian X, Yu C, Shi L, et al. MicroRNA-199a-5p aggravates primary hypertension by damaging vascular endothelial cells through inhibition of autophagy and promotion of apoptosis. Exp Ther Med. 2018;16(2):595–602. doi:10.3892/etm.2018.6252
36. Zhang Z, Pan X, Yang S, et al. miR-155 promotes ox-LDL-induced autophagy in human umbilical vein endothelial cells. Mediators Inflamm. 2017;2017:9174801. doi:10.1155/2017/9174801
37. Sun Y, Liu XL, Zhang D, et al. Platelet-derived exosomes affect the proliferation and migration of human umbilical vein endothelial cells via miR-126. Curr Vasc Pharmacol. 2019;17(4):379–387. doi:10.2174/1570161116666180313142139
38. Yao Y, Jia H, Wang G, Ma Y, Sun W, Li P. miR-297 protects human umbilical vein endothelial cells against LPS-induced inflammatory response and apoptosis. Cell Physiol Biochem. 2019;52(4):696–707.
39. Wan Y, Fei X, Wang Z, et al. miR-423-5p knockdown enhances the sensitivity of glioma stem cells to apigenin through the mitochondrial pathway. Tumour Biol. 2017;39(4):1010428317695526. doi:10.1177/1010428317695526
40. Shi L, Tian C, Sun L, Cao F, Meng Z. The lncRNA TUG1/miR-145-5p/FGF10 regulates proliferation and migration in VSMCs of hypertension. Biochem Biophys Res Commun. 2018;501(3):688–695. doi:10.1016/j.bbrc.2018.05.049
41. Mou X, Wang J, Wang L, Wang S. Correlation between single nucleotide polymorphisms of the rs664589 locus in the long-chain noncoding RNA lung adenocarcinoma metastasis-associated gene 1, hypertension, and its mechanism. Genet Test Mol Biomarkers. 2020;24(3):120–130. doi:10.1089/gtmb.2019.0193
42. Xu Y, Zhang J, Fan L, He X. miR-423-5p suppresses high-glucose-induced podocyte injury by targeting Nox4. Biochem Biophys Res Commun. 2018;505(2):339–345. doi:10.1016/j.bbrc.2018.09.067
43. Bouabout G, Ayme-Dietrich E, Jacob H, et al. Nox4 genetic inhibition in experimental hypertension and metabolic syndrome. Arch Cardiovasc Dis. 2018;111(1):41–52. doi:10.1016/j.acvd.2017.03.011
44. Lu W, Kang J, Hu K, et al. The role of the Nox4-derived ROS-mediated RhoA/Rho kinase pathway in rat hypertension induced by chronic intermittent hypoxia. Sleep Breath. 2017;21(3):667–677. doi:10.1007/s11325-016-1449-2
45. Liang YF, Zhang DD, Yu XJ, et al. Hydrogen sulfide in paraventricular nucleus attenuates blood pressure by regulating oxidative stress and inflammatory cytokines in high salt-induced hypertension. Toxicol Lett. 2017;270:62–71. doi:10.1016/j.toxlet.2017.02.004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.