Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Liver Injury and Its Impact on Prognosis in Patients with HBV-Related Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitors Plus Immune Checkpoint Inhibitors
Authors Shen J, Wang X, Yang G, Li L, Fu J, Xu W , Zhang Q, Pan X
Received 30 July 2023
Accepted for publication 13 January 2024
Published 24 January 2024 Volume 2024:11 Pages 207—217
DOI https://doi.org/10.2147/JHC.S431191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Jiaming Shen,1,2,* Xia Wang,2,* Guangde Yang,2,* Li Li,2 Juanjuan Fu,2 Wei Xu,3 Qingqiao Zhang,3 Xiucheng Pan2
1Department of Gastroenterology, People’s Hospital of Jingjiang, Taizhou, People’s Republic of China; 2Department of Infectious Disease, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, People’s Republic of China; 3Department of Interventional Radiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiucheng Pan, Tel +8618052268129, Email [email protected]
Purpose: Recently, the triple therapy of transarterial chemoembolization (TACE) combined with tyrosine kinase inhibitors (TKIs) plus immune checkpoint inhibitors (ICIs) has become a new treatment option for advanced or unresectable hepatocellular carcinoma (HCC) patients. We aimed to explore the liver injury and its effect on overall survival (OS) in patients treated with this combination therapy.
Patients and Methods: Patients with HBV-related HCC who were treated with TACE-TKIs-ICIs from January 2020 to December 2021 were enrolled. Liver injury and survival time were the main endpoints of the study. Logistic regression analysis was used to analyze the factors associated with liver injury. Cox regression and Kaplan–Meier analysis were used to determine prognostic factors for OS.
Results: As of March 2022, 52 of the 119 enrolled patients developed any grade hepatotoxicity: 15 cases with grade 1, 19 cases with grade 2, 16 cases with grade 3 and 2 cases with grade 4. Our analysis indicated that lack of antiviral prevention was a risk factor for liver injury (OR = 0.149; 95% CI: 0.050– 0.442; P = 0.001). The findings suggested that liver injury events (HR = 1.912; 95% CI: 1.031– 3.546; P = 0.040) was associated with patient death. The median OS of patients without liver injury, grade 1– 2 and grade 3– 4 liver injury were undefined, 13.7 months and 11.1 months, respectively (log-rank P = 0.034).
Conclusion: Liver injury adverse events are common in HBV-related HCC patients treated with TACE-TKIs-ICIs. Patients who developed liver injury had a poor prognosis. For HBV-related HCC patients, effective prophylactic antiviral therapy and regular liver function testing are required before and during this triple therapy.
Keywords: HBV-related HCC, TACE, TKI, ICI, liver injury, survival time
Graphical Abstract:

Introduction
Primary liver cancer is one of the most common cancers in the world, of which hepatocellular carcinoma (HCC) accounts for the vast majority.1 HBV infection is a major risk factor for HCC, especially in most Asian countries and regions.2 Despite improvements in monitoring and treatment of hepatitis B, many HCC patients are firstly diagnosed with unresectable or advanced stage, which is not suitable for early radical treatment and has a poor prognosis. Transarterial chemoembolization (TACE) is commonly recommended by various guidelines for local treatment of intermediate-stage or unresectable HCC.3–5 However, repeated TACE treatment may damage liver function, and embolism-induced hypoxia can lead to expression of vascular endothelial growth factor and promote tumor recurrence and metastasis.6 Tyrosine kinase inhibitors (TKIs), such as sorafenib, lenvatinib and apatinib, can reduce tumor angiogenesis by inhibiting vascular endothelial growth factor receptor.7 In this setting, TACE combined with TKI has synergistic anticancer effect and improves the prognosis of patients with advanced HCC.6,8,9 The combination of TKI and ICI such as “atezolizumab-bevacizumab” regimen has been recommended as the first-line therapy for patients with unresectable or advanced HCC because of its remarkable efficacy.10,11 Since TACE can cause tumor tissue necrosis and activate tumor-specific immune responses,12 the triple therapy of TACE combined with TKIs plus ICIs has become a new treatment option for patients with advanced or unresectable HCC in recent years.
However, previous studies have found that TACE alone, single TKI agent or immunotherapy may lead to hepatic adverse events.13–15 A systematic review reported that the incidence of elevated liver enzymes after TACE therapy was 52% in HCC patients.15 Griffiths et al16 summarized that the incidence of hepatotoxicity under ICIs or TKIs treatment in advanced HCC patients was 28% and 21%, respectively. In LAUNCH prospective trial, the incidence of liver injury in advanced HCC patients treated with TACE and lenvatinib was significantly higher than that in the lenvatinib monotherapy group, and the dose of lenvatinib was reduced due to serious hepatic adverse events.9 Similarly, Dai et al17 noted a 13.8% incidence of liver injury in patients with unresectable HCC treated with sintilimab alone or in combination. Severe hepatotoxicity can lead to interruption or cessation of anticancer treatment, even liver failure and death of patients. Therefore, it is necessary to pay attention to liver injury in HCC patients under anti-tumor therapy.
At present, there are few reports on liver injury under the treatment of TACE combined with TKIs plus ICIs. Therefore, the main purpose of this study is to investigate the incidence of liver injury and its influencing factors, and to further determine the impact of liver injury on the survival of HCC patients treated with TACE-TKIs-ICIs therapy.
Materials and Methods
Patient Selection and Study Design
The present research was a real-world retrospective study, which was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University, and was exempted from written informed consent (Ethics number: xyfy2022-KL085-01). Data of patients diagnosed with HCC and treated with TACE-TKIs-ICIs at our institution between 1 January 2020 to 31 December 2021 were retrospectively analyzed. Sintilimab (Innovent Biologics), camrelizumab (Hengrui Medicine) or tislelizumab (BeiGene) at a fixed dose of 200 mg were given for every 3 weeks. Sorafenib (400 mg twice daily, Bayer), lenvatinib (8 to 12 mg/day, Eisai) and apatinib (250 mg/day, Hengrui Medicine) were orally administered according to body weight. The main inclusion criteria were as follows: 1) aged between 18 and 85 years old; 2) diagnosed with HCC; 3) be seropositive for hepatitis B core antibody (HBcAb); 4) received at least one cycle of TACE combination with TKI plus ICI; 5) Child-Pugh grade A or B7; 6) be in stage B or C according to the Barcelona Clinic Liver Cancer (BCLC) staging system. Patients were excluded if they 1) combined with other viral infections; 2) had other malignancies in addition to HCC; 3) survival time less than 3 months; 4) incomplete data.
Clinical and Laboratory Variables
Patient demographic characteristics and treatment history were collected before initiation of ICI therapy. Data related to routine blood, blood biochemistry, alpha-fetoprotein (AFP), HBV DNA, HBV serum infection markers and imaging were collected before and during anticancer treatment. Clinical variables in relation to white blood cell (WBC), neutrophils, lymphocytes, platelets (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), albumin (ALB), alpha fetoprotein (AFP), HBV serological markers and BCLC stage were obtained from the electronic medical record system of the Affiliated Hospital of Xuzhou Medical University. The calculation formula of albumin-bilirubin (ALBI) score was as follows: ALBI = log10 bilirubin × 0.66 − albumin × 0.085, where the bilirubin is expressed in µmol/L and the albumin in g/L. Grading according to ALBI score: Grade 1, ALBI score ≤ −2.60; Grade 2, −2.60 < ALBI score ≤ −1.39; Grade 3, ALBI score > −1.39. The lower limit of HBV deoxyribonucleic acid (DNA) detection was 20 IU/mL in our institution.
Outcome Assessments
The primary endpoint of the study was the occurrence of liver injury. Assessment and classification of liver injury was in accordance with Common Terminology Criteria for Adverse Events (version 5.0).18 Serious hepatic adverse events were defined as grade 3 or greater hepatotoxicity. Other endpoints of this study were death from any causes, end of last treatment, date of last contact, 31 March 2022. Overall survival (OS) was defined as the period from initiation of ICI to death or 31 March 2022.
Statistical Analysis
All statistical analyses were done with SPSS 26.0 software. Categorical variables were expressed as the frequency (percent), quantitative variables were expressed as mean (range) or mean ± standard deviation. We used Chi-squared or Fisher's exact test to analyze categorical variables, while quantitative variables were compared using t-test or Mann–Whitney U-test, as appropriate. Univariate and multivariate logistic or Cox analyses were used to identify the risk factors for liver injury or prognostic factors for overall survival. The survival curves were analyzed using Kaplan–Meier method and log rank test. All the needed images have been generated by GraphPad Prism (version 9.0). The statistical significance of P < 0.05 (2-tailed) significant.
Results
Baseline Characteristics of Patients
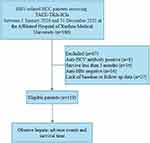
During the study period, a total of 186 HCC patients treated with TACE in combination with TKIs plus ICIs were screened for eligibility. After excluding 67 patients, 119 patients were finally enrolled (see Figure 1). Table 1 summarizes detail characteristics of enrolled patients. Of 119 recruited patients, the median age was 57 years and 91.6% were male. At baseline, 47.9% patients (57/119) had detectable HBV DNA levels (median 2170 IU/mL), 25.2% (30/119) had serum HBV DNA levels >2000 IU/mL, and 95.0% (113/119) were positive for HBsAg. There were 95 patients who were on antiviral prophylaxis therapy prior to receiving ICIs. A total of 104 cases (87.4%) and 15 cases (12.6%) were Child-Pugh grade A and B, respectively. Seventy-five (63.0%) and 44 (37.0%) of patients had BCLC stage B and C, respectively.
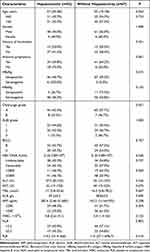 |
Table 1 Baseline Characteristics of Enrolled Patients |
Hepatic Adverse Events
There were 52 patients (43.7%) who developed liver injury of any grade: 15 cases with grade 1, 19 cases with grade 2, 16 cases with grade 3, and 2 cases with grade 4. Serious hepatic adverse events accounted for 15.1% in this population. The median time of hepatotoxicity was 2.5 months (range: 0.1–23 months) after the patients received ICI for the first time. There were statistically significant differences among patients with liver injury in terms of preventive antiviral treatment (35.8% vs 75.0%, P = 0.001) or baseline HBsAg (40.7% vs 100%, P = 0.015). Results of univariate and multivariate logistic regression analysis for liver injury are displayed in Table 2. The multivariate logistic analysis showed that failure to administer prophylactic antiviral (OR = 0.149; 95% CI: 0.050–0.442; P = 0.001) and low baseline WBC count (OR = 0.814; 95% CI: 0.677–0.980; P = 0.030) were risk factors for hepatic adverse events.
 |
Table 2 Univariate and Multivariate Logistic Regression Analysis for Risk Factors of Liver Injury |
Survival Time
Overall, we observed 45 deaths during the whole study period, and Kaplan–Meier survival curve showed a median survival time (MST) of 22.4 months for patients treated with TACE-TKI-ICI therapy (Figure 2A). The mortality rates of patients with or without liver injury were 53.8% (28/52) and 25.4% (17/67). Compared with patients without hepatotoxicity, patients with hepatotoxicity had a shorter survival time (MST: 13.7 months vs undefined, log-rank P = 0.015; Figure 2B). The MST of patients without liver injury, grade 1–2 and grade 3–4 liver injury were undefined, 13.7 months and 11.1 months, respectively (log-rank P = 0.034; Figure 2C). Results of univariate and multivariate Cox analysis for OS are displayed in Table 3. In addition to liver injury, the baseline high Child-Pugh grade (HR = 2.450; 95% CI: 1.162–5.164; P = 0.019), high AFP level (HR = 2.392; 95% CI: 1.241–4.611; P = 0.009) and younger age group (HR = 0.422; 95% CI: 0.211–0.844; P = 0.015) were also associated with death (see Table 3).
 |
Table 3 Univariate and Multivariate Analysis for Prognostic Factors of Survival Time |
Discussion
In our study, we investigated the occurrence rate and influencing factors of liver injury in HBV-related HCC patients who received a combination treatment of TACE-TKIs-ICIs. The study also further identified the prognostic factors for patient survival under the treatment of this regimen. Our findings indicated that the rates of any grade or 3–4 grade hepatic adverse events were 43.7% and 15.1%, respectively. Lack of prophylactic antiviral treatment was a risk factor for liver injury. Moreover, we found that hepatotoxicity, Child-Pugh grade and AFP levels are prognostic factors for survival time.
ICI is increasingly used in the treatment of advanced HCC.10 HCC patients treated with immunotherapy usually developed liver injury within 1–3 months following initiation of ICIs.14 The results of this study showed that the median time to liver injury was 2.5 months after the patients first received ICI treatment. The incidence of immune-related hepatitis caused by PD-1 inhibitors was about 5% and hepatic adverse events of grade 3–4 accounted for 1–2%, which was previously reported in the literature.19 In the current study, we indicated that nearly half of HCC patients would encounter hepatotoxicity (grade 3–4: 15.1%) during the treatment of TACE-TKI-ICI, which was higher than the incidence of hepatotoxicity reported by most clinical trials on ICIs.20,21 Combination therapy is likely to be the main reason for this difference, as more liver injury events occurred with ICI-based combined anticancer therapy than with immunomonotherapy.22 In a study exploring the efficacy and safety of sintilimab-dominated combination therapy, Dai et al17 noted that the incidence of liver injury in triple therapy with TACE- sorafenib-sintilimab, sintilimab-sorafenib and sintilimab monotherapy was 22.9%, 21.7% and 13.6%, respectively. Furthermore, clinical trials required HBV-infected patients to receive antiviral treatment, and the viral load is lower than the lower limit of detection or less than 100 IU/mL.23 However, in our study, 57 patients had detectable baseline HBV DNA levels and 24 patients did not receive prophylactic antiviral treatment. The rates of hepatotoxicity were 35.8% and 75.0% in patients with and without antiviral prophylaxis, respectively. Further analysis showed that the lack of prophylactic antiviral treatment was a risk factor for liver toxicity. In addition, HBV reactivation and even hepatitis activity could occur in HBV infection-related cancer patients during or after treatment with TACE, TKIs, and ICIs.23–26 Our previous study27 found that 12 HBV-related HCC patients who were treated with TACE-TKIs-ICIs encountered HBV reactivation, and 9 of them developed hepatotoxicity. Combination therapy may increase the incidence of HBV reactivation-associated hepatitis.
Regarding the risk factors of liver injury event, data analysis showed that lack of antiviral prophylaxis therapy is a risk factor for hepatic adverse events in patients with HCC receiving TACE-TKIs-ICIs combination therapy. The risk of liver injury was significantly higher in those who did not receive prophylactic antiviral therapy prior to their first ICI, approximately 6.711 times higher than those with prophylactic antiviral therapy. Previous research results also confirmed that the prophylactic use of entecavir antiviral therapy can significantly reduce the risk of developing liver injury events after TACE treatment.28 In addition, Lin et al29 found that the risk of developing any grade hepatotoxicity in cancer patients treated with PD-1 inhibitors was associated with HBsAg positivity. Our results of logistic analysis, however, showed that HBsAg has no effect on the occurrence of liver injury. On the one hand, a possible reason was that the sample size of our study is relatively small. On the other hand, the criteria for enrolling patients in the two studies differed, with the former being PD-1 inhibitors-treated cancer patients. According to these results, regular and effective antiviral therapy is necessary for HCC patients treated with TACE-TKI-ICI combination to reduce the risk of hepatotoxicity, whether HBsAg positive or negative.
Our current study revealed that patients who encountered liver toxicity had a significantly shorter survival time than those who did not, with an MST of 13.7 months. Besides, the higher the severity of the liver injury, the worse the median OS of the patients. This phenomenon was also seen in a previous study in Hong Kong,30 which pointed out that hepatic adverse events during ICIs treatment affected the prognosis of cancer patients, especially those with liver cancer. We believed that liver injury is a prognostic factor for HCC patients under treatment of TACE-TKI-ICI and recommended that liver function should be closely monitored to identify adverse events of liver injury in a timely manner. In addition to liver injury events, survival analysis also indicated that baseline high Child-Pugh grade and high AFP levels were relevant risk factors for patient survival time, which was consistent with previous research results.31–34 Interestingly, we also found that age group is an independent predictor for OS, which we believe to be a novel finding.35–37
Worthy of note, a previous review38 reported that baseline HBV DNA levels were associated with survival prognosis. High HBV DNA levels may influence the prognosis of HBV-related HCC patients under local and systemic treatment. Similarly, our results indicated that patients with detectable baseline HBV DNA levels have a significantly worse prognosis under the TACE-TKIs-ICIs combination regimen, but baseline HBV DNA >2000 IU/mL is not a risk predictor for OS when 2000 IU/mL is used as the cut-off value for HBV DNA levels. This was consistent with the findings of Sun et al39 that there was no significant difference in OS between HCC patients receiving PD-1 inhibitors immunotherapy with baseline HBV DNA > or ≤2000 IU/mL. Whether the HBV DNA levels can predict the survival prognosis of patients under TACE-TKIs-ICIs combination therapy requires further large-scale prospective studies.
This research had certain limitations. First, this study was a single-center retrospective study with a small sample size, which might limit the statistical efficacy. Second, due to the complexity of the treatment modality, we cannot specify the exact cause of liver injury. Third, the monitoring interval of liver function was inconsistent in the same patient or different patients, which may affect the observation of liver injury events. Fourth, all ICIs in this study were PD-1 checkpoint inhibitors, and whether the results are applicable to other ICIs remains to be further explored.
Conclusions
In conclusion, liver injury adverse events are not uncommon in HBV-related HCC patients treated with TACE-TKIs-ICIs. The more severe the hepatotoxicity, the worse the median survival. Before and during the triple treatment, it is necessary for patients with HBV-related HCC to take effective prophylactic antiviral therapy and examine liver function regularly.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (Ethics number: xyfy2022-KL085-01). Given that this was a retrospective observational study of the patient’s anonymous clinical data and that no identifying information was used, the written informed consent was not necessary. We certify that this study was performed in accordance with the ethical standards outlined in the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
Jiaming Shen, Xia Wang and Guangde Yang are co-first authors of this study. The authors report no conflicts of interest related to this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Chan SL, Wong VW, Qin S, Chan HL. Infection and cancer: the case of hepatitis B. J Clin Oncol. 2016;34(1):83–90. doi:10.1200/JCO.2015.61.5724
3. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
4. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
5. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi:10.1007/s12072-017-9799-9
6. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
7. Cucarull B, Tutusaus A, Rider P, et al. Hepatocellular carcinoma: molecular pathogenesis and therapeutic advances. Cancers. 2022;14(3):621. doi:10.3390/cancers14030621
8. Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15(3):663–675. doi:10.1007/s12072-021-10184-9
9. Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol. 2023;41(1):117–127. doi:10.1200/JCO.22.00392
10. Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38(36):4317–4345. doi:10.1200/jco.20.02672
11. Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi:10.6004/jnccn.2021.0022
12. Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70(5):999–1007. doi:10.1016/j.jhep.2019.01.027
13. Teo YL, Ho HK, Chan A. Risk of tyrosine kinase inhibitors-induced hepatotoxicity in cancer patients: a meta-analysis. Cancer Treat Rev. 2013;39(2):199–206. doi:10.1016/j.ctrv.2012.09.004
14. Sangro B, Chan SL, Meyer T, Reig M, El-Khoueiry A, Galle PR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. 2020;72(2):320–341. doi:10.1016/j.jhep.2019.10.021
15. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
16. Griffiths CD, Zhang B, Tywonek K, Meyers BM, Serrano PE. Toxicity profiles of systemic therapies for advanced hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Network Open. 2022;5(7):e2222721. doi:10.1001/jamanetworkopen.2022.22721
17. Dai L, Cai X, Mugaanyi J, et al. Therapeutic effectiveness and safety of sintilimab-dominated triple therapy in unresectable hepatocellular carcinoma. Sci Rep. 2021;11(1):19711. doi:10.1038/s41598-021-98937-2
18. National Cancer Institute. Common terminology criteria for Adverse Events (CTCAE) Version 5.0. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
19. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi:10.1093/annonc/mdv383
20. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
21. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
22. Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, Version 1.2019. J Natl Compr Canc Netw. 2019;17(3):255–289. doi:10.6004/jnccn.2019.0013
23. Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72(2):307–319. doi:10.1016/j.jhep.2019.09.025
24. Jang JW. Hepatitis B virus reactivation in patients with hepatocellular carcinoma undergoing anti-cancer therapy. World J Gastroenterol. 2014;20(24):7675–7685. doi:10.3748/wjg.v20.i24.7675
25. Iacovelli R, Palazzo A, Procopio G, et al. Incidence and relative risk of hepatic toxicity in patients treated with anti-angiogenic tyrosine kinase inhibitors for malignancy. Br J Clin Pharmacol. 2014;77(6):929–938. doi:10.1111/bcp.12231
26. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152(6):1297–1309. doi:10.1053/j.gastro.2017.02.009
27. Shen J, Wang X, Wang N, et al. HBV reactivation and its effect on survival in HBV-related hepatocarcinoma patients undergoing transarterial chemoembolization combined with tyrosine kinase inhibitors plus immune checkpoint inhibitors. Front Cell Infect Microbiol. 2023;13:1179689. doi:10.3389/fcimb.2023.1179689
28. Yoo SH, Jang JW, Kwon JH, Jung SM, Jang B, Choi JY. Preemptive antiviral therapy with entecavir can reduce acute deterioration of hepatic function following transarterial chemoembolization. Clin Mol Hepatol. 2016;22(4):458–465. doi:10.3350/cmh.2016.0054
29. Lin Z, Zhang X, Zhou Y, et al. Hepatotoxicity associated with PD-1 blockade antibodies in cancer patients co-infected with hepatitis B virus. Cancer Immunol Immunother. 2022;71(5):1247–1255. doi:10.1007/s00262-021-03082-4
30. Chan SL, Yip TC, Wong VW, et al. Pattern and impact of hepatic adverse events encountered during immune checkpoint inhibitors - A territory-wide cohort study. Cancer Med. 2020;9(19):7052–7061. doi:10.1002/cam4.3378
31. Wu CJ, Lee PC, Hung YW, et al. Lenvatinib plus pembrolizumab for systemic therapy-naive and -experienced unresectable hepatocellular carcinoma. Cancer Immunol Immunother. 2022;71(11):2631–2643. doi:10.1007/s00262-022-03185-6
32. Yang X, Chen B, Wang Y, et al. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol Int. 2023;17(1):1–11. doi:10.1007/s12072-022-10480-y
33. Iavarone M, Cabibbo G, Biolato M, et al. Predictors of survival in patients with advanced hepatocellular carcinoma who permanently discontinued sorafenib. Hepatology. 2015;62(3):784–791. doi:10.1002/hep.27729
34. Lui TKL, Cheung KS, Leung WK. Machine learning models in the prediction of 1-year mortality in patients with advanced hepatocellular cancer on immunotherapy: a proof-of-concept study. Hepatol Int. 2022;16(4):879–891. doi:10.1007/s12072-022-10370-3
35. Gao B, Yang F, Zheng D, et al. Transarterial chemoembolization combined with tyrosine kinase inhibitors plus immune checkpoint inhibitors for advanced hepatocellular carcinoma: a propensity score matching analysis. J Hepatocell Carcinoma. 2023;10:2265–2276. doi:10.2147/jhc.S443041
36. Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi:10.3389/fimmu.2022.848387
37. Han Z, Yang F, Zhang Y, et al. Prognostic efficacy and prognostic factors of TACE plus TKI with ICIs for the treatment of unresectable hepatocellular carcinoma: a retrospective study. Front Oncol. 2022;12:1029951. doi:10.3389/fonc.2022.1029951
38. Yu SJ, Kim YJ. Hepatitis B viral load affects prognosis of hepatocellular carcinoma. World J Gastroenterol. 2014;20(34):12039–12044. doi:10.3748/wjg.v20.i34.12039
39. Sun X, Hu D, Yang Z, et al. Baseline HBV loads do not affect the prognosis of patients with hepatocellular carcinoma receiving anti-programmed cell death-1 Immunotherapy. J Hepatocell Carcinoma. 2020;7:337–345. doi:10.2147/JHC.S278527
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.