Back to Journals » OncoTargets and Therapy » Volume 14
LncRNA ANRIL Regulates Ovarian Cancer Progression and Tumor Stem Cell-Like Characteristics via miR-324-5p/Ran Axis
Authors Wang K, Hu YB, Zhao Y, Ye C
Received 13 August 2020
Accepted for publication 11 November 2020
Published 19 January 2021 Volume 2021:14 Pages 565—576
DOI https://doi.org/10.2147/OTT.S273614
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
This paper has been retracted.
Ke Wang, 1 Yu-Bo Hu, 2 Ye Zhao, 3 Cong Ye 1
1Department of Gynaecology and Obstetrics, The Third Hospital of Jilin University, Changchun, Jilin 130000, People’s Republic of China; 2Department of Anesthesiology, The Third Hospital of Jilin University, Changchun, Jilin 130000, People’s Republic of China; 3Department of Dermatology, The Third Hospital of Jilin University, Changchun, Jilin 130000, People’s Republic of China
Correspondence: Cong Ye
Department of Gynaecology and Obstetrics, The Third Hospital of Jilin University, Changchun, Jilin 130000, People’s Republic of China
Email [email protected]
Objective: Long non-coding RNA (lncRNA) ANRIL is emerging as a crucial role in ovarian cancer progression and prognosis. However, the precise molecular mechanism of ANRIL on ovarian cancer is not known. Thus, we aim to study the underlying mechanism of ANRIL on the action.
Methods: The MTT assay assessed cell viability. Cell migration and invasion were determined using the wound healing assay, Transwell migration, and invasion assay. The relationships of ANRIL, miR-324-5p, and RAN were evaluated using luciferase activity assay and RNA pull-down assay. Cancer stem cell was identified by flow cytometry. Sphere formation assay was conducted to determine the stem-like properties. Xenograft tumor was established to assess tumor growth in vivo. qRT-PCR and Western blot were used to detect gene expression.
Results: ANRIL was elevated while miR-324-5p was decreased in ovarian cancer tissues and cells. Besides, downregulated ANRIL enhanced miR-324-5p expression, and the luciferase reporting experiment and RNA pull-down assay showed the binding interaction between ANRIL and miR-324-5p. miR-324-5p directly targeted Ran and negatively modulated the expression of Ran. Besides, Ran was promoted by overexpressed ANRIL, which was reversed by overexpression of miR-324-5p. Furthermore, decreased ANRIL and increased miR-324-5p suppressed tumor growth, migration capacity, drug resistance, and alleviated stem-like characteristics in vitro and in vivo. Ran mediated the regulation of ANRIL on cell viability, stem-like properties, and drug resistance of ovarian cancer cells.
Conclusion: The ANRIL/miR-324-5p/Ran axis regulated ovarian cancer development, making the axis meaningful targets for ovarian cancer therapy.
Keywords: ANRIL, ovarian cancer, tumorigenicity, migration, drug resistance, cancer stem cell
Introduction
Ovarian cancer is a significant cause of death from gynecologic cancer. There are about 14,000 cases died of ovarian cancer each year in the United States. Overall survival of women with advanced ovarian cancer was rarely improved in recent years. Recurrence and chemotherapy resistance are the two most common causes of high mortality for ovarian cancer patients. The acquisition of cisplatin resistance usually occurs in about 25% of patients within six months of chemotherapy. The standard therapy for ovarian cancer is surgery combined with cisplatin chemotherapy at present.
In recent years, the roles of lncRNAs in various cancers were discussed in many reports. Emerging evidence revealed that lncRNA ANRIL was elevated in tumor tissues, and it served as oncogene roles in numerous malignancies.1–4 Besides, ANRIL regulated cancer cell proliferation, invasion, migration and apoptosis.3–5 Furthermore, the study has reported that ANRIL was upregulated in ovarian cancer.6 Qiu et al revealed that ANRIL regulated ovarian cancers carcinogenesis and might have diagnostic value in ovarian cancer.3 In addition, the resistance of ovarian cancer cells to cisplatin was affected by ANRIL.7 Additionally, ANRIL facilitated ovarian cancer cell proliferation and cell cycle progression.5 It is well documented that ANRIL interacted with miRNAs to modulate cancer progression, such as miR-186,6 miR-99a/miR-449a,8 miR-125a,9 miR-323.10 Thus, we speculated that ANRIL might regulate ovarian cancer progression via sponging miRNAs.
A study confirmed that ANRIL was upregulated while miR-324-5p was down-regulated in laryngeal squamous cell cancer.11 Of note, the effect of the ANRIL/miR-324-5p axis on ovarian cancer was unclear, which might be similar to that in laryngeal squamous cell cancer. According to the TargetScan prediction of RNA action sites, Ran was considered as a target of miR-324-5p. Interestingly, the highly expressed Ran also accelerated the development of ovarian cancer.12,13 However, the association between miR-324-5p and Ran was undefined. Hence, we aimed to investigate the role of the ANRIL/miR-324-5p/Ran axis in ovarian cancer progression. The malignant behaviors such as migration, invasion, cancer stem characteristics, and cell tumorigenicity were discussed in this study.
This study identified the regulatory mechanism of ANRIL/miR-324-5p/Ran axis on the malignant progression of ovarian cancer, making ANRIL a meaningful target for ovarian cancer therapy.
Materials and Methods
Cancer Samples
The ovarian cancer tissues and adjacent normal tissues were collected from 96 ovarian cancer patients who attended the Third Hospital of Jilin University. The harvested paired tissues were immediately frozen in liquid nitrogen and preserved at −80°C freezer. The research was permitted by the Ethical Committee of the Third Hospital of Jilin University. Each patient has written informed consent.
Cell Culture
Human normal ovarian surface epithelial cells (HOSEPiCs) and human ovarian cancer cells (cisplatin-sensitive strain SKOV3, cisplatin-resistant strain SKOV3/DDP and A2780) were acquired from American type culture collection (ATCC; Manassas, VA, USA). All cells were maintained in RPMI-1640 medium (Gibco) complemented with 10% FBS (Life Technologies, Grand Island, USA) and 1% penicillin and streptomycin at 37°C environment with 5% CO2.
Cell Transfection
The si-ANRIL and miR-324-5p mimics were obtained from Ribobio (Guangzhou, China). SKOV3 were inoculated into 6-well plates (1×104 cells/well) for 24 h. Then, 50 nM si-ANRIL or miR-324-5p mimics were transfected to SKOV3 using Lipofectamine® 3000 (Invitrogen, USA) following the manufacture’s protocols. The scrambled negative control of siRNAs (si-NC) and mimic control (miR-NC) were negative controls.
RNA Isolation and qRT-PCR
RNA isolation was conducted using TRIzol (Invitrogen, Carlsbad, USA) following the standard protocol. Reverse transcription was performed with the SuperScript Reverse Transcriptase Kit (Vazyme, Nanjing, China) and TaqMan™ MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific). Q-PCR assay was conducted by SuperScript III Platinum SYBR Green One-Step qPCR kit and mirVana™ qRT-PCR miRNA Detection Kit (Thermo Fisher Scientific). The primers sequences were: ANRIL: F, 5ʹ- GGAACCAAGCAGACCGAAGAC −3ʹ, R, 5ʹ- CCCCAACCCACAGGAACATAA −3ʹ; miR-324-5p: F, 5ʹ- CGCGGATCCGGGTGGATGTAAGGGATGAG-3ʹ, R, 5ʹ- CCGGAATTCTTGGGCTGATCCAGGAGAAG-3ʹ;14 Ran: F, 5ʹ-TGGCTTGCTAGGAAGCTCAT-3ʹ, R: 5ʹ- CACCGCTGACATCACAGGAC-3ʹ; β-actin: F, 5ʹ-GACCTCTATGCCAACACAGT-3ʹ, R, 5ʹ-AGTACTTGCGCTCAGGAGG-3ʹ.15 The relative gene expression was obtained using the 2−ΔΔCt method by normalizing to β-actin or U6.
Luciferase Activity Assay
Wild and mutant sequences of ANRIL and Ran containing predicted miR-324-5p binding sites were amplified and inserted into the pMIR vector (Promega) using PCR. Then, vectors and miR-324-5p mimics were co-transfected into SKOV3 cells using Lipofectamine 3000. Forty-eight hours later, the luciferase activities were measured by a dual-luciferase reporter system (Promega) based on the manufacturer’s protocols and analyzed by normalizing Renilla activities.
RNA Pull-Down Assay with Biotinylated miR-324-5p
SKOV3 cells were transfected with biotinylated wild miR-424-5p, mutant miR-424-5p, or negative control (NC). Cell lysates were harvested after 48 h post-transfection and incubated with Dynabeads M-280 Streptavidin (Invitrogen, USA) at 4°C overnight. After the washing step, the bound RNAs were extracted by TRIzol reagents, and the expression of ANRIL and RAN was determined by qRT-PCR.
Western Blot
Cell lysates were extracted using RIPA lysis buffer (Thermo Fisher Scientific). The same amount of protein samples (40 µg) were denatured at 100 °C for 5 min. Protein samples were subjected to SDS-PAGE and transferred to PVDF membranes. After blocked by 5% skim milk at 25°C for 1 h, the membrane was incubated with the corresponding primary antibody for 12 h at 4 °C. Next, the membrane was incubated with horseradish peroxidase-labeled secondary antibody at 25 °C for 1 h. Finally, the luminescent solution was added, and the exposure was taken in the gel imager. The gray value was quantified by Image J software. β-actin was the internal reference protein. The experiments were conducted at least three times.
Cell Viability Assay
SKOV3 cells were inoculated into 96-well plates (5×103 cells/well). Then, cells were incubated with 10 µL MTT (Sigma-Aldrich; Merck KGaA) for 4 h at 37 °C. After the culture medium was removed, 100 µL DMSO (Sigma-Aldrich; Merck KGaA) was added to each well. The absorbance was analyzed at 490 nm using a multiwell microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Wound Healing Assay
For wound healing assay, SKOV3 cells were cultured in a 6-well chamber. The confluent cell monolayer was scratched using a 10-µL pipette tip. The cell migration was observed under a DM2500 bright field microscope (LEICA, Wetzlar, Germany) after 24 h cultivation and analyzed using ImageJ software.
Migration and Transwell Invasion Assay
For migration assay, SKOV3 cells were inoculated in the upper chamber, and the medium plus 10% FBS was added to the lower chamber. Twenty-four hours later, the migrated cells were fixed with 95% ethanol, dyed with hematoxylin, and observed under a DM2500 bright-field microscope at 400 × magnification.
For invasion assay, SKOV3 cells were inoculated in the upper chamber, which was pre-coated with Matrigel, and the medium plus 10% FBS was added to the lower chamber. Twenty-four hours later, the invasion cells were fixed with 95% ethanol, dyed with hematoxylin, and observed under a DM2500 bright-field microscope at 400 × magnification.
Sphere Formation Assay
Each well of the 6-well culture dish were covered using 2 mL bottom agar mixture (DMEM, 10% (v/v) fetal calf serum, 0.6% (w/v) agar). After the bottom layer was solidified, a top agar-medium mixture containing 2 × 104 cells was added and incubated at 37 °C for 4 weeks, followed by crystal violet staining. The sphere (diameter ≥100 μm) numbers were calculated from 5 fields per well.
Flow Cytometry for Cancer Stem Cell Isolation
Cells were incubated with aldehyde dehydrogenase 1 (ALDH1; StemCell Technologies Inc., Vancouver, BC, Canada), CD117 (Miltenyi Biotech, Auburn, CA, USA), and Notch1 antibodies (BioLegend, San Diego, CA, USA) followed by separating the cancer stem cells or analyze their expression using fluorescence-activated cell sorting analysis (FACS).
Xenografts Tumor Establishment
Female BALB/c nude mice aged 4 weeks were bought from the Jilin University Animal Center (China). The recombinant lentiviruses and the negative control (NC) lentivirus were used to mediate animal gene expression. Twelve mice were divided into four groups, including the KD-NC group, KD-ANRIL group, OE-NC group, and OE-miR-324-5p group (n=3). All mice were subcutaneously injected with SKOV3 cells in their flanks and feed for 35 days. Another 12 mice were also divided into four groups, including the KD-NC group, KD-ANRIL group, OE-NC group, and OE-miR-324-5p group (n=3), and all mice were subcutaneously injected with A2780 cells in their flanks and feed for 35 days. Mice were maintained in standard conditions (25±2°C, 70% humidity, and 12-h light-dark periods) with a regular sterile chow diet and free to water. The experiments were conducted following the National Institutes of Health guide for the care and use of laboratory animals. The Animal Care and Use Committee of The Third Hospital of Jilin University permitted the study. The tumor volume was assessed every 7 days until 35 days. After the mice were sacrificed, the tumor was resected to determine gene expression.
Statistical Analysis
Data were described as mean ± standard deviation (SD) and analyzed using SPSS 19.0 software. The group differences were compared using the ANOVA test or student’s t-test. P < 0.05 was regarded as statistically significant.
Results
The Expression of ANRIL and miR-324-5p Were Abnormal in Ovarian Cancer
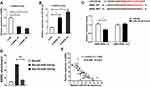
To explore whether ANRIL and miR-324-5p involve regulating ovarian cancer, we assessed the expression of ANRIL and miR-324-5p using qRT-PCR. As shown in Figure 1A, ANRIL was elevated in ovarian cancer tissues. Besides, the expression of ANRIL was associated with tumor size, FIGO stage, and pathological grade, while not associated with age and pathological subtype of ovarian cancer patients (Table 1). Furthermore, miR-324-5p was decreased in ovarian cancer tissues. The expression of miR-324-5p was associated with FIGO stage and pathological grade, while not associated with age, tumor size and pathological subtype (Table 2). Moreover, the expression of ANRIL and miR-324-5p in ovarian cancer cells were determined. Compared to the HOSEPiCs cells, the human ovarian cancer cells (cisplatin-sensitive strain SKOV3 and cisplatin-resistant strain SKOV3/DDP) presented increased ANRIL and decreased miR-324-5p (Figure 1B).
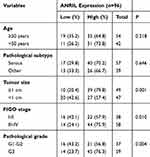 |
Table 1 Association of ANRIL Expression and Clinicopathological Variables in Ovarian Cancer Patients |
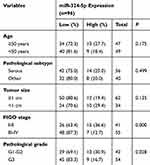 |
Table 2 Association of miR-324-5p Expression and Clinicopathological Variables in Ovarian Cancer Patients |
Targeting Relationship of ANRIL and miR-324-5p Was Found in Ovarian Cancer
To knockdown the ANRIL expression in SKOV3 cells, two siRNA were constructed (si-ANRIL R1: 5ʹ-GCAAGAAACATTGCTGCTAGC-3ʹ; si-ANRIL R2: 5ʹ-GCCCAATTATGCTGTGGTAAC-3ʹ). As a result, compared with si-NC, si-ANRIL-R1 and si-ANRIL-R2 resulted in an obvious reduction for the expression of ANRL (Figure 2A). Besides, knockdown of the ANRIL enhanced the expression of miR-324-5p (Figure 2B). The results indicated a negative regulation between miR-324-5p and ANRIL. In addition, the targeting relation between ANRL and miR-324-5p was demonstrated by the Luciferase reporter assay. miR-324-5p mimics considerably reduced the relative luciferase activity in cells transfected with ANRIL wild sequence while had no effects in cells transfected with ANRIL mutant sequence (Figure 2C). Furthermore, RNA pull-down assay results revealed that the enrichment of ANRIL by biotinylated miR-324-5p was higher than biotinylated mutant miR-324-5p (Figure 2D). Spearman correlation analysis revealed a negative correlation between the expression of ANRIL and miR-324-5p in ovarian cancer (Figure 2E).
miR-324-5p Negatively Modulates the Ran Expression in Ovarian Cancer Cells
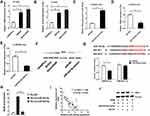
To explore the downstream protein of miR-324-5p, we searched the TargetScan website and found Ran, which is associated with ovarian cancer development, was a potential target of miR-324-5p. In SKOV3 and SKOV3/DDP cells, the mRNA and protein levels of Ran were upregulated compared to HOSEPiCs (Figure 3A and B). miR-324-5p mimics were introduced to analyze the regulatory mechanism. miR-324-5p mimics remarkably upregulated the expression of miR-324-5p (Figure 3C). Besides, miR-324-5p mimics declined the mRNA and protein levels of Ran (Figure 3C–F). Furthermore, the protein level of Ran was upregulated in SKOV3 and SKOV3/DDP cells (Figure 3F). To further confirm the relationship between Ran and miR-324-5p, the luciferase reporter assay was performed. Results indicated the target binding relations between Ran and miR-324-5p (Figure 3G). Besides, RNA pull-down assay showed that the enrichment of Ran by biotinylated miR-324-5p was higher than biotinylated mutant miR-324-5p (Figure 3H). Spearman correlation analysis found a negative correlation between the expression of miR-324-5p and Ran mRNA level in ovarian cancer patients (Figure 3I). Furthermore, Western blot results indicated that the protein level of Ran was promoted by overexpressed ANRIL, which was reversed by miR-324-5p mimics (Figure 3J).
Decreased ANRIL and Increased miR-324-5p Inhibits Ovarian Cancer Cell Growth, Migration, and EMT in vitro
To investigate the role of ANRIL and miR-324-5p in ovarian cancer malignant behaviors, SKOV3 cells were transfected with si-ANRIL or miR-324-5p mimics. The knockout efficiency of 3 siRNAs against ANRIL was determined using the qRT-PCR. Results showed that the knockout efficiency of si-ANRIL 2 was better than the other two siRNAs (Figure 4A). Therefore, si-ANRIL 2 was used for subsequent experiments. MTT assay result showed that both si-ANRIL and miR-324-5p mimics inhibited cell viability of SKOV3 cells (Figure 4B). As shown in Figure 4C–E, both si-ANRIL and miR-324-5p mimics inhibited the cell migration and invasion ability of SKOV3 cells. Furthermore, both si-ANRIL and miR-324-5p mimics inhibited the expression of EMT-related proteins, including N-cadherin, Vimentin, Snail, and cyclinD1. Reversely, si-ANRIL and miR-324-5p mimics upregulated the level of E-cadherin (Figure 4F). Moreover, si-ANRIL and miR-324-5p mimics increased chemosensitivity of ovarian cancer cells to cisplatin (Figure 4G) and doxorubicin (Figure 4H). The results indicated that the downregulation of ANRIL and overexpression of miR-324-5p inhibited tumor growth, migration, and EMT.
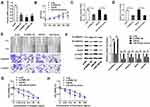
Decreased ANRIL and Increased miR-324-5p Alleviates Stem-Like Properties in vitro
As shown in Figure 5A and B, sphere formation assay demonstrated that si-ANRIL and miR-324-5p mimics significantly inhibited the sphere formation. In addition, si-ANRIL and miR-324-5p mimics inhibited cancer stem cell numbers (Figure 5C) and suppressed the mRNA levels of cancer stem related genes (Figure 5D). Moreover, the effects of ANRIL and miR-324-5p on tumorigenicity were determined by xenograft assay using SKOV3 and A2780 cells. Results showed that stable silencing of ANRIL and overexpression miR-324-5p inhibited ovarian cancer cell tumorigenicity in vivo (Figure 5E and F). The mRNA levels of cancer stem related genes were suppressed by decreased ANRIL and overexpressed miR-324-5p in tumor tissues established using SKOV3 and A2780 cells (Figure 5G).
Ran Participates in the ANRIL-Mediated Modulation of Stem-Like Properties in Ovarian Cancer Cells
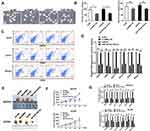
To investigate the role of Ran in the regulation of ANRIL on stem-like properties, Ran was overexpressed in SKOV3 cells. As a result, the mRNA and protein levels of Ran and RhoA were inhibited by si-ANRIL, while were reversed by Ran overexpression (Figure 6A and B). MTT results indicated that si-ANRIL inhibited cell viability, and Ran overexpression increased the phenomenon (Figure 6C). In addition, si-ANRIL reduced the cancer cell spheres and size, while Ran abrogated the effect (Figure 6D and E). To confirm the stem-like properties, the mRNA levels of stem-related genes were determined. The mRNA expression levels of CD24/44/117/133, ALDH, and Norch1 were inhibited by si-ANRIL but reversed by overexpression of Ran (Figure 6F). Finally, si-ANRIL increased chemosensitivity of ovarian cancer cells to cisplatin and doxorubicin, while abrogated by overexpression of Ran (Figure 6G). Thus, Ran mediated the regulation of ANRIL on cell viability, stem-like properties, and drug resistance of ovarian cancer cells.
Discussion
Ovarian cancer is one of the most lethal gynecological malignant owing to the late discovery and high recurrence rate.16 Resistance to conventional cisplatin therapy is attributed to populations of cancer stem cells that possess stem-like characteristics and high tumorigenicity.17 It was well documented that the biology of ovarian cancer stem cells and identified various markers and signaling pathways for their self-renewal ability in recent years. Targeting cancer stem cells is the most prospective strategy to overcome ovarian cancer resistance and reduce mortality.18 Thus, it is meaningful to explore the underlying mechanism of malignant behaviors, including EMT, invasion, stem-like properties, and drug resistance in ovarian cancer cells.
LncRNAs have been broadly studied in cancer biology, and many researches indicated that lncRNAs might be potential biomarkers in cancers.19 LncRNA ANRIL served as oncogene roles in some cancers, including pancreatic cancer,20 bladder cancer21 and breast cancer,22 and so on. ANRIL was related to cell proliferation, cell cycle, poor prognosis, and the sensitivity to cisplatin in ovarian cancer cells.3,5,7,23 A lot of literature showed that overexpressed ANRIL promoted cell invasion ability, EMT process, and stem-like properties in cancers.24,25 In this study, ANRIL was elevated in ovarian cancer, and knockdown of ANRIL significantly suppressed cell invasion, stem-like properties, and drug resistance to cisplatin or doxorubicin, which were consistent with the previous studies.
Furthermore, ANRIL interacted with a variety of miRNAs in several cancers. For instance, ANRIL functioned as miR-125a sponge in CAL27 cells,26 miR-199a sponge in Triple-negative breast cancer,27 and miR-186 sponge in cervical cancer.28 miR-324-5p was proved to exert tumor-suppressive functions.29 Decreased miR-324-5p was observed in laryngeal squamous cell cancer,11 lung cancer,30,31 breast cancer,32 gastric cancer,33 cervical cancer,34 bladder cancer,35 and colon cancer.36,37 miR-324-5p inhibited the Warburg effect and repressed tumorigenesis of ovarian cancer.38 We revealed that miR-324-5p was decreased in ovarian cancer. The relationship between miR-324-5p and ANRIL has been investigated in laryngeal squamous cell cancer, in which highly expressed ANRIL promoted overall survival, advanced clinical stage, and lymph node metastasis.11 Consistent with previous studies,11 our study revealed that the modulation of ANRIL on ovarian cancer was mediated by sponging miR-324-5p. Most of the aforementioned studies demonstrated that miR-324-5p suppressed cell growth and migration. Besides, miR-324-5p suppressed drug tolerance to cisplatin in non-small cell lung cancer.39 In this study, we further discussed the role of miR-324-5p in EMT and stem-like properties and drug resistance. MTT results suggested that the increased miR-324-5p induced by si-ANRIL inhibited the drug resistance. Cell invasion inhibition was also observed in various cancers by up-regulated miR-324-5p.40–42 Our results agree with the previous studies. Furthermore, we first revealed that miR-324-5p upregulation inhibited the stem-like properties in ovarian cancer.
To investigate the mechanism of miR-324-5p suppressing ovarian cancer cell malignant behaviors, we screened the target genes of miR-324-5p using prediction software. We primarily identified that Ran could be targeted by miR-324-5p, which was consistent with previous study.43 Small GTPase Ran was a carcinogenic protein. Several miRNAs, including miR-802, miR-2, miR-197, miR-203 could directly bind to Ran to modulate cancer cell behaviors in cancers,15,44–47 which indicated that Ran involved in the regulation of malignant behaviors in cancers. Until now, some articles have revealed the regulatory mechanism of Ran in ovarian cancer, including stem-like properties,48 cell invasion,49 cell survival,50 and prognosis,13 which further indicated the carcinogenic role of Ran in ovarian cancer. In this study, we reported that ran mediated the regulation of ANRIL on cell viability, stem-like properties, and drug resistance of ovarian cancer cells.
Taken together, the downregulation of ANRIL suppressed the cell proliferation, metastasis, drug resistance, and stem-like properties of ovarian cancer cells. In conclusion, this study supplemented the tumorigenic role of ANRIL in ovarian cancer. Excitingly, we revealed a new regulatory mechanism of ANRIL in ovarian cancer malignant behaviors, including excessive proliferation, invasion, metastasis, EMT, and chemotherapy resistance. LncRNA ANRIL involves the progression of ovarian cancer through miR-324-5p/Ran axis, making the axis meaning targets for ovarian cancer therapy.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Funding
There is no funding to report.
Disclosure
The authors declare no competing interests.
References
1. Huang MD, Chen WM, Qi FZ, et al. Long non-coding RNA ANRIL is upregulated in hepatocellular carcinoma and regulates cell apoptosis by epigenetic silencing of KLF2. J Hematol Oncol. 2015;8:50. doi:10.1186/s13045-015-0146-0
2. Naemura M, Murasaki C, Inoue Y, Okamoto H, Kotake Y. Long noncoding RNA ANRIL regulates proliferation of non-small cell lung cancer and cervical cancer cells. Anticancer Res. 2015;35(10):5377–5382.
3. Qiu JJ, Lin YY, Ding JX, Feng WW, Jin HY, Hua KQ. Long non-coding RNA ANRIL predicts poor prognosis and promotes invasion/metastasis in serous ovarian cancer. Int J Oncol. 2015;46(6):2497–2505. doi:10.3892/ijo.2015.2943
4. Zhu H, Li X, Song Y, Zhang P, Xiao Y, Xing Y. Long non-coding RNA ANRIL is up-regulated in bladder cancer and regulates bladder cancer cell proliferation and apoptosis through the intrinsic pathway. Biochem Biophys Res Commun. 2015;467(2):223–228. doi:10.1016/j.bbrc.2015.10.002
5. Qiu JJ, Wang Y, Liu YL, Zhang Y, Ding JX, Hua KQ. The long non-coding RNA ANRIL promotes proliferation and cell cycle progression and inhibits apoptosis and senescence in epithelial ovarian cancer. Oncotarget. 2016;7(22):32478–32492. doi:10.18632/oncotarget.8744
6. Zhang JJ, Wang DD, Du CX, Wang Y. Long noncoding RNA ANRIL promotes cervical cancer development by acting as a sponge of miR-186. Oncol Res. 2017;26(3):345–352. doi:10.3727/096504017x14953948675449
7. Miao JT, Gao JH, Chen YQ, Chen H, Meng HY, Lou G. LncRNA ANRIL affects the sensitivity of ovarian cancer to cisplatin via regulation of let-7a/HMGA2 axis. Biosci Rep. 2019;39(7). doi:10.1042/bsr20182101
8. Zhang EB, Kong R, Yin DD, et al. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5(8):2276–2292. doi:10.18632/oncotarget.1902
9. Hu X, Jiang H, Jiang X. Downregulation of lncRNA ANRIL inhibits proliferation, induces apoptosis, and enhances radiosensitivity in nasopharyngeal carcinoma cells through regulating miR-125a. Cancer Biol Ther. 2017;18(5):331–338. doi:10.1080/15384047.2017.1310348
10. Zhang H, Wang X, Chen X. Potential role of long non-coding RNA ANRIL in pediatric medulloblastoma through promotion on proliferation and migration by targeting miR-323. J Cell Biochem. 2017;118(12):4735–4744. doi:10.1002/jcb.26141
11. Liu F, Xiao Y, Ma L, Wang J. Regulating of cell cycle progression by the lncRNA CDKN2B-AS1/miR-324-5p/ROCK1 axis in laryngeal squamous cell cancer. Int J Biol Markers. 2020;35(1):47–56. doi:10.1177/1724600819898489
12. Zaoui K, Boudhraa Z, Khalifé P, Carmona E, Provencher D, Mes-Masson AM. Ran promotes membrane targeting and stabilization of RhoA to orchestrate ovarian cancer cell invasion. Nat Commun. 2019;10(1):2666. doi:10.1038/s41467-019-10570-w
13. Cáceres-Gorriti KY, Carmona E, Barrès V, et al. RAN nucleo-cytoplasmic transport and mitotic spindle assembly partners XPO7 and TPX2 are new prognostic biomarkers in serous epithelial ovarian cancer. PLoS One. 2014;9(3):e91000. doi:10.1371/journal.pone.0091000
14. Bamodu OA, Yang CK, Cheng WH, et al. 4-acetyl-antroquinonol B suppresses SOD2-enhanced cancer stem cell-like phenotypes and chemoresistance of colorectal cancer cells by inducing hsa-miR-324 re-expression. Cancers. 2018;10(8):269. DOI:10.3390/cancers10080269
15. Zhang F, Yang Z, Cao M, et al. MiR-203 suppresses tumor growth and invasion and down-regulates MiR-21 expression through repressing Ran in esophageal cancer. Cancer Lett. 2014;342(1):121–129. doi:10.1016/j.canlet.2013.08.037
16. Ghoneum A, Gonzalez D, Abdulfattah AY, Said N. Metabolic plasticity in ovarian cancer stem cells. Cancers. 2020;12(5):1267. doi:10.3390/cancers12051267
17. Belur Nagaraj A, Kovalenko O, Avelar R. Mitotic exit dysfunction through the deregulation of APC/C characterizes cisplatin-resistant state in epithelial ovarian cancer. Clin Cancer Res. 2018;24(18):4588–4601. doi:10.1158/1078-0432.Ccr-17-2885
18. Muñoz-Galván S, Carnero A. Targeting cancer stem cells to overcome therapy resistance in ovarian cancer. Cells. 2020;9(6):1402. doi:10.3390/cells9061402
19. Jiang W, Xia J, Xie S. Long non-coding RNAs as a determinant of cancer drug resistance: towards the overcoming of chemoresistance via modulation of lncRNAs. Drug Resist Updates. 2020;50:100683. doi:10.1016/j.drup.2020.100683
20. Chen S, Zhang J-Q, Chen J-Z. The over expression of long non-coding RNA ANRIL promotes epithelial-mesenchymal transition by activating the ATM-E2F1 signaling pathway in pancreatic cancer: an in vivo and in vitro study. Int J Biol Macromol. 2017;102:718–728. doi:10.1016/j.ijbiomac.2017.03.123
21. Nie F-Q, Sun M, Yang J-S. Long noncoding RNA ANRIL promotes non-small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther. 2015;14(1):268–277. doi:10.1158/1535-7163.Mct-14-0492
22. Mehta-Mujoo PM, Cunliffe HE, Hung NA, Slatter TL. ANRILLong non-coding RNA in the nucleus associates with periostin expression in breast cancer. Front Oncol. 2019;9:885. doi:10.3389/fonc.2019.00885
23. Zhang D, Ding L, Li Y, et al. Midkine derived from cancer-associated fibroblasts promotes cisplatin-resistance via up-regulation of the expression of lncRNA ANRIL in tumour cells. Sci Rep. 2017;7(1):16231. doi:10.1038/s41598-017-13431-y
24. Dong X, Jin Z, Chen Y. Knockdown of long non-coding RNA ANRIL inhibits proliferation, migration, and invasion but promotes apoptosis of human glioma cells by upregulation of miR-34a. J Cell Biochem. 2018;119(3):2708–2718. doi:10.1002/jcb.26437
25. Poi MJ, Li J, Sborov DW. Polymorphism in ANRIL is associated with relapse in patients with multiple myeloma after autologous stem cell transplant. Mol Carcinog. 2017;56(7):1722–1732. doi:10.1002/mc.22626
26. Chai L, Yuan Y, Chen C, Zhou J, Wu Y. The role of long non-coding RNA ANRIL in the carcinogenesis of oral cancer by targeting miR-125a. Biomed Pharmacother. 2018;103:38–45. doi:10.1016/j.biopha.2018.01.105
27. Xu S-T, Xu J-H, Zheng Z-R. Long non-coding RNA ANRIL promotes carcinogenesis via sponging miR-199a in triple-negative breast cancer. Biomed Pharmacother. 2017;96:14–21. doi:10.1016/j.biopha.2017.09.107
28. Zhang -J-J, Wang -D-D, Du C-X, Wang Y. Long noncoding RNA ANRIL promotes cervical cancer development by acting as a sponge of miR-186. Oncol Res. 2018;26(3):345–352. doi:10.3727/096504017x14953948675449
29. Kuo WT, Yu SY, Li SC, et al. MicroRNA-324 in human cancer: miR-324-5p and miR-324-3p have distinct biological functions in human cancer. Anticancer Res. 2016;36(10):5189–5196. doi:10.21873/anticanres.11089
30. Ba Z, Zhou Y, Yang Z, Xu J, Zhang X. miR-324-5p upregulation potentiates resistance to cisplatin by targeting FBXO11 signalling in non-small cell lung cancer cells. J Biochem. 2019;166(6):517–527. doi:10.1093/jb/mvz066
31. Lin MH, Chen YZ, Lee MY, et al. Comprehensive identification of microRNA arm selection preference in lung cancer: miR-324-5p and −3p serve oncogenic functions in lung cancer. Oncol Lett. 2018;15(6):9818–9826. doi:10.3892/ol.2018.8557
32. Song L, Liu D, Zhao Y, et al. Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-kappaB activation mediated by IL-4/miR-324-5p/CUEDC2 axis. Biochem Biophys Res Commun. 2015;464(3):705–710. doi:10.1016/j.bbrc.2015.07.004
33. Lin H, Zhou AJ, Zhang JY, Liu SF, Gu JX. MiR-324-5p reduces viability and induces apoptosis in gastric cancer cells through modulating TSPAN8. J Pharm Pharmacol. 2018;70(11):1513–1520. doi:10.1111/jphp.12995
34. Jiang H, Huang G, Zhao N, et al. Long non-coding RNA TPT1-AS1 promotes cell growth and metastasis in cervical cancer via acting AS a sponge for miR-324-5p. J Exp Clin Cancer Res. 2018;37(1):169. doi:10.1186/s13046-018-0846-8
35. Eissa S, Safwat M, Matboli M, Zaghloul A, El-Sawalhi M, Shaheen A. Measurement of urinary level of a specific competing endogenous RNA network (FOS and RCAN mRNA/miR-324-5p, miR-4738-3p,/lncRNA miR-497-HG) enables diagnosis of bladder cancer. Urol Oncol. 2019;37(4):292e19–292 e27. doi:10.1016/j.urolonc.2018.12.024
36. Chen Y, Wang SX, Mu R, et al. Dysregulation of the miR-324-5p-CUEDC2 axis leads to macrophage dysfunction and is associated with colon cancer. Cell Rep. 2014;7(6):1982–1993. doi:10.1016/j.celrep.2014.05.007
37. Gu C, Zhang M, Sun W, Dong C. Upregulation of miR-324-5p inhibits proliferation and invasion of colorectal cancer cells by targeting ELAVL1. Oncol Res. 2019;27(5):515–524. doi:10.3727/096504018X15166183598572
38. Zheng X, Zhou Y, Chen W, et al. Ginsenoside 20(S)-Rg3 prevents PKM2-targeting miR-324-5p from H19 sponging to antagonize the Warburg effect in ovarian cancer cells. Cell Physiol Biochem. 2018.
39. Ba Z, Zhou Y, Yang Z, Xu J, Zhang X. miR-324-5p upregulation potentiates resistance to cisplatin by targeting FBXO11 signalling in non-small cell lung cancer cells. J Biochem. 2019;166(6):517–527. doi:10.1093/jb/mvz066
40. Gu C, Zhang M, Sun W, Dong C. Upregulation of miR-324-5p inhibits proliferation and invasion of colorectal cancer cells by targeting ELAVL1. Oncol Res. 2019;27(5):515–524. doi:10.3727/096504018x15166183598572
41. Song L, Liu D, Zhao Y, et al.Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-κB activation mediated by IL-4/miR-324-5p/CUEDC2 axis. Biochem Biophys Res Commun. 2015;464(3):705–710. doi:10.1016/j.bbrc.2015.07.004
42. Cao L, Xie B, Yang X, et al. MiR-324-5p suppresses hepatocellular carcinoma cell invasion by counteracting ECM degradation through post-transcriptionally downregulating ETS1 and SP1. PLoS One. 2015;10(7):e0133074. doi:10.1371/journal.pone.0133074
43. Gu J, Gui S, Hu L, Kong L, Di M, Wang Y. Downregulated miRNA-324-5p aggravates neuronal injury induced by oxygen-glucose deprivation via modulating RAN. Exp Ther Med. 2020;19(1):658–664. doi:10.3892/etm.2019.8249
44. Feng H, Liu L, Xu L, Wang H, Hua Q, He P. MiR-802 suppresses colorectal cancer cell viability, migration and invasion by targeting RAN. Cancer Manag Res. 2020;12:2291–2300. doi:10.2147/cmar.S231709
45. Wang X, Li D, Sun L, et al.Regulation of the small GTPase Ran by miR-802 modulates proliferation and metastasis in colorectal cancer cells. Br J Cancer. 2020;122(11):1695–1706. doi:10.1038/s41416-020-0809-7
46. Yao L, Zhou Y, Sui Z. HBV-encoded miR-2 functions as an oncogene by downregulating TRIM35 but upregulating RAN in liver cancer cells. EBioMedicine. 2019;48:117–129. doi:10.1016/j.ebiom.2019.09.012
47. Tang WF, Huang RT, Chien KY, et al. Host microRNA miR-197 plays a negative regulatory role in the enterovirus 71 infectious cycle by targeting the RAN protein. J Virol. 2016;90(3):1424–1438. doi:10.1128/jvi.02143-15
48. Zhong Y, Cao L, Ma H, et al. Lin28A regulates stem-like properties of ovarian cancer cells by enriching RAN and HSBP1 mRNA and up-regulating its protein expression. Int J Biol Sci. 2020;16(11):1941–1953. doi:10.7150/ijbs.43504
49. Zaoui K, Boudhraa Z, Khalife P, Carmona E, Provencher D, Mes-Masson AM. Ran promotes membrane targeting and stabilization of RhoA to orchestrate ovarian cancer cell invasion. Nat Commun. 2019;10(1):2666. doi:10.1038/s41467-019-10570-w
50. Barres V, Ouellet V, Lafontaine J, Tonin PN, Provencher DM, Mes-Masson AM. An essential role for Ran GTPase in epithelial ovarian cancer cell survival. Mol Cancer. 2010;9:272. doi:10.1186/1476-4598-9-272
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.