Back to Journals » International Journal of General Medicine » Volume 14
LncRNA CASC15 Promotes Cerebral Ischemia/Reperfusion Injury via miR-338-3p/ETS1 Axis in Acute Ischemic Stroke
Authors Chen C, Wang L, Wang L, Liu Q, Wang C
Received 3 June 2021
Accepted for publication 24 August 2021
Published 2 October 2021 Volume 2021:14 Pages 6305—6313
DOI https://doi.org/10.2147/IJGM.S323237
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Chen Chen,1 Linjing Wang,1 Li Wang,2 Qi Liu,1 Chunying Wang1
1Institute of Chinese Medicine, Heilongjiang University of Chinese Medicine, Harbin, 150040, People’s Republic of China; 2Harbin Traditional Chinese Medicine Hospital, Harbin, 150076, People’s Republic of China
Correspondence: Chunying Wang
Institute of Chinese Medicine, Heilongjiang University of Chinese Medicine, 24 Heping Road, Harbin, 150040, People’s Republic of China
Tel/Fax +86-18644086608
Email [email protected]
Purpose: Acute ischemic stroke (AIS) is a leading health problem caused by cerebral ischemia/reperfusion (CI/R). This study aimed to unveil the potential clinical value and mechanism of lncRNA CASC15.
Patients and Methods: The expression of CASC15, miR-338-3p was detected by quantitative real-time PCR (qRT-PCR). The correlations between CASC15 and national institutes of health stroke scale (NIHSS) scores or miR-338-3p were evaluated by Pearson correlation. A receiver operating characteristic (ROC) curve was performed to provide the diagnostic value of CASC15. Cell Counting Kit-8 (CCK-8) and flow cytometer were used to detect the condition of cell viability and apoptosis. The levels of interleukin-1beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) was detected by enzyme-linked immunosorbent assay (ELISA) assay.
Results: The expression of CASC15 was increased and the levels of miR-338-3p were decreased in AIS patients. A positive association between CASC15 and NIHSS score and an inverse association between CASC15 and miR-338-3p were revealed by Pearson correlation. CASC15 might discriminate AIS patients from healthy people. Silenced CASC15 exerted neuroprotective roles on cell viability, apoptosis, and inflammation via the miR-338-3p/ETS1 axis.
Conclusion: CASC15 might act as a potential diagnostic biomarker for AIS patients. CASC15/miR-338-3p/ETS1 axis played an essential role in cell viability, apoptosis, and neuroinflammation.
Keywords: CASC15/miR-338-3p/ETS1, acute ischemic stroke, biomarker, inflammation
Introduction
Stroke is one of the most dangerous vascular diseases, leading to public economic challenges and family burdens.1 Acute ischemic stroke (AIS) is the common type of stroke induced by blood occlusion.2 The treatments of stroke were constituted by emergency reperfusion of blood flow and other precautionary methods.3,4 However, recent achievement underlines that the reperfusion after acute ischemia of the brain might lead to even severe injury.5 Due to less understanding of the pathology of AIS and prompt responsive measures to the emergency of AIS, AIS still intimidates the lives of all patients. The immune inflammation response and injury of cell biological activities are important aspects among AIS disorders.6 Thus, a predictive biomarker involving the mechanism of AIS and cerebral ischemia/reperfusion (CI/R) damage is promising in the realms pertinent to AIS clinical therapy.
Long non-coding RNAs (lncRNAs) participate in diseases via interlaced regulatory networks.7 Substantial research has been published to unveil the significance of all sorts of lncRNAs in AIS.8,9 For instance, lncRNA HULC is associated with national institutes of health stroke scale (NIHSS) scores and may function as a biomarker.10 Besides, the abnormal expression of ANRIL alters the inflammatory situation of AIS patients.11 A recent academic achievement concerning lncRNA GAS5 proposes the impacts of GAS5 in AIS. It is reported that GAS5 is highly expressed in the animal models and cell models caused by CI/R and silenced GAS5 improves cell viability by miR-137/Notch1 axis.12 Xiang et al provide a further substantiation, namely, they verify that lncRNA MEG3 leads to CI/R damage by sponging miR-424-5p and MAPK pathway.13 LncRNA CASC15, as a common lncRNA, has been explored by investigators in studies of cancer and diseases.14,15 Differently expressed CASC15 is elucidated in several diseases. In hepatocellular carcinoma, bladder cancer, and prostate cancer, CASC15 is identified to be an oncogene by accelerating cell migration, expediting cell invasion, and attenuating cell apoptosis by specific signal pathways.16–18 It is known to all, some sustained cardiac disorders are increased AIS susceptibility, like cardiac hypertrophy.19 Upregulation of CASC15 is evaluated in the animal models of cardiac hypertrophy.20 But yet, there is still a loss of data about CASC15 in AIS and their potential association.
Thus, we collected AIS patients and revealed the expression of CASC15 in these patients. The association of CASC15 and national institutes of health stroke scale (NIHSS) scores was identified and the clinical predictive accuracy of CASC15 was made clear. The significance of CASC15 on cell inflammation, cell viability, and cell apoptosis was investigated. Significantly, the underlying molecular mechanism of CASC15 in CI/R damage was substantiated.
Patients and Methods
Patients and Cells
We collected 98 patients with AIS and 85 control patients in the Heilongjiang University of Chinese Medicine and all these patients provided informed consent for the blood collection. AIS patients were diagnosed by Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. The inclusion criteria were: hospitalization within 48 h of onset, voluntary signing of informed consent, and complete medical records. The exclusion criteria of AIS patients were: (1) transient ischemic attack, cerebral hemorrhage, subarachnoid hemorrhage, and space-occupying lesions; (2) consciousness disturbance; (3) serious diseases such as heart, liver, kidney, blood system, and tumor. For the healthy group, individuals who met with the following conditions were included, including the voluntary signing of informed consent and no history of stroke. The exclusion criteria of controls were as follows: tumor, severe inflammation, pregnancy, and severe liver or kidney failure. Besides, detailed information, like baseline demographics and clinical examination data of participants were gathered. The Institutional Ethics Committee of Heilongjiang University of Chinese Medicine approved our experiments. A total of 85 healthy controls with a mean age of 55.81 ± 9.45 years old and 98 AIS patients (mean age of 56.27 ± 11.00 years old) were recruited in this investigation. No significant difference was observed between the healthy group and the AIS group in age, body mass index (BMI), and Sex (all P > 0.05, Table 1). Moreover, the data of diabetes, hypertension, hyperuricemia, and hyperlipidemia in AIS individuals were showed no difference when compared to control subjects (all P > 0.05, Table 1). The mean NIHSS score was 11.73 ± 5.78 in the AIS group (Table 1).
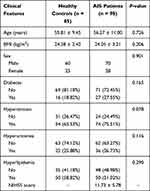 |
Table 1 Baseline Data of AIS Patients and the Healthy Group |
Human neuroblastoma SH-SY5Y cells were obtained from EK-Bioscience (Shanghai, China) and cultivated in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% FBS and 1% compounded P/S. The atmosphere of cell culture was a moist air with 5% CO2 at 37°C. Our experiments complied with the Declaration of Helsinki.
Sample Collection
The blood specimens were isolated from each AIS patient at the arrival of hospitalization. And healthy controls contributed to their blood samples after 8-h fasting. All samples were placed vertically for 2 hours and then centrifuged to collect plasma specimens.
Oxygen Glucose Deprivation/Reoxygenation (OGD/R) Cells Establishment
According to a previous publication, SH-SY5Y cells were applied to conduct OGD/R cell models.21 Briefly, the cells were transferred to the DMEM medium without glucose and serum at an atmosphere with 94% N2, 5% CO2, and 1% O2 for 8 hours. Then, the cells were returned to normal culture conditions for 24 hours.
Cell Transfection
Small interfering CASC15 (si-CASC15) and its negative control (si-NC), together with miR-338-3p mimic, miR-338-3p inhibitor, and their negative control (miR-NC) were attained from IBSBIO (Shanghai, China). Transfection and co-transfection were conducted by using Lipofectamine 3000 (Invitrogen, USA).
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
We isolated RNA samples from each individual and experimental cells by TRIzol reagent (Invitrogen, USA). RNA with an OD260/OD280 value of 1.8–2.0 would only be reverse transcribed into DNA if it was content. A Titan one tube RT-PCR kit (Roche, Basel, Switzerland) and HyperScript III miRNA 1st Strand cDNA Synthesis Kit (NovaBio, Shanghai, China) were used to synthesize cDNA. In order to detect relative levels of CASC15 and miR-338-3p, cDNA samples were mixed with 2 x S6 Universal SYBR qPCR Mix (NovaBio, Shanghai, China) on ABI 7500 system (ThermoFisher, USA). β-actin and U6 were served as internal housekeeping genes. The obtained data were calculated with 2−∆∆CT.
Enzyme-Linked Immunosorbent Assay (ELISA)
Emerging neuroinflammation was given priority in the injury of CI/R. Thus, the levels of interleukin-1beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) were detected by ELISA assay Kits (all from Boster, Wuhan, China).
Cell Viability
A 96-well plate was seeded SH-SY5Y cells with a suitable concentration (5000/well). Then, the wells were added with a 10 μL Cell Counting Kit-8 (CCK-8, Dojindo, Japan). These experimental cells were incubated at 37°C for further 2 hours. After that, a microplate reader was performed to determine the absorbance values under 450 nm.
Cell Apoptosis
About 1×105 cells were gathered by centrifugation and washed by PBS twice. Then 500 μL binding buffer was added to suspend cells and a 5 μL Annexin V-FITC was mixed with suspension liquid. After a 30-min incubation, the reaction was interrupted with phosphate-buffered saline (PBS). Subsequently, an addition of 500 μL PBS suspended cells. A flow cytometer was carried out to observe the condition of the apoptotic cell.
Luciferase Activity Assay
Target genes were forecasted by bioinformatics. Wide CASC15 (WT-CASC15) including binding region was amplified by PCR and its mutation sequences (MUT-CASC15) were synthesized from Sangon (Shanghai, China). In the same way, the sequences of WT-ETS1 and MUT- ETS1 were procured from Sangon (Shanghai, China). pGL3 plasmids were inserted previous sequences and co-transfected with miR-338-3p mimics, miR-338-3p inhibitors, and miR-NCs into 293T cells respectively. The luciferase in cells was released by lysis reagent. Firefly luciferase reagent was added and the firefly luciferase data were recorded. Then, an Renilla luciferase reagent was added and the firefly luciferase data were recorded. The detection reagents were from the dual-luciferase reporter assay system (Promega, USA).
Statistical Analysis
The comparisons of different groups were analyzed by Student’s t-test, one-way ANOVA, and χ2 test. The correlations between CASC15 and NIHSS score as well as CASC15 and miR-338-3p were unveiled by Pearson Correlation. Receiver operating characteristic (ROC) curve was performed to provide the diagnostic value of CASC15 by the area under the ROC curve (AUC), specificity, and sensitivity. All these results were analyzed by SPSS and GraphPad and expressed as mean ± standard deviation (SD).
Results
Elevated Expression of CASC15 Was Related to NIHSS Score in AIS Patients
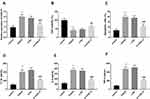
As expressed in Figure 1A, CASC15 was highly expressed in the AIS patients compared with healthy controls, suggesting CASC15 might be a regulator in AIS (P < 0.001). Besides, the Pearson correlation analysis demonstrated that the expression of CASC15 was increasingly elevated along with the increasing NIHSS scores in AIS patients (R = 0.617, P < 0.001; Figure 1B).
 |
Figure 1 (A) CASC15 is upregulated in AIS patients. (B) CASC15 was proportionate to the NIHSS score. (C) CASC15 could serve as a diagnostic biomarker for AIS patients. ***P < 0.001. |
Diagnostic Accuracy of CASC15 for AIS Patients
Considering the aberrant expression of CASC15 in AIS patients, the indicative value of CASC15 for AIS patients was proposed by the ROC curve. Figure 1C exhibited this result, which provided the AUC was 0.902 as well as the high sensitivity (0.847) and promising specificity (0.824) when the cut-off value was 1.339.
CASC15 Contributed to Cell Injury and Inflammation
To research the possible mechanism of CASC15, the levels of CASC15 were artificially regulated by the interference genes. Figure 2A provided that the expression of CASC15 was raised in OGD/R cell models and successfully inhibited in the si-CASC15 group (P < 0.001). Cell viability was reduced in the group induced by OGD/R and reversed by the inhibition of CASC15, indicating silenced CASC15 might exert protective effects in CI/R damage (P < 0.01, Figure 2B). This inference was reinforced by the results of cell apoptosis, that is, OGD/R led to increased apoptotic cells, whereas, silenced CASC15 alleviated this tendency (P < 0.001, Figure 2C). Beyond these, OGD/R contributed to the inflammation by the result that pro-inflammatory indicators, such as IL-1β, IL-6, and TNF-α, were raised (P < 0.001, Figure 2D–F). Interestingly, the underexpression of CASC15 ameliorated cell inflammation, indicating CASC15 could protect SH-SY5Y against neuroinflammation (P < 0.001, Figure 2D–F).
MiR-338-3p Was a ceRNA of CASC15
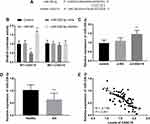
Figure 3A exhibited a complementary region between CASC15 and miR-338-3p, including 8 sites. Luciferase report further determined the relationship between CASC15 and miR-338-3p. In the WT-CASC15 group, a reduction of luciferase activity was found in the group transfected with miR-338-3p mimics, and an abundance of luciferase activity was found when cells were transfected with miR-338-3p inhibitors, which testified miR-338-3p was a ceRNA of CASC15 (P < 0.01, Figure 3B). Furthermore, knockdown of CASC15 increased the relative expression of miR-338-3p, further suggesting CASC15 sponged miR-338-3p (P < 0.01, Figure 3C).
MiR-338-3p Lowly Expressed in AIS Patients
Taken the correlation of CASC15 and miR-338-3p into consideration, we assessed the expression of miR-338-3p in AIS patients. The results of qRT-PCR showed that the relative expression of miR-338-3p was decreased in the plasma specimens from patients with AIS (P < 0.001, Figure 3D). In addition to the aberrantly expressed miR-338-3p, a negative correlation between CASC15 and miR-338-3p was found in AIS patients (R = −0.718, P < 0.001; Figure 3E).
MiR-338-3p Mediated the Function of CASC15 on Cell Models
The expression of miR-338-3p in the OGD/R group was decreased compared to the control group (P < 0.001, Figure 4A). Silenced CASC15 prevented the decreased trend of miR-338-3p, while the co-transfection of si-CASC15 and miR-338-3p inhibitors reversed the influence of reduced CASC15 (P < 0.001, Figure 4A). In addition, the silenced miR-338-3p mitigated the abundance of cell viability caused by reduced CASC15 expression (P < 0.01, Figure 4B). Downregulation of CASC15 contributed to the decreased apoptotic cells, however, miR-338-3p knockdown suppressed this effect (P < 0.001, Figure 4C). Besides, reduced miR-338-3p expression destroyed the protective effects of CASC15 on cell inflammation reaction (P < 0.001, Figure 4D–F).
ETS1 Serves as a Target Gene of miR-338-3p
As shown in Figure 5A, the putative target bases between ETS1 and miR-338-3p were exhibited. The target relation between ETS1 and miR-338-3p was identified by luciferase report assay. In the WT-ETS1 group, the luciferase activity was lowly expressed in the miR-338-3p mimic group and was highly expressed in the miR-338-3p inhibitor group (P < 0.001, Figure 5B).
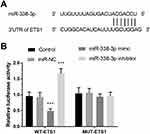 |
Figure 5 (A) The binding region between ETS1 and miR-338-3p. (B) The consequence of luciferase assay uncovered ETS1 was a target of miR-338-3p. ***P < 0.001. |
Discussion
As one of the leading agents of death or disability, the incidence of AIS is increasing recently globally.22 In a recent investigation, AIS accounts for 60–80% of stroke in China.23 In view of this situation, it is urgent to improve the diagnosis and risk judgment of AIS and to find the target of drug action. Recently, many pieces of research on the AIS and lncRNA have been conducted by several investigators. A sequencing data observed by Zhu et al determine 3009 abnormal expressed lncRNAs in AIS patients.24 LncRNA SNHG12 is highly expressed in the mice exposed to cerebral artery occlusion and it participates angiogenesis process and neuroinflammation via miR-150/VEGF axis.25 This previous achievement underlines the fundamental roles of lncRNAs in AIS.
In our study, we found that the expression of CASC15 was raised in the AIS patients relative to healthy controls, which proposed that CASC15 was a probable regulator in AIS. The levels of CASC15 were in direct proportion to the alternation of NIHSS scores, reporting the close association between CASC15 and AIS. Additionally, CASC15 showed a promising likelihood as a diagnostic biomarker for AIS patients. Research conducted by Zhang et al uses the same method to discover the value of ANRIL and their results imply the diagnostic importance of ANRIL in ischemic stroke.26 Significantly, mechanistic researches further indicate the consequence of the lncRNAs of vascular disorders.27 lncRNA SNHG6 represses the cell viability and inhibits cell apoptosis via the miR-181c-5p/BIM pathway.28 In the current investigation, the knockdown of CASC15 restored the percentage of viable cells and apoptotic cells destroyed by OGD/R, indicating the protective value of CASC15. Surprisingly, the silenced CASC15 attenuated cell immune inflammation caused by OGD/R, validating CASC15 might function as a neuroprotective factor.
The present study predicted and determined miR-338-3p was a sponge of CASC15 and the abnormal expression of CASC15 could change the levels of miR-338-3p. Furthermore, the expression of miR-338-3p was lessened and it was inversely proportional to the expression of CASC15 in AIS patients, suggesting the opposite function of miR-338-3p and CASC15 in AIS. These findings were further confirmed by the result obtained by Teng et al, which gives the decreased expression of miR-338-3p in patients with acute cerebral infarction, a higher risk of AIS.29 Additionally, the miR-338-3p mediated the function of CASC15 on the viability, apoptosis, and inflammation of SH-SY5Y cell models, providing CASC15 exerted regulatory effects per sponging miR-338-3p. In research about osteosarcoma, CASC15 controls osteosarcoma cell biological progression by functioning as a sponge of miR-338-3p.30
ETS1, namely ETS proto-oncogene 1 is reported as a mediator in neuroinflammatory progression. In a study conducting CI/R rat models, ETS1 expresses highly in the hippocampus and regulates neuronal apoptosis.31 In several disorders, ETS1 is identified to be a downstream targeting component of miR-338-3p, like bladder cancer and clear cell renal cell carcinoma.32,33 We predicted ETS1 might be a direct gene of miR-338-3p and the results of the luciferase report assay further determined the previous conjecture. One limitation of this investigation was no further mechanism of ETS1.
Conclusion
To sum up, elevated levels of CASC15 were found in the AIS patients and it was closely associated with NIHSS scores. CASC15 might function as an indicative biomarker in distinguishing AIS patients. MiR-338-3p, a ceRNA of CASC15, was expressed at a lower level in AIS patients. As expected, there was a negative correlation between CASC15 and miR-338-3p. In vitro conclusion substantiated silenced CASC15 could mitigate the damage of OGD/R on cell viability, cell apoptosis, and inflammation responses through accommodating miR-338-3p/ETS1 axis.
Ethics Statement
The Institutional Ethics Committee of Heilongjiang University of Chinese Medicine approved our experiments.
Funding
This work was financially supported by Scientific Research Foundation of Health Commission of Heilongjiang Province (2018234).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–211. doi:10.1055/s-0038-1649503
2. Boling B, Keinath K. Acute ischemic stroke. AACN Adv Crit Care. 2018;29(2):152–162. doi:10.4037/aacnacc2018483
3. Hankey GJ. Stroke. Lancet. 2017;389(10069):641–654. doi:10.1016/S0140-6736(16)30962-X
4. Rabinstein AA. Update on treatment of acute ischemic stroke. Continuum. 2020;26(2):268–286.
5. Patel RAG, McMullen PW. Neuroprotection in the treatment of acute ischemic stroke. Prog Cardiovasc Dis. 2017;59(6):542–548. doi:10.1016/j.pcad.2017.04.005
6. Zhi SM, Fang GX, Xie XM, et al. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur Rev Med Pharmacol Sci. 2020;24(3):1524–1536.
7. Qian X, Zhao J, Yeung PY, Zhang QC, Kwok CK. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem Sci. 2019;44(1):33–52. doi:10.1016/j.tibs.2018.09.012
8. Bao MH, Szeto V, Yang BB, Zhu SZ, Sun HS, Feng ZP. Long non-coding RNAs in ischemic stroke. Cell Death Dis. 2018;9(3):281. doi:10.1038/s41419-018-0282-x
9. Wang Y, Luo Y, Yao Y, et al. Silencing the lncRNA Maclpil in pro-inflammatory macrophages attenuates acute experimental ischemic stroke via LCP1 in mice. J Cereb Blood Flow Metab. 2020;40(4):747–759. doi:10.1177/0271678X19836118
10. Chen X, Zhang X, Su C, Huang S. Long noncoding RNA HULC in acute ischemic stroke: association with disease risk, severity, and recurrence-free survival and relation with IL-6, ICAM1, miR-9, and miR-195. J Clin Lab Anal. 2020;34(11):e23500.
11. Feng L, Guo J, Ai F. Circulating long noncoding RNA ANRIL downregulation correlates with increased risk, higher disease severity and elevated pro-inflammatory cytokines in patients with acute ischemic stroke. J Clin Lab Anal. 2019;33(1):e22629. doi:10.1002/jcla.22629
12. Chen F, Zhang L, Wang E, Zhang C, Li X. LncRNA GAS5 regulates ischemic stroke as a competing endogenous RNA for miR-137 to regulate the Notch1 signaling pathway. Biochem Biophys Res Commun. 2018;496(1):184–190. doi:10.1016/j.bbrc.2018.01.022
13. Xiang Y, Zhang Y, Xia Y, Zhao H, Liu A, Chen Y. LncRNA MEG3 targeting miR-424-5p via MAPK signaling pathway mediates neuronal apoptosis in ischemic stroke. Aging. 2020;12(4):3156–3174. doi:10.18632/aging.102790
14. Wang B, Xu W, Cai Y, Guo C, Zhou G, Yuan C. CASC15: a tumor-associated long non-coding RNA. Curr Pharm Des. 2021;27:127–134.
15. Zhang Y, Zhang L, Lu S, et al. Long non-coding RNA CASC15 promotes intrahepatic cholangiocarcinoma possibly through inducing PRDX2/PI3K/AKT axis. Cancer Res Treat. 2021;53(1):184–198. doi:10.4143/crt.2020.192
16. Wang C, Zi H, Wang Y, Li B, Ge Z, Ren X. RETRACTED ARTICLE: lncRNA CASC15 promotes tumour progression through SOX4/Wnt/β-catenin signalling pathway in hepatocellular carcinoma. Artif Cells Nanomed Biotechnol. 2020;48(1):763–769. doi:10.1080/21691401.2019.1576713
17. Yu X, Wang ZL, Han CL, et al. LncRNA CASC15 functions as an oncogene by sponging miR-130b-3p in bladder cancer. Eur Rev Med Pharmacol Sci. 2019;23(22):9814–9820.
18. Zhang C, Wang GX, Fu B, Zhou XC, Li Y, Li YY. LncRNA CASC15 promotes migration and invasion in prostate cancer via targeting miR-200a-3p. Eur Rev Med Pharmacol Sci. 2019;23(19):8303–8309.
19. Lee TM, Harn HJ, Chiou TW, et al. Remote transplantation of human adipose-derived stem cells induces regression of cardiac hypertrophy by regulating the macrophage polarization in spontaneously hypertensive rats. Redox Biol. 2019;27:101170. doi:10.1016/j.redox.2019.101170
20. Li C, Zhou G, Feng J, Zhang J, Hou L, Cheng Z. Upregulation of lncRNA VDR/CASC15 induced by facilitates cardiac hypertrophy through modulating miR-432-5p/TLR4 axis. Biochem Biophys Res Commun. 2018;503(4):2407–2414. doi:10.1016/j.bbrc.2018.06.169
21. Chai Z, Gong J, Zheng P, Zheng J. Inhibition of miR-19a-3p decreases cerebral ischemia/reperfusion injury by targeting IGFBP3 in vivo and in vitro. Biol Res. 2020;53(1):17. doi:10.1186/s40659-020-00280-9
22. Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. doi:10.1038/s41572-019-0118-8
23. Liu Z, Zhao Y, Liu D, et al. Effects of nursing quality improvement on thrombolytic therapy for acute ischemic stroke. Front Neurol. 2018;9:1025. doi:10.3389/fneur.2018.01025
24. Zhu W, Tian L, Yue X, Liu J, Fu Y, Yan Y. LncRNA expression profiling of ischemic stroke during the transition from the acute to subacute stage. Front Neurol. 2019;10:36. doi:10.3389/fneur.2019.00036
25. Zhao M, Wang J, Xi X, Tan N, Zhang L. SNHG12 promotes angiogenesis following ischemic stroke via regulating miR-150/VEGF pathway. Neuroscience. 2018;390:231–240. doi:10.1016/j.neuroscience.2018.08.029
26. Zhang K, Qi M, Yang Y, Xu P, Zhua Y, Zhang J. Circulating lncRNA ANRIL in the serum of patients with ischemic stroke. Clin Lab. 2019;65(8). doi:10.7754/Clin.Lab.2019.190143
27. Zhang X, Zhu XL, Ji BY, et al. LncRNA-1810034E14Rik reduces microglia activation in experimental ischemic stroke. J Neuroinflammation. 2019;16(1):75. doi:10.1186/s12974-019-1464-x
28. Zhang X, Liu Z, Shu Q, Yuan S, Xing Z, Song J. LncRNA SNHG6 functions as a ceRNA to regulate neuronal cell apoptosis by modulating miR-181c-5p/BIM signalling in ischaemic stroke. J Cell Mol Med. 2019;23(9):6120–6130. doi:10.1111/jcmm.14480
29. Teng L, Meng R. Long non-coding RNA MALAT1 promotes acute cerebral infarction through miRNAs-mediated hs-CRP regulation. J Mol Neurosci. 2019;69(3):494–504. doi:10.1007/s12031-019-01384-y
30. Zhang H, Wang J, Ren T, et al. LncRNA CASC15 is upregulated in osteosarcoma plasma exosomes and CASC15 knockdown inhibits osteosarcoma progression by regulating miR-338-3p/RAB14 axis. Onco Targets Ther. 2020;13:12055–12066. doi:10.2147/OTT.S282053
31. Yu HL, Wang LZ, Zhang LL, et al. ESE1 expression correlates with neuronal apoptosis in the hippocampus after cerebral ischemia/reperfusion injury. Neural Regen Res. 2019;14(5):841–849.
32. Zhang L, Yan R, Zhang SN, et al. MicroRNA-338-3p inhibits the progression of bladder cancer through regulating ETS1 expression. Eur Rev Med Pharmacol Sci. 2019;23(5):1986–1995.
33. Yang X, Zhang Y, Fan H. Downregulation of SBF2-AS1 functions as a tumor suppressor in clear cell renal cell carcinoma by inhibiting miR-338-3p-targeted ETS1. Cancer Gene Ther. 2021;28:813–827.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.