Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Liver Stiffness Measurement is Useful in Predicting Type 2 Diabetes Mellitus Among Nonalcohol Fatty Liver Disease Patients
Authors Ding Y , Wang G, Deng Q, Yang M, Li J, Wang Z, Niu H, Xia S
Received 8 November 2023
Accepted for publication 11 January 2024
Published 22 January 2024 Volume 2024:17 Pages 295—304
DOI https://doi.org/10.2147/DMSO.S448626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Yuping Ding,1,2 Guiqiang Wang,3,4 Quanjun Deng,1,2 Mei Yang,1,2 Jinghua Li,5 Zuoyu Wang,1,2 Haiyan Niu,1,2 Shihai Xia1,2
1Department of Gastroenterology and Hepatology, Characteristic Medical Center of the Chinese People’s Armed Police Force, Tianjin, 300162, People’s Republic of China; 2Tianjin Key Laboratory of Hepatopancreatic Fibrosis and Molecular Diagnosis & Treatment, Tianjin, 300162, People’s Republic of China; 3Department of Infectious Disease, Center for Liver Disease, Peking University First Hospital, Beijing, People’s Republic of China; 4Department of Infectious Diseases, Peking University International Hospital, Beijing, People’s Republic of China; 5Department of Endocrinology and Hematology, Characteristic Medical Center of the Chinese People’s Armed Police Force, Tianjin, 300162, People’s Republic of China
Correspondence: Shihai Xia, Department of Gastroenterology and Hepatology, Characteristic Medical Center of the Chinese People’s Armed Police Force, 220, Chenglin Road, Dongli, Tianjin, 300162, People’s Republic of China, Email [email protected]
Background: Type 2 diabetes mellitus (T2DM) and non-alcoholic fatty liver disease (NAFLD) are closely related conditions.
Aim: This study investigated a group of individuals with NAFLD to evaluate if liver fibrosis, identified by FibroScan, correlated with T2DM.
Methods: 154 NAFLD patients obtained FibroScan, liver ultrasonography (US), and a thorough assessment of clinical implications and chemical biomarkers.
Results: In comparison to the NAFLD without T2DM group, the hemoglobin A1c(HBA1c)(mmol/mol%), homeostasis model of assessment for insulin resistance index (HOMA-IR), gamma-glutamyl transferase (GGT), fibrosis indices, and liver stiffness measurement (LSM) values were all considerably higher in the NAFLD with T2DM group. Patients with NAFLD and T2DM had considerably lower serum uric acid(SUA) levels than those with NAFLD alone.Those with severe fibrosis (79.3%, 23/29) in the NAFLD group showed a greater frequency of T2DM than those with mild fibrosis (45.6%, 21/46) or no fibrosis (27.85%, 22/79) (P=0.000). LSM value and elements of the metabolic syndrome (MetS) were independent risk factors for incident T2DM among NAFLD patients (OR=1.466, 95% CI [1.139-1.888], P=0.003; and OR=0.273, 95% CI [0.081-0.916], P=0.036).
Conclusion: FibroScan can identify significant fibrosis, which is independently linked to a higher prevalence of T2DM. As a result, it is crucial to make use of this technology to predict T2DM in NAFLD patients.
Keywords: liver stiffness measurement, NAFLD, T2DM, risk factors, early warning
Introduction
The main characteristic of non-alcoholic fatty liver disease (NAFLD) is excessive fat deposition in the liver. It has a close connection to metabolic syndrome (MetS), whose prevalence has increased as obesity has become more widespread. Type 2 diabetes mellitus (T2DM) and obesity are two metabolic risk factors that are associated with NAFLD, which is defined by 5% hepatocyte steatosis. The exclusion of other chronic liver disorders and excessive alcohol use (over 30 g/day for men and 20 g/day for women) are additional qualifiers.1 In 2020, a global panel of experts categorized fatty liver as metabolic dysfunction-associated fatty liver disease (MAFLD), changing it from a negative exclusion diagnosis.
NAFLD is the most common chronic liver disease worldwide, whereas non-alcoholic steatohepatitis (NASH) is the more severe form of NAFLD that can lead to liver fibrosis and cirrhosis. T2DM is a recognized risk factor for the development of NASH in NAFLD. Thus, the development of T2DM may potentially be influenced by NAFLD and fibrosis.2,3 It had been determined that patients with NAFLD and T2DM had an approximately 37% global prevalence of NASH and an approximately 17% global prevalence of advanced fibrosis, respectively.4 NAFLD increases the probability of becoming T2DM.5,6 The risk of acquiring T2DM at its early starting doubles in patients with NAFLD, despite the fact that NAFLD is present in more than 70% of patients with T2DM.2,7 The emergence of hyperglycemia and T2DM is the most clear consequence of NAFLD’s association with extrahepatic insulin since insulin resistance (IR) and NAFLD are connected.8 The risk of developing T2DM is higher in patients with NAFLD and extensive fibrosis.4 Several pharmacological strategies have been evaluated in the treatment of NASH and NAFLD utilizing already available medications such as antidiabetic and anti-obesity medicines.9 At different stages of clinical trials, a number of pharmacological treatments are presently being investigated, including FXR agonist (MET409), GLP-1 analogue (XW003), and GLP-1/glucagon agonist (DD01).10 Physicians’ interest in employing natural products as an alternative to conventional treatments for NAFLD has grown recently.11 Given its potential benefits, further investigation and study should be done on this topic.
Currently, liver biopsy is the gold standard for diagnosing and staging liver fibrosis. This invasive technical operation, however, limits its clinical utility. Recently, a trustworthy non-invasive approach for identifying hepatic steatosis and fibrosis in patients with various stages of NAFLD has emerged: transient elastography with FibroScan, which gives controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) parameters.12,13 It is rare to examine the association between liver stiffness value and diabetes among individuals with NAFLD, despite the fact that hemoglobin A1c (HbA1c), the fasting blood glucose level(FBG), is straightforward and immediately useful in the diagnosis of diabetes.We sought to examine if FibroScan can evaluate the relationship between fibrosis and the incidence of T2DM in a group of individuals with NAFLD.
Materials and Methods
Patients
This observational cross-sectional study included adult inpatients having a diagnosis of NAFLD by Fibro-Scan screened from Department of Gastroenterology and Hepatology, Characteristic Medical Center of the Chinese People’s Armed Police Force between January 2016 and January 2021. Patients with chronic liver diseases, such as steatosis-inducing drugs, viral B and C hepatitis, autoimmune hepatitis, Wilson’s disease, genetic hemochromatosis, or alpha 1-antitrypsin deficiency, as well as excessive alcohol consumption (defined as 30 g/day for men and 20 g/day for women) were also excluded from the study. There were 154 inpatients participants in total in this research (Figure 1).
 |
Figure 1 Flowchart of study population enrollment. |
Clinical and Laboratory Assessments
Clinical, anthropometric, and laboratory data were acquired throughout the FibroScan evaluation. Based on the diagnostic criteria (from clinical history, or according to ADA criteria) for T2DM, patients were classified as either having NAFLD without T2DM or having NAFLD with T2DM. Low-density lipoprotein(LDL) -cholesterol>3.37mmol/L, triglycerides(TG)>1.7 mmol/L, total cholesterol(TC)>5.2mmol/L, high-density lipoprotein (HDL)-cholesterol<1.55 mmol/L, or use of lipid-lowering drugs were the local laboratory cut-offs used to identify dyslipidemia. The blood pressure threshold for hypertension was 140/90 mmHg.14 Transaminase levels above 40 U/L for aspartate aminotransferase (AST) and 50 U/L for alanine aminotransferase(ALT), respectively, and gamma glutamyl transferase (GGT) readings above 60 U/L were considered abnormal. Smoking history and age at diagnosis were reported.
Liver Assessment
One professional physician evaluated the existence and severity of hepatic fibrosis and steatosis using the FibroScan 502. When a measurement failed, the expert physician used the M probe or the XL probe while being blinded to the subjects’ clinical data.15 The final values were obtained by the use of standardized procedures.16 The existence of hepatic steatosis was detected using CAP value of 248 obtained by the M probe and described in the literature (severe steatosis is defined as 280 dB/m).17 While the LSM value was used to assess the level of fibrosis (F0, ≤5.9 kPa), mild fibrosis (F1,6–6.9kPa; F2,7–9kPa); and severe fibrosis (F3,9.1–10.3kPa; F4,≥10.4kPa), respectively.18,19
Vascular Complications and T2DM Clinical Status
For all patients, information was collected on the development of macrovascular disorders like coronary artery disease, hypertension, the MetS, and T2DM whether or not they had microangiopathy.
Statistical Analysis
The chi-squared test or Fisher’s exact test was used to express categorical variables as absolute and relative frequencies (n,%) and continuous variables as means standard deviation (SD) or medians (range values). To compare the incidence of T2DM among the various NAFLD severity levels, chi-square segmentation was employed. For continuously distributed variables with normal and non-normal distributions, respectively, the unpaired Student’s t-test and Mann–Whitney U-test were applied. In order to investigate the risk factors connected to T2DM, multivariate logistic correlation analysis was employed. SPSS for Windows (version 18.0; Chicago, Illinois, USA) was used for data analysis and quality assurance.
Results
Demographic and Clinical Complications Characteristics
The participants’ mean age was 51.27±12.16 years, as indicated in Table 1. Among them, 59.7% patients (92/154) were male. A total of 71.0% patients (88/124) had MetS, 65.3% patients (96/147) had dyslipidemia and 38.5% patients (45/117) had hypertension. Coronary atherosclerotic heart disease (CHD) was present in 22.2% (26/117) of patients. Of these, 29.9% (35/117) were current smokers. In the NAFLD cohort, 66 patients (42.86%) had NAFLD and T2DM. The prevalence of MetS, Hypertension, CHD was higher in the T2DM group than in the group without T2DM (80.0% (48/60) vs 62.5% (40/64), P=0.032; 51.9% (28/54)vs 27%(17/63), P=0.006;33.3% (18/54) vs 12.7% (8/63), P=0.007). The age of NAFLD with T2DM patients was 55.71±10.62 years old which was older than that of NAFLD without T2DM patients (P=0.000). The Systolic blood pressures (SBP) of NAFLD with T2DM patients were 131.22±14.71 mmHg, higher than that in the group of NAFLD without T2DM patients (124.37±14.49 mmHg) (P=0.013)(Table 1).
 |
Table 1 Demographic Data of NAFLD Patients Without T2DM and NAFLD with T2DM Patients |
Chemical Characteristics and LSM Values or Fibrosis Indices
The homeostasis model of assessment for insulin resistance index (HOMA-IR) and HbA1c values between NAFLD patients with and without T2DM were significantly different (HOMA-IR: median 6.76 (2,26) vs 4.37 (2,12), P=0.019; HbA1c: 8.25±1.66% vs 5.65±1.97%, P=0.000). Similar results were seen at all GGT levels. P=0.017 (55 μmol/L (6,1385 μmol/L) vs 40.5 μmol/L (11,711 μmol/L). In comparison to NAFLD patients without T2DM, individuals with T2DM had reduced serum uric acid (SUA) levels (324.32±90.94 mol/L vs 382.69±98.84 mol/L, P=0.004). The LSM by FibroScan (10.37±10.26 vs 5.73±2.58, P=0.000), AST to platelet ratio index (APRI) (P=0.044), and Fibrosis-4 score (FIB-4) (P=0.001) were higher in the NAFLD with T2DM group than the NAFLD without T2DM individuals. There were no discernible differences in ALT and AST levels, TG, TC, LDL, and HDL levels between NAFLD patients with and without T2DM. Hepatic steatosis (CAP value) between the two groups did not differ significantly (Table 2).
 |
Table 2 Laboratory Variables of NAFLD Without and with T2DM in NAFLD Patients |
The Correlation Between the Degree of Fibrosis and the Frequency of T2DM in NAFLD Patients
Non fibrosis was seen in 79 (51.3%) of the NAFLD patients at enrollment (F0,≤LSM 5.9 kPa), mild fibrosis was present in 46 (29.9%) of the patients (F1,6–6.9kPa; F2,7–9kPa), and severe fibrosis was present in 29 instances (18.83%) of the patients (F3,9.1–10.3kPa; F4,≥10.4kPa) (Table 3). Greater LSM values were also associated with a significantly greater incidence of T2DM. Patients with severe fibrosis (23/29) exhibited a higher prevalence of T2DM compared to patients without fibrosis (22/79) (79.3% vs 27.8%, χ2=23.11, P<0.0001) and patients with mild fibrosis (21/46) (79.3% vs 45.7%, χ2=8.31, P<0.0001). In addition, we found that the frequency of T2DM events varied significantly between the groups with mild and non fibrosis NAFLD (45.7% vs 27.8%, χ2=4.084, P=0.043). (Figure 2).
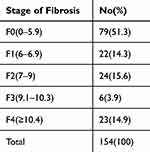 |
Table 3 Distribution of Patients According to the Stage of Liver Fibrosis |
 |
Figure 2 Fibrosis level and the rate of type 2 diabetes in NAFLD patients. |
Regression Analysis for Presence of T2DM in NAFLD
We evaluated the association between T2DM in NAFLD patients and each clinical consequence after controlling for recognized risk variables and new possible confounders. We discovered that the existence of T2DM in NAFLD patients was still substantially correlated with age, CHD, hypertension, MetS, SBP, LSM, GGT, and SUA levels (P<0.01). Also shown in Table 4 was an independent relationship between the prevalence of T2DM in NAFLD patients and LSM value (OR 1.466, 95% CI 1.4–1.888, P=0.003) and MetS (OR 0.273, 95% CI 0.081–0.916, P=0.036).
 |
Table 4 Multivariate Analysis of Factors Associated with Diabetes in NAFLD Patients |
Discussion
According to previous epidemiological reports,20 NAFLD is currently regarded as the most common cause of chronic liver disease in Western countries, with prevalence rates in the general population ranging from 13.5% in Africa to 31.8% in the Middle East, 29.62% in Asia, and 29.81% on the Chinese mainland.21,22 According to recent studies, NAFLD affects several organ systems, including the hepatic, renal, cardiovascular, and cerebrovascular systems.23 NAFLD has also been connected to a number of extra-hepatic tumors.24 Most recently, bladder cancer-one of the most deadly diseases for the elderly-has been found associated to NAFLD and insulin resistance(IR).25 In the most recent guidelines for patients with NAFLD, T2DM screening is frequently been recommended.1,26 The risk of developing T2DM in NAFLD patients was thus examined in our study utilizing clinical laboratory tests, FibroScan, and other techniques.
In terms of fundamental clinical characteristics, we discovered that individuals with NAFLD and T2DM had considerably higher incidence rates of the MetS, hypertension, and CHD. Additionally, they were older and had greater SBP than the participants. When metabolic markers are tested, NAFLD and T2DM patients had lower SUA levels and higher HOMA-IR. These results imply that metabolic variables may be important in explaining the association between liver fat content and T2DM prevalence. A key role for IR and visceral fat is played in the pathophysiology of CHD, T2DM, and NAFLD.27 Patients with NAFLD and T2DM had considerably higher GGT levels than those without T2DM. One of the important enzymes involved in the metabolism of glutathione and cysteine, GGT is strongly linked to central obesity,28 diabetes,29 and cardiovascular30 risk. These findings imply that in individuals with NAFLD, GGT may act as a critical diagnostic marker for T2DM. According to the findings, those with T2DM had greater levels of the fibrosis index, APRI, FIB-4, and LSM when it evolved to liver fibrosis. The incidence of T2DM in individuals with NAFLD also increased in a stepwise fashion with increasing NAFLD fibrosis severity, which is interesting. It was seemingly shown that liver fibrosis and MetS were independent risk factors for the development of NAFLD to T2DM.
In this study, the high rate of T2DM accompany with the high prevalence of increased liver stiffness in NAFLD patients, prompts physicians to consider IR and inflammation disorder as an important comorbidity that could impact on NAFLD and T2DM.27 Therefore, it is absolutely not possible to define on this basis a cause-effect relationship between measurement of liver stiffness and risk of T2DM.All we can conclude is that, while evaluating NAFLD, individuals with greater liver stiffness values require special attention to the identification of T2DM diagnostic markers and ongoing awareness about the potential onset of T2DM.Can NAFLD fibrosis serve as a trigger for type 2 diabetes?It is necessary to do more prospective studies that support our findings.
While, recent research has revealed that fatty liver fibrosis and T2DM are related.31 Mantovani et al found that the magnitude of the risk of incident T2DM mirrored the severity of NAFLD, especially the severity of liver fibrosis, which was consistent with our results32 T2DM and NASH both develop and progress as a result of insulin resistance. NASH was present in 37.3% of those with T2DM worldwide.4 Some worldwide professionals renamed NAFLD to MAFLD in 202033 due to the significant correlation between NAFLD and T2DM.
This recommends that patients with NAFLD, especially those who have clinical indications of MetS, should have liver fibrosis screenings. A clinical experiment with a significant sample size found that the severity of NAFLD may influence the probability of becoming T2DM in the future, and conversely, its resolution may lower that risk of development diabetes.34 Transient elastography (TE) is a simple, reproducible, and noninvasive tool used to measure liver steatosis by CAP value and measure liver fibrosis by LSM. Liver fibrosis may be the primary risk factor for T2DM. TE screening has important clinical significance as a NAFLD monitoring method, and LSM can be used to dynamically monitor the grade of fibrosis in relation to IR and the emergence of T2DM during follow-up.
It is important to recognize the limitations of this study. First, the research sample may be biased toward older participants due to its near 60% male composition and average age of 51 years. Additionally, the sample may not be sufficiently balanced or have a high clinical comorbidity status when participants who are eligible for admission to tertiary hospitals are included. Consequently, the individuals in the sample frequently have higher rates of T2DM than the general population.35 Second, The association between LSM values and T2DM-related biomarkers was examined in this study using a cross-sectional design. Additionally, the degree of liver fibrosis as assessed by FibroScan assessment was used to predict the occurrence of T2DM, and follow-up data on the dynamic changes of LSM value and T2DM are absent.Thirdly, the individuals were enrolled in a single study center, and the sample size was small. To verify our findings, multicenter controlled and randomized investigations are required. In addition, a large sample cohort with a well-balanced range of ages and clinical comorbidity status can be used to design this study in a longitudinal prospective way.
Abbreviations
NAFLD, Non-alcoholic fatty liver disease; NASH, Non-alcoholic steatohepatitis; T2DM, Type 2 diabetes mellitus; BMI Body mass index; DBP, Diastolic blood pressure; SBP, Systolic blood pressure; ALT, Alanine aminotransferase; AST, Aspartate Transaminase; TBL, total bilirubin; GGT, Gamma glutamyl transpeptidase; HDL, High-density lipoprotein cholesterol; TC, Total cholesterol; LDL, Low-density lipoprotein cholesterol; TG, Triglyceride; HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model of assessment for insulin resistance index; APRI, AST to platelet ratio index; FIB-4, Fibrosis-4 score; CAP, Controlled attenuation parameter; LSM, Liver stiffness measurement.
Ethics Declarations
All patients provided written informed permission to participate in the trial, which followed to the 1975 Helsinki Declaration and was approved by the Characteristic Medical Center of the Chinese People’s Armed Police Force ethics committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Ph. D. Startup Fund from Logistics University of PAP (WHB201517); The Independent Innovation Science Fund Project (2024-2025) in Characteristic Medical Center of the Chinese People’s Armed Police Force.
Disclosure
The authors report no conflicts of interest in this work.
References
1. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi:10.1016/j.jhep.2015.11.004
2. Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612. doi:10.1038/s41575-021-00448-y
3. Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–296. doi:10.1016/S2213-8587(22)00003-1
4. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi:10.1016/j.jhep.2019.06.021
5. Khneizer G, Rizvi S, Gawrieh S. Non-alcoholic Fatty Liver Disease and Diabetes Mellitus. Adv Exp Med Biol. 2021;1307:417–440.
6. Lallukka S, Yki-Järvinen H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract Res Clin Endocrinol Metab. 2016;30(3):385–395. doi:10.1016/j.beem.2016.06.006
7. Lee CH, Lui DT, Lam KS. Non-alcoholic fatty liver disease and type 2 diabetes: an update. J Diabetes Investig. 2022;13(6):930–940. doi:10.1111/jdi.13756
8. Rinaldi L, Pafundi PC, Galiero R, et al. Mechanisms of Non-Alcoholic Fatty Liver Disease in the Metabolic Syndrome. A Narrative Review. Antioxidants (Basel). 2021;10(2):270. doi:10.3390/antiox10020270
9. Ding Y, Deng Q, Yang M, Niu H, Wang Z, Xia S. Clinical Classification of Obesity and Implications for Metabolic Dysfunction-Associated Fatty Liver Disease and Treatment. Diabetes Metab Syndr Obes. 2023;16:3303–3329. doi:10.2147/DMSO.S43125
10. Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. 2022;126:154925. doi:10.1016/j.metabol.2021.154925
11. Tarantino G, Balsano C, Santini SJ, et al. It Is High Time Physicians Thought of Natural Products for Alleviating NAFLD. Is There Sufficient Evidence to Use Them? Int J Mol Sci. 2021;22(24):13424. doi:10.3390/ijms222413424
12. Sporea I, Mare R, Popescu A, et al. Screening for Liver Fibrosis and Steatosis in a Large Cohort of Patients with Type 2 Diabetes Using Vibration Controlled Transient Elastography and Controlled Attenuation Parameter in a Single-Center Real-Life Experience. J Clin Med. 2020;9(4):1032. doi:10.3390/jcm9041032
13. Lombardi R, Airaghi L, Targher G, et al. Liver fibrosis by FibroScan® independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020;40(2):347–354. doi:10.1111/liv.14274
14. Katsimardou A, Imprialos K, Stavropoulos K, Sachinidis A, Doumas M, Athyros V. Hypertension in Metabolic Syndrome: novel Insights. Curr Hypertens Rev. 2020;16(1):12–18. doi:10.2174/1573402115666190415161813
15. Oeda S, Takahashi H, Imajo K, et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: a multicenter prospective study. J Gastroenterol. 2020;55(4):428–440. doi:10.1007/s00535-019-01635-0
16. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology. 2017;152(6):1544–1577. doi:10.1053/j.gastro.2017.03.016
17. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. doi:10.1016/j.jhep.2016.12.022
18. Fallatah H, Akbar H, Fallatah A . Fibroscan Compared to FIB-4, APRI, and AST/ALT Ratio for Assessment of Liver Fibrosis in Saudi Patients With Nonalcoholic Fatty Liver Disease. Hepat Mon. 2016;16(7):e38346.
19. Ding Y, Wang Z, Niu H, Deng Q, Wang Y, Xia S. FIB-4 is closer to FibroScan screen results to detecting advanced liver fibrosis and maybe facilitates NAFLD warning. Medicine (Baltimore). 2023;102(34):e34957. doi:10.1097/MD.0000000000034957
20. Kasper P, Martin A, Lang S, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110(7):921–937. doi:10.1007/s00392-020-01709-7
21. Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: implications for Liver Transplantation. Transplantation. 2019;103(1):22–27. doi:10.1097/TP.0000000000002484
22. Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi:10.1016/S2468-1253(19)30039-1
23. Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6(7):578–588. doi:10.1016/S2468-1253(21)00020-0
24. Sanna C, Rosso C, Marietti M, Bugianesi E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int J Mol Sci. 2016;17(5):
25. Tarantino G, Crocetto F, Di Vito C, et al. Association of NAFLD and Insulin Resistance with Non Metastatic Bladder Cancer Patients: a Cross-Sectional Retrospective Study. J Clin Med. 2021;10(2):346. doi:10.3390/jcm10020346
26. Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28(5):528–562. doi:10.1016/j.eprac.2022.03.010
27. Caturano A, Acierno C, Nevola R, et al. Non-Alcoholic FattyLiver Disease: from Pathogenesis to Clinical Impact. Processes. 2021;9:135. doi:10.3390/pr9010135
28. Neuman MG, Malnick S, Chertin L. Gamma glutamyl transferase - an underestimated marker for cardiovascular disease and the metabolic syndrome. J Pharm Pharm Sci. 2020;23(1):65–74. doi:10.18433/jpps30923
29. Hua S, Qi Q, Kizer JR, et al. Association of liver enzymes with incident diabetes in US Hispanic/Latino adults. Diabet Med. 2021;38(8):e14522. doi:10.1111/dme.14522
30. Martínez-Quintana E, Pardo-Maiza J, Déniz-Alvarado B, Riaño-Ruiz M, González-Martín JM, Rodríguez-González F. Gamma-glutamyl transferase and cardiovascular events in patients with congenital heart disease. Eur J Clin Invest. 2022;52(4):e13720. doi:10.1111/eci.13720
31. Drożdż K, Nabrdalik K, Hajzler W, Kwiendacz H, Gumprecht J, Lip GYH. Metabolic-Associated Fatty Liver Disease (MAFLD), Diabetes, and Cardiovascular Disease: associations with Fructose Metabolism and Gut Microbiota. Nutrients. 2021;14(1):103. doi:10.3390/nu14010103
32. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70(5):962–969. doi:10.1136/gutjnl-2020-322572
33. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
34. Cho HJ, Hwang S, Park JI, et al. Improvement of Nonalcoholic Fatty Liver Disease Reduces the Risk of Type 2 Diabetes Mellitus. Gut Liver. 2019;13(4):440–449. doi:10.5009/gnl18382
35. Schlesinger S, Neuenschwander M, Barbaresko J, et al. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65(2):275–285. doi:10.1007/s00125-021-05592-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
