Back to Journals » Clinical Epidemiology » Volume 9
Liver-related morbidity and mortality in patients with chronic hepatitis C and cirrhosis with and without sustained virologic response
Authors Hallager S, Ladelund S, Christensen PB, Kjær M , Thorup Roege B, Grønbæk KE , Belard E, Barfod TS, Madsen LG , Gerstoft J, Tarp B , Krarup HB , Weis N
Received 10 January 2017
Accepted for publication 5 July 2017
Published 24 October 2017 Volume 2017:9 Pages 501—516
DOI https://doi.org/10.2147/CLEP.S132072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Henrik Sørensen
Sofie Hallager,1 Steen Ladelund,2 Peer Brehm Christensen,3 Mette Kjær,4,5 Birgit Thorup Roege,6 Karin Elmegaard Grønbæk,7 Erika Belard,8 Toke S Barfod,9 Lone Galmstrup Madsen,10 Jan Gerstoft,11 Britta Tarp,12 Henrik Bygum Krarup,13 Nina Weis,1,5
1Department of Infectious Diseases, Copenhagen University Hospital, Hvidovre, 2Clinical Research Center, Copenhagen University Hospital, Hvidovre, 3Department of Infectious Diseases and Clinical Institute, Odense University Hospital, University of Southern Denmark, Odense, 4Department of Hepatology, Copenhagen University Hospital, Rigshospitalet, 5Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, 6Department of Internal Medicine, Kolding Hospital, Kolding, 7Department of Gastroenterology, Copenhagen University Hospital, Hvidovre, 8Department of Gastroenterology, Copenhagen University Hospital, Herlev, 9Department of Internal Medicine, Zealand University Hospital, Roskilde, 10Department of Gastroenterology, Zealand University Hospital, Køge, 11Department of Infectious Diseases, Copenhagen University Hospital, Rigshospitalet, 12Diagnostic Centre, University Research Clinic for Innovative Patient Pathways, Silkeborg Regional Hospital, Silkeborg, 13Section of Molecular Diagnostics, Clinical Biochemistry and Department of Medical Gastroenterology, Aalborg University Hospital, Aalborg, Denmark
Background: Chronic hepatitis C (CHC) causes liver cirrhosis in 5%–20% of patients, leading to increased morbidity and mortality. This study aimed to estimate liver-related morbidity and mortality among patients with CHC and cirrhosis in Denmark with and without antiviral treatment and sustained virologic response (SVR). Furthermore we aimed to estimate the rate of hepatocellular carcinoma (HCC) and decompensation associated with certain prognostic factors.
Materials and methods: Patients with CHC and cirrhosis registered in the Danish Database for Hepatitis B and C were eligible. Cirrhosis was based on liver biopsy, transient elastography, and clinical cirrhosis. Data were extracted from nationwide registries. The study period was from 2002 until 2013.
Results: Of 1,038 patients included, 716 (69%) were male and the median age was 52 years. Median follow-up was 3.8 years, 360 patients died, and 233 of 519 treated patients achieved SVR. Alcohol overuse and hepatitis C virus genotype 3 were associated with an increased incidence rate (IR) of HCC, whereas diabetes and alcohol overuse were associated with increased IRs of decompensation. Achieving SVR reduced all-cause mortality (adjusted mortality rate ratio 0.68 [95% CI 0.43–1.09]) and liver-related mortality (mortality rate ratio 0.6 [95% CI 0.36–1]), as well as liver-related morbidity with adjusted IR ratios of 0.37 (95% CI 0.22–0.62) for HCC and 0.31 (95% CI 0.17–0.57) for decompensation. The IRs of HCC and decompensation remained elevated in patients with alcohol overuse after SVR.
Conclusion: Alcohol overuse, hepatitis C genotype 3, and diabetes were associated with liver-related morbidity in patients with CHC and cirrhosis. SVR markedly reduced liver-related morbidity and mortality; however, special attention to patients with alcohol overuse should continue after SVR.
Keywords: chronic hepatitis C, cirrhosis, liver-related morbidity, cohort study, sustained virologic response
Plain-language summary
Chronic infection with hepatitis C virus (HCV) can cause liver cirrhosis in 5%–20% of patients. With cirrhosis, the risk of liver cancer and complications increases dramatically; however, successful antiviral treatment of chronic hepatitis C (CHC) reduces this risk. Little is known about the risk of liver cancer, complications, and death in patients with CHC and cirrhosis in Denmark. Therefore, this study aimed to estimate the incidence of liver cancer, liver complications, and liver-related death and how these were affected by successful antiviral treatment in patients with CHC and cirrhosis in Denmark. We performed an observational cohort study of nationwide data, and found that among patients with CHC and cirrhosis, HCV genotype 3 and alcohol overuse were associated with development of liver cancer, whereas diabetes mellitus was associated with development of liver complications. Curing CHC significantly reduced the rate of liver cancer and complications, as well as the rate of death. There is an urgent need to cure patients with CHC and cirrhosis, especially those with HCV genotype 3, and special attention needs to be given to patients with diabetes mellitus or alcohol overuse.
Introduction
Chronic infection with hepatitis C virus (HCV) is a disease of global importance with a large burden of morbidity and mortality.1–6 For 5%–20% of chronic hepatitis C (CHC) patients, the disease will progress to liver cirrhosis7 associated with complications, such as liver decompensation, hepatocellular carcinoma (HCC), and increased mortality.8 The commonest causes of death among patients with CHC and cirrhosis are liver-related, particularly HCC.8,9 Several prognostic factors, such as diabetes mellitus, male sex, alcohol abuse, and HCV genotype 3, are thought to be associated with the development of HCC, decompensation, and mortality.10–14
Absence of HCV RNA 24 weeks after end of treatment (EOT24) is considered a cure for CHC, and is termed sustained virologic response (SVR).15 Patients with CHC and cirrhosis have previously been difficult to cure; however, achieving a cure for CHC has been shown to be associated with decreased morbidity and mortality.10,12 With the introduction of new, very effective antiviral drugs that cure CHC in nearly all treated patients,16 large cohorts of patients with CHC and cirrhosis who fail treatment in comparison with patients with SVR will be difficult to assemble.
Moreover, our knowledge of liver-related morbidity and mortality and how SVR affects these among patients with CHC and cirrhosis on a nationwide scale is limited. Most previous studies of HCC, decompensation, and liver-related mortality in CHC patients with cirrhosis have been single- or few-center studies, and included fairly selected cohorts from tertiary centers.5,8,10,12,13
The aims of this nationwide cohort study were to estimate liver-related morbidity and mortality among patients with CHC and cirrhosis in Denmark with and without antiviral treatment, and to estimate liver-related morbidity and mortality associated with SVR in a relatively large cohort of treated CHC patients with cirrhosis. Furthermore, we aimed to estimate the rates of HCC and decompensation associated with certain prognostic factors.
Materials and methods
This observational cohort study used prospectively collected data. The Danish health-care system is publicly funded and provides health-care services free of charge to the individual. Patients were identified in the Danish Database for Hepatitis B and C (DANHEP). DANHEP is a nationwide database with ongoing enrolment established January 1, 2002.17 It contains demographic, clinical, liver-biopsy, laboratory, and treatment data, and transient-elastography measurements on patients seen with CHC and/or CHB in any of the specialized outpatient clinics responsible for CHC patients in Denmark after January 1, 2002. Cross-linkage between nationwide registries is possible due to the unique 10-digit personal identification number18 given to all residents in Denmark and registered in the Danish civil registration system, along with date of birth, sex, vital and migration status, and address. All hospital-admission dates and diagnoses are registered in the Danish national patient registry (NPR),19 histopathologic diagnoses are registered in the national Danish pathology database (Patobank),20 causes of death are registered in the Danish registry of causes of death,21 and cancer diagnoses are registered in the Danish cancer registry.22 Further information about the Danish registry of causes of death is provided in the Supplementary material, and the remaining registries are described in more detail elsewhere.23
Patient cohort
Eligible patients were registered in DANHEP and fulfilled the following criteria: a positive HCV RNA test, cirrhosis and enrolment in DANHEP before December 31, 2013, ≥18 years of age, and a valid personal identification number and address in Denmark. All ICD and systematized nomenclature of medicine (SNOMED)24 codes used in the definitions of inclusion and exclusion criteria, outcomes, and covariates are provided in the Supplementary material. To avoid reverse causation and inclusion of prevalent cases of decompensation and HCC, the first 6 months of observation were excluded. Baseline was thus defined as 6 months (183 days) after the first date of a diagnosis of cirrhosis and enrolment in DANHEP, whichever occurred last, and could not precede January 1, 2002. Cirrhosis was defined as: liver biopsy (Metavir fibrosis score of F4),25 transient elastography (FibroScan; Echosens, Paris, France) median elasticity ≥17 kPa26,27 (10 or more valid measurements and an interquartile range ≤30% of median elasticity),28 or clinical cirrhosis (cirrhosis registered in NPR, ascites, hepatic encephalopathy, esophageal varices, esophageal variceal hemorrhage, spontaneous bacterial peritonitis). Patients were excluded if they had CHB, HIV infection, autoimmune hepatitis, or hemochromatosis at baseline.
Definition of outcomes and covariates
The two primary outcomes in the separate analyses of liver-related morbidity were HCC and decompensation. We defined HCC as a diagnosis registered in Patobank, the Danish registry of causes of death, the Danish cancer registry, or the NPR. Decompensated liver cirrhosis was defined as a diagnosis registered in the NPR, the Danish registry of causes of death, and/or DANHEP of ascites, esophageal variceal hemorrhage, hepatic encephalopathy, or spontaneous bacterial peritonitis.
In mortality analyses, the primary outcome was liver-related and all-cause mortality. All-cause mortality was defined as death due to any cause with a date of death registered in the Danish civil registration system, which was updated throughout the study period. Liver-related mortality was based on the underlying cause of death registered on the death certificate and divided into liver-related and liver-unrelated. Because the Danish registry of causes of death was only updated through December 2012 at the time of data extraction, analyses of liver-related mortality were limited to before December 31, 2012.
Antiviral treatment was registered in DANHEP and defined as reception of at least one dose of antiviral therapy. Based on information registered in DANHEP, successful treatment was defined as the absence of HCV RNA (SVR) at EOT24. Only patients who were alive and had negative HCV RNA samples at ≥EOT24 were categorized as having SVR. Non-SVR was defined as a treatment with known treatment response that did not result in SVR. Reinfection after SVR was defined as at least one positive HCV RNA after a period with undetectable HCV RNA after the EOT24 date.
Comorbidity was assessed by a time-updated, cumulative Charlson comorbidity index (CCI)29 score calculated based on ICD codes assigned to hospital contacts registered in the NPR (ICD codes modified by Quan et al30 are provided in the Supplementary material). Originally, the CCI score was developed to predict short-term, in-hospital mortality, but has subsequently been shown to predict long-term mortality.31 Liver disease, HCC, and HIV diagnoses were excluded from the CCI score. Orthotopic liver transplantation (LTx) was defined as an LTx procedure registered in the NPR. Diabetes mellitus was based on ICD codes registered in the NPR. Intravenous drug use (IDU; ever) was defined as having an IDU-related diagnosis registered in the NPR prior to the end of follow-up, self-reported IDU, or IDU as route of HCV transmission registered in DANHEP. Alcohol overuse was defined as any of the following prior to the end of follow-up: alcohol abuse-related diagnosis in NPR, a liver biopsy with a histopathologic diagnosis of alcoholic hepatitis or alcoholic cirrhosis, or self-reported daily alcohol consumption of >36 g alcohol for men and >24 g alcohol for women32 registered in DANHEP. Psychiatric disease was defined as a diagnosis of psychiatric disease registered in the NPR.
Statistical analysis
Patient characteristics were compared between patient groups with Mann–Whitney U test, c2 test, or c2 test with Monte Carlo simulations using 10,000 samples. All rates and rate ratios were calculated using Poisson regression, where time at risk and number of events in different strata were calculated using the stratify macro.33
Patients were followed from baseline until the primary outcome, exit for other reasons, or end of the study period, whichever occurred first. The study ended on December 31, 2013 except for liver-related mortality, where the last day of observation was December 31, 2012. When estimating rates in addition to reaching the end of the study period, infection with HIV, CHB, autoimmune hepatitis, or hemochromatosis led to censoring. Patients with prevalent cases of the primary outcome or LTx at baseline were excluded from morbidity analyses. A diagnosis of HCC before decompensation when decompensation was the primary outcome did not lead to the end of follow-up and vice versa. Cumulative-incidence functions of liver-related morbidity in all patients were calculated with death, coinfection with HIV or HBV, autoimmune hepatitis, hemochromatosis, and LTx as competing risks. Incidence rate ratios (IRRs) of HCC and decompensation were calculated to estimate the rates associated with predefined prognostic factors (sex, diabetes, alcohol overuse, and HCV genotype). In all Poisson regression analyses, CCI score (0, 1, 2, 3, 4, 5, ≥6), LTx, diabetes, psychiatric disease, HCC, and decompensation were introduced as time-dependent categorical covariates, whereas sex, alcohol overuse, IDU, and genotype (1, 2, 3, 4–6, multiple genotypes) were introduced as baseline categorical covariates. Age (<40, 40–44,45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, 85–89, and ≥90 years) was used as a continuous, time-dependent covariate after testing for log linearity or as a categorical variable. In cases of insufficient events, appropriate covariate strata were combined to ensure events in all strata.
In estimates of rates of HCC, decompensation and mortality when comparing patients with SVR vs non-SVR time at risk were calculated from the first EOT24 date with known treatment response or baseline, whichever occurred last, until the primary outcome or end of follow-up. In addition to the reasons mentioned previously, patients were censored at reinfection. SVR was introduced as a time-dependent covariate, and the clock-reset approach was applied. Cumulative-incidence functions in SVR, non-SVR, and untreated patients treated death, LTx, SVR, treatment, coinfection with HIV or HBV, and other liver diseases as competing events. Untreated patients were followed from baseline until initiation of event, treatment, or end of follow-up. All statistical tests were two-sided with a=0.05. Data handling and statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA), except for cumulative-incidence functions and figures, where R (version 3.2.2)34 was used.
Ethics
This study was approved by the Danish Data Protection Agency (2013-41-2323) in accordance with Danish law.
Results
Data were extracted from nationwide registries on 5,779 patients with positive HCV RNA. Of these, 1,306 patients had cirrhosis, and after the exclusion of 268 patients, 1,038 patients were eventually included (inclusion and exclusion of patients can be seen in Figure S1). One patient (0.1%) was lost to follow-up with unknown address and vital status in the Danish civil registration system. Baseline characteristics of all patients, untreated and treated, are shown in Table 1. Before the end of follow-up, 537 (52%) patients had initiated antiviral treatment, of whom 519 patients reached an EOT24 date at least once. The majority of treatment courses with a known treatment outcome consisted of a backbone of IFN (with or without Ribavirin).15 Only 45 (7%) patients were treated with polyethylene glycolated IFN combined with Ribavirin and a direct-acting antiviral (DAA) drug. Two patients were treated with Ribavirin monotherapy and failed to achieve SVR. Overall, 233 patients achieved SVR (45%), 276 patients achieved non-SVR (53%) at least once, and 10 patients had unknown treatment response (2%). A total of 27 patients achieved SVR24 after initially failing therapy. Three patients were reinfected and did not achieve a second SVR24. Characteristics of patients at their first EOT24 are listed in Table S1.
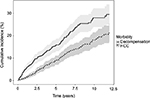
Patients were followed for a median of 3.8 (range 0.01–12) and 4,909 years total. After the exclusion of patients with prevalent cases of HCC and/or LTx at baseline, among 989 patients at risk of HCC, 115 patients were diagnosed with HCC during follow-up. Similarly among 811 patients at risk of decompensation, 153 decompensated during follow-up. Of 1,038 patients included, 360 eventually died during follow-up. Of 299 deaths prior to December 31, 2012, 186 (62%) died of liver-related causes. The cumulative-incidence functions of liver-related morbidity are shown in Figure 1. Liver-related morbidity IRs and mortality rates (MRs) for all patients and for SVR, non-SVR, and untreated patients are shown in Table 2. Adjusted liver-related morbidity IRRs are shown in Table 3 for all patients. In adjusted analyses, alcohol overuse was associated with increased rates of both HCC and decompensation among patients at risk, and compared to patients with genotype 1 or 2, genotype 3 was associated with an increased rate of HCC. In contrast, male sex and diabetes were not significantly associated with HCC. Genotype was not associated with decompensation, but diabetes was associated with an IRR of 1.65 for decompensation after adjusting for confounding.
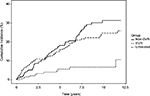
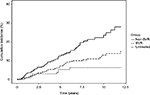
Median and total follow-up years were 4.8 (range 0.04–12) and 1,197 after SVR and 3.8 (range 0.04–12) and 1,444 after non-SVR. Cumulative-incidence functions of liver-related morbidity for SVR, non-SVR, and untreated patients are shown in Figures 2 and 3. Of the 226 patients who achieved SVR and were at risk of HCC, 10 developed HCC maximum 6.1 years after SVR24. In contrast, of 289 patients at risk after non-SVR, 47 were diagnosed with HCC. After non-SVR, 56 of 266 patients at risk decompensated, whereas only 11 of 211 patients decompensated after SVR. One patient with SVR and 11 with non-SVR received LTx. Adjusted liver-related morbidity IRRs and MRRs for SVR vs non-SVR and untreated patients are presented in Table 4. Cumulative incidences for 1-, 5-, and 10-year liver-related morbidity with death and LTx as competing risks are presented in Table S2. Achieving SVR was associated with a reduced rate of HCC, even after adjusting for sex, age, alcohol overuse, genotype, and diabetes. The rate of decompensation was also reduced substantially in the presence of SVR, and of the 11 patients who decompensated after SVR, seven had a history of alcohol overuse. Adjusted all-cause and liver-related MRs were reduced by 34% and 40% with SVR, respectively, when taking confounding into account.
Discussion
This nationwide cohort study based on prospectively collected data adds to our knowledge of liver-related morbidity and mortality among CHC patients with cirrhosis in a real-life cohort. We found major differences between untreated and treated patients with regard to baseline characteristics associated with mortality. A history of alcohol overuse and genotype 3 were associated with increased rates of HCC, whereas diabetes increased the rate of decompensation, as did alcohol overuse. Achieving SVR was associated with markedly reduced rates of liver-related morbidity and mortality after adjustment for confounding. However, a history of alcohol overuse was associated with diagnoses of HCC and decompensation after SVR.
Patients who did not initiate treatment differed from treated patients with respect to several baseline characteristics, such as comorbidity, decompensated cirrhosis, HCC, and a history of alcohol overuse or IDU. These differences were reflected in the high all-cause mortality among untreated patients, even compared to patients with non-SVR24. These differences are probably primarily a result of the selection of healthier patients for antiviral treatment, in part due to contraindications to polyethylene glycol–IFN-based therapy15 and other possible factors, such as continuous links to care.
We found IRs of HCC ranging from 0.85 after SVR to 3.53 after non-SVR per 100 person-years (PYs). Some studies have reported an increased rate of HCC associated with HCV genotype 3 independently of alcohol overuse, obesity, and diabetes.12,35 The confirmed increased rate of HCC associated with genotype 3 observed in our adjusted analyses did not take SVR into account. However, given the fact that both genotypes 2 and 3 were associated with higher chances of SVR36 in the era of IFN and Ribavirin compared with genotype 1, we find it unlikely that differences in SVR rates among patients with different genotypes should explain the observed increased rate of HCC associated with genotype 3. We found no statistically significant association between sex or diabetes and HCC, as has been described elsewhere,10,11,13,37,38 whereas diabetes did increase the rate of decompensation. Previously, it has been suggested that diabetes and genotype 3 are associated with fibrosis progression39–41 and complications in cirrhotic patients.14 The complex carcinogenic biological mechanisms involved in the development of HCC in the presence of diabetes and HCV genotype 3 are not fully understood. However, steatosis is thought to play a key role,42 and diabetes and HCV show signs of a synergistic effect in the formation of HCC.13,43 Apart from being associated with obesity and diabetes, steatosis is also generally accepted to be associated with HCV genotype 3,44 which may partly explain the increased risk of HCC. Nonetheless, the absence of information on steatosis and steatohepatitis in this study precludes any further conclusions on this matter. Alcohol overuse is a well-known risk factor,38 and was also strongly associated with HCC in this study. We did not observe an association between male sex and HCC, as demonstrated elsewhere,8,10,12,13,45 and we have no good explanation for this (crude rate male vs female 1.18 [95% CI 0.83–1.68] vs adjusted rate 1.21 [95% CI 0.86–1.69] per 100 PYs). While it was unclear which covariates were included in the final multivariate Cox regression models performed in previously published studies,8,10,12,13,45 we find it unlikely that the lack of an association in our study is the result of confounding. It may simply be due to chance. Death would be considered a competing event with HCC, and the MR was higher among men than women (adjusted MRR 1.39 [95% CI 1.07–1.8]); however, a rate is independent of competing events. Similarly, we found no association between diabetes and HCC, which has been demonstrated consistently,11–13,38 though mainly after SVR. In our study, diabetes was based entirely on the NPR. Misclassification in cases of diabetes not registered in the hospital registry may have occurred, which would bias the results toward the null. The positive predictive value of a diagnosis of diabetes in the NPR has been demonstrated to be good.46,47 However, the prevalence of diabetes of 12% at baseline in our cohort was at the lower end compared to the 12%–24% in other cohorts,11–13,48 which could suggest that we have underestimated the true diabetes prevalence. Alternatively, since other studies have mainly been performed in single or few tertiary centers, it may also be that the association between diabetes and HCC demonstrated by some could be the result of selection bias. Because diabetes was introduced as a time-dependent variable in our regression analyses, one could argue that this could lead to reverse causation of diabetes and HCC, if diabetes was more likely to be diagnosed in patients with yet-undiagnosed HCC. However, this would lead to a stronger association between diabetes and HCC, which was not the case in our study. Furthermore, the median time from diabetes to HCC was 4.6 years and 3.9 years from diabetes to decompensation, which makes reverse causation unlikely. Interestingly, in our cohort diabetes was associated with HCC in an unadjusted analysis and when adjusting for everything but age (data not shown). Patients with diabetes were also slightly older than patients without diabetes, and the rate of HCC increased significantly with age. Therefore, at least in this study, an association between diabetes and HCC was confounded by age. The HCC IR of 0.85/100 PYs after SVR observed in our cohort is consistent with the HCC IRs of 0.55–1.39/100 PYs described by others after SVR.10,12,38,49,50 The lowest HCC IR after SVR was reported in a study by van der Meer et al (0.55/100 [95% CI 0.14–0.96] PYs),12 which had a long median follow-up (8.4 years), but included 46% patients with advanced fibrosis, slightly younger patients (median age 48 years), and only patients with compensated cirrhosis. The highest HCC IR estimate reported of 1.39/100 PYs was found in a large cohort consisting of 95% males with a relatively high prevalence of diabetes and alcohol abuse,38 all risk factors previously described to be associated with HCC.10,12,13,38 Our results on the continuously elevated rate of HCC after non-SVR are well within the estimates reported in other cohorts of 1.44–5.85/100 PYs.10,12,49–51 We were able to confirm the reduced rate of HCC in cirrhotic CHC patients associated with SVR reported previously.10,12,49,50 These results underscore the benefits associated with a cure for CHC in cirrhotic patients. Despite small differences in the definition of liver complications applied here and elsewhere, we also found a markedly reduced rate of decompensation associated with SVR, in line with previous results.10,12,49,50 It is noteworthy that the annualized risk of HCC of 1.08% during the first 5 years after SVR found in our cohort is below the threshold of cost-effectiveness of 1.5%/year for HCC surveillance.52,53 However, the cutoff was estimated in the 1990s based on patients with multiple etiologies and exclusively cirrhosis Child–Pugh A. This was during in an era when CHC was difficult to cure, with presumably shorter life expectancies. At this point, the European Association for the Study of the Liver recommends continued HCC surveillance after SVR in cirrhotic patients.16 Further studies are needed to determine whether surveillance for HCC is cost-effective after SVR, and if so, for how long after SVR surveillance should be recommended.
The fact that adjusted all-cause and liver-related MRRs associated with SVR did not reach statistical significance in this study may have been due to underpowering when adjusting for confounding. We are confident that SVR was truly associated with reduced mortality in this real-life cohort, as has been demonstrated previously.23 With highly effective DAAs, even patients with advanced liver disease and comorbidities can undergo treatment and achieve very high SVR rates.16 Therefore, it will be of interest to follow CHC patients cured with IFN-free DAA regimens to study the long-term effects of eradicating HCV in patients with advanced liver disease, comorbidity, and/or substance abuse that would have precluded them from IFN and Ribavirin-based therapy.
The unique tradition for nationwide health registries and opportunity to cross-link between registries ensured complete follow-up in this cohort study of prospectively collected data. Unfortunately, only a fraction of the total number of patients with CHC in Denmark are aware of their CHC-status and attend specialized care,54 which limits the generalizability of our morbidity-rate and MR estimates, due to potential selection bias. For example, if only the sickest patients attend specialized care with symptoms of and complications due to CHC, we could have overestimated the rates of liver-related morbidity and mortality associated with CHC.
Reporting cancer diagnoses to the Danish cancer registry by physicians is mandatory, and since hospitals in Denmark are reimbursed based on admissions and procedures registered in the NPR, the number of HCC cases diagnosed in hospitals not registered in the cancer registry, Patobank, or NPR is likely to be low. A recent study from Sweden suggests that relying on a single registry could lead to substantial underestimation of primary liver cancers, possibly due to the noninvasive nature of HCC diagnosis.55 In our study, we extracted information from four different registries, which confers validity to our results. However, it is possible that we underestimated the number of HCC cases due to unrecognized HCC in deceased patients in the absence of diagnostic imaging or autopsy. Only two patients were diagnosed with HCC exclusively in the registry of causes of death. With regard to decompensation, it is likely that the registration of this complication is less rigorous than HCC, since we had to rely on only two registries, and thus it is possible that we underestimated the occurrence of decompensation.
Other limitations include the fact that we did not have information about smoking status, valid HCV RNA concentrations, body-mass index, nonalcoholic fatty-liver disease, nonalcoholic steatohepatitis, CHC disease duration, Child–Pugh or MELD (model for end-stage liver disease) score, systematic information about cirrhosis regression, quantification and duration of alcohol intake, or information about current alcohol overuse or IDU. These are all important potential confounding factors.
In conclusion, we found high rates of liver-related morbidity and mortality among patients with CHC and cirrhosis, in particular among untreated patients. Genotype 3 and alcohol overuse were associated with increased rates of HCC, and diabetes and alcohol overuse increased the rate of decompensation. Successful eradication of HCV was associated with substantially reduced rates of liver-related mortality, HCC, and liver decompensation when adjusting for confounding. There continues to be an urgent need to cure patients with CHC and cirrhosis before the development of complications, particularly in those with genotype 3. Special attention should be given to patients with a history of alcohol overuse in whom an elevated rate of HCC and decompensation remain after SVR.
Acknowledgments
SH received financial support from the Bonén Foundation. NW received financial support from the Danish Innovation Foundation, The Liver Score Project (ID 139-2012-3). None of the funding sources was involved in the design of the study, data collection or analysis, or writing of the final manuscript. We would like to acknowledge the DANHEP group and all patients in DANHEP and their families. The abstract of this paper was presented at the Liver Meeting held by the American Association for the Study of Liver Diseases (AASLD), November 11–15, 2016, Boston, MA, as an oral presentation with interim findings. The presentation’s abstract was published in the AASLD abstract book (abstract 176: http://onlinelibrary.wiley.com/doi/10.1002/hep.28796/epdf).
Author contributions
NW, PBC, SH, and HBK contributed to the concept and design of this study. SH managed and sorted data and performed statistical analyses in collaboration with statistician SL, who also contributed to the design of the study. SH wrote the manuscript in collaboration with NW. All authors contributed toward data collection and analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
SH has served as an unpaid speaker at an event sponsored by MSD. PBC has received unrestricted grants from Gilead, AbbVie, Roche, and Schering and served as an advisory board member for Roche. MK has served as a speaker for AbbVie and received travel grants from BMS, AbbVie, and Bayer. BTR has served as advisory board member for BMS and received travel grants from BMS. EB has received travel grants from Norgine. TSB has served as a speaker and advisory board member for AbbVie and received travel grants from Gilead, AbbVie, and BMS. LGM has served as an advisory board member for BMS and AbbVie and served as a speaker for Medivir and BMS. JG has received grants, participated in advisory boards, or served as speaker for AbbVie, ViiV, Gilead, BMS, MSD, and Medivir. BT has received grants from Gilead and served as an advisory board member for AbbVie. HBK has received travel grants from Gilead, Medivir, MSD, and BMS. NW has received lecture honoraria from AbbVie, BMS, Gilead, Janssen, and MSD; has served as an advisory board member for AbbVie, BMS, Gilead, Medivir, and MSD; and has worked as a clinical Investigator for AbbVie, BMS, and MSD. The other authors report no conflicts of interest in this work.
References
Hanafiah KM, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. | ||
El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273.e1. | ||
Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362(9401):2095–2100. | ||
Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21 Suppl 1:34–59. | ||
Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–472. | ||
Omland LH, Krarup H, Jepsen P, et al. Mortality in patients with chronic and cleared hepatitis C viral infection: a nationwide cohort study. J Hepatol. 2010;53(1):36–42. | ||
Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–S46. | ||
Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43(6):1303–1310. | ||
Benvegnu L. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53(5):744–749. | ||
Aleman S, Rahbin N, Weiland O, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis. 2013;57(2):230–236. | ||
Hedenstierna M, Nangarhari A, Weiland O, Aleman S. Diabetes and cirrhosis are risk factors for hepatocellular carcinoma after successful treatment of chronic hepatitis C. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63(6):723–729. | ||
van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. | ||
Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47(6):1856–1862. | ||
Elkrief L, Chouinard P, Bendersky N, et al. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60(3):823–831. | ||
Christensen PB, Clausen MR, Krarup H, Laursen AL, Schlichting P, Weis N. Treatment for hepatitis B virus (HBV) and hepatitis C virus (HCV) infection: Danish national guidelines 2011. Dan Med J. 2012;59(6):C4465. | ||
European Association for Study of Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63(1):199–236. | ||
Hansen N, Obel N, Christensen PB, et al. Predictors of antiviral treatment initiation in hepatitis C virus-infected patients: a Danish cohort study. J Viral Hepat. 2009;16(9):659–665. | ||
Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–25. | ||
Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish national hospital register: a valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–268. | ||
Patobank [website on the Internet]. 2017. Available from: http://www.patobank.dk. Accessed November 9, 2015. | ||
Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39(7 Suppl):26–29. | ||
Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39(7 Suppl):42–45. | ||
Hallager S, Christensen PB, Ladelund S, et al. Mortality in patients with chronic hepatitis C and cirrhosis compared to the general population: a Danish cohort study. J Infect Dis. 2017;215(2):192–201. | ||
Côté RA, Robboy S. Progress in medical information management: systematized nomenclature of medicine (SNOMED). JAMA. 1980;243(8):756–762. | ||
Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C: the METAVIR cooperative study group. Hepatology. 1996;24(2):289–293. | ||
Degos F, Perez P, Roche B, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53(6):1013–1021. | ||
Christiansen KM, Mössner BK, Hansen JF, Jarnbjer EF, Pedersen C, Christensen PB. Liver stiffness measurement among patients with chronic hepatitis B and C: results from a 5-year prospective study. PloS One. 2014;9(11):e111912. | ||
Boursier J, Zarski JP, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57(3):1182–1191. | ||
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. | ||
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005:1130–1139. | ||
Ganna A, Ingelsson E. 5 Year mortality predictors in 498,103 UK Biobank participants: a prospective population-based study. Lancet. 2015;386(9993):533–540. | ||
Danish Health Authority. Anbefalinger om alkohol. 2010. Available from: https://sundhedsstyrelsen.dk/da/sundhed-og-livsstil/alkohol/anbefalinger. Accessed February 25, 2016. | ||
Rostgaard K. Methods for stratification of person-time and events: a prerequisite for Poisson regression and SIR estimation. Epidemiol Perspect Innov. 2008;5:7. | ||
R Foundation. The R project for statistical computing. 2017. Available from: https://www.r-project.org. Accessed January 21, 2016. | ||
Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. veterans with HCV. Hepatology. 2014;60(1):98–105. | ||
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55(2):245–264. | ||
van der Meer AJ, Feld JJ, Hofer H, et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66(3):485–493. | ||
El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virologic response in veterans with HCV-infection: HCC after SVR. 2016 Available from: https://dissem.in/p/80365084/risk-of-hepatocellular-carcinoma-after-sustained-virologic-response-in-veterans-with-hcv-infection-hcc-after-svr. Accessed August 31, 2017 | ||
Ratziu V, Munteanu M, Charlotte F, Bonyhay L, Poynard T. Fibrogenic impact of high serum glucose in chronic hepatitis C. J Hepatol. 2003;39(6):1049–1055. | ||
Bochud PY, Cai T, Overbeck K, et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009;51(4):655–666. | ||
McMahon BJ, Bruden D, Townshend-Bulson L, et al. Infection with hepatitis C virus genotype 3 is an independent risk factor for end-stage liver disease, hepatocellular carcinoma, and liver-related death. Clin Gastroenterol Hepatol. 2017;15(3):431–437.e2. | ||
Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56(6):1384–1391. | ||
Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54(4):533–539. | ||
Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: why does it really matter? Gut. 2006;55(1):123–130. | ||
Degos F, Christidis C, Ganne-Carrie N, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47(1):131–136. | ||
Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish national registry of patients. BMC Med Res Methodol. 2011;11:83. | ||
Nielsen GL, Sørensen HT, Pedersen AB, Sabroe S. Analyses of data quality in registries concerning diabetes mellitus: a comparison between a population based hospital discharge and an insulin prescription registry. J Med Syst. 1996;20(1):1–10. | ||
Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136(1):138–148. | ||
Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-α is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45(3):579–587. | ||
Cardoso AC, Moucari R, Figueiredo-Mendes C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52(5):652–657. | ||
Dienstag JL, Ghany MG, Morgan TR, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54(2):396–405. | ||
European Association for Study of Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. | ||
Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101(4):422–434. | ||
Christensen PB, Hay G, Jepsen P, et al. Hepatitis C prevalence in Denmark: an estimate based on multiple national registers. BMC Infect Dis. 2012;12:1. | ||
Törner A, Stokkeland K, Svensson A, et al. The underreporting of hepatocellular carcinoma to the cancer register and a log-linear model to estimate a more correct incidence. Hepatology. 2017;65(3):885–892. |
Supplementary materials
Data sources
Danish civil registration system, Danish Database for Hepatitis B and C (DANHEP), Danish national patient registry (NPR), national Danish pathology database (Patobank), and Danish cancer registry provided in supplementary material published previously.1
The Danish register of causes of death (DAR) is a nationwide registry that contains information on nearly every death in Denmark since 1943. On each death certificate, a physician has registered one or more diagnoses considered to be the cause(s) of death, and coded with appropriate ICD-10 codes during this study period.2
Definition of covariates
Lists of ICD-8, ICD-10, and SNOMED codes used in the definition of inclusion and exclusion criteria, covariates, and causes of death are supplied in Tables S1–S5.
  | Table S2 ICD codes used in definition of Charlson comorbidity index score Notes: aAIDS-related diagnoses were not relevant, since patients and controls with HIV/AIDS were excluded. Liver disease-related diagnoses and hepatocellular carcinoma were considered to be part of the causal path between cirrhosis and death, and thus were not included. All ICD-10 codes are according to Quan et al.3 Abbreviation: ICD-10, 10th revision of the International Statistical Classification of Diseases and Related Health Problems. |
  | Table S3 Causes of death Abbreviation: ICD-10, 10th revision of the International Statistical Classification of Diseases and Related Health Problems. |
References
Hallager S, Christensen PB, Ladelund S, et al. Mortality in patients with chronic hepatitis C and cirrhosis compared to the general population: a Danish cohort study. J Infect Dis. 2017;215(2):192–201. | ||
Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39(7 Suppl):26–29. | ||
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.