Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Liraglutide Improves Nonalcoholic Fatty Liver Disease in Diabetic Mice by Activating Autophagy Through AMPK/mTOR Signaling Pathway
Authors Liao Z, Huang L, Chen J, Chen T, Kong D, Wei Q, Chen Q, Deng B, Li Y, Zhong S, Huang Z
Received 7 November 2023
Accepted for publication 11 January 2024
Published 5 February 2024 Volume 2024:17 Pages 575—584
DOI https://doi.org/10.2147/DMSO.S447182
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Zhanlin Liao,1,* Liangzhi Huang,1,* Jun Chen,2 Ting Chen,1 Dezhi Kong,1 Qifeng Wei,1 Qiao Chen,1 Bin Deng,1 Yanyan Li,1 Shuai Zhong,1 Zugui Huang1
1Department of Endocrine, Affiliated Nanping First Hospital of Fujian Medical University, Nanping, Fujian, 353006, People’s Republic of China; 2Department of Ophthalmology, Affiliated Nanping First Hospital of Fujian Medical University, Nanping, Fujian, 353006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zugui Huang, Department of Endocrine, Affiliated Nanping First Hospital of Fujian Medical University, No. 317 Zhongshan Road, Nanping, Fujian, 353006, People’s Republic of China, Email [email protected]
Background: Type 2 diabetes (T2DM) combined nonalcoholic fatty liver disease (NAFLD) are characterized by metabolic disruptions. Liraglutide has been proved to be effective in T2DM. If LRG could regulate NAFLD combined T2DM has not been reported.
Methods: Intraperitoneal injection of 1% streptozotocin (STZ) plus high-sugar and high-fat diet was used to induce NAFLD combined T2DM animal model. Palmitic acid (200 μmol/L) and glucose (25 mmol/L) incubation were used to induce cell model. The cell apoptosis, mRNA and protein expression were measured through flow cytometry, PCR, and Western blotting, respectively.
Results: Liraglutide significantly improved the liver injury of NAFLD combined T2DM rats, but Com-C reversed the effect of liraglutide. The decreased AMPK/mTOR signaling pathway in the NAFLD combined T2DM animals was greatly activated by liraglutide. Com-C reversed the protection effects of liraglutide on palmitic acid+glucose induced cell damage.
Conclusion: Liraglutide could greatly alleviate the damage caused by NAFLD+T2DM and palmitic acid+glucose. The protection effects of liraglutide were greatly inhibited by suppressing AMPK/mTOR signaling pathway. This research might provide a novel therapeutic strategy for the prevention and treatment of NAFLD combined T2DM disease.
Keywords: type 2 diabetes, nonalcoholic fatty liver disease, liraglutide, AMPK/mTOR, autophagy
Introduction
Type 2 diabetes (T2DM) and nonalcoholic fatty liver disease (NAFLD) are common chronic diseases characterized by metabolic disruptions, and their mutual influence can lead to adverse consequences.1 Epidemiological studies conducted abroad have also shown a close relationship between diabetes and NAFLD, with a significantly higher prevalence of NAFLD in individuals with abnormal glucose metabolism compared to those with normal glucose metabolism.2,3 In 2017, the International Diabetes Federation (IDF) reported that there are approximately 425 million adults worldwide living with diabetes, with China having an alarmingly high number of 114 million diabetic patients, among whom the prevalence of comorbid nonalcoholic fatty liver disease ranges from 28% to 70%.4 Therefore, research on the co-occurrence of T2DM and NAFLD is of paramount importance.
Adenosine monophosphate-activated protein kinase (AMPK) and mammalian target of rapamycin complex 1 (mTORC1) are two central kinase complexes responsible for sensing intracellular nutrient levels, regulating metabolic balance, and controlling cell growth.5 AMPK plays a crucial role in maintaining cellular energy homeostasis and is a major regulator of both lipid and glucose metabolism.6 It is closely associated with various metabolic disorders such as obesity and diabetes. AMPK is activated by sensing changes in the ratio of ATP to AMP.7 It detects states of lower nutrient and energy levels and becomes activated under these conditions. AMPK inhibits synthetic metabolic pathways to reduce ATP consumption, promotes catabolic pathways to increase ATP production, and inhibits cell growth to ultimately ensure energy homeostasis.8 mTORC1 is a protein kinase complex that is activated at the surface of lysosomes. It promotes cell growth and proliferation by enhancing protein translation and synthesis, inhibiting autophagy, and promoting the synthesis of fatty acids and nucleotides.9 Therefore, mTORC1 activation requires the simultaneous presence of growth factors, glucose, amino acids, sufficient oxygen, and high energy levels within the cell.
It has been confirmed that the relative activities of mTORC1 and AMPK under different cellular conditions largely determine the extent of autophagy induction.10 Several studies have shown a protective role of the AMPK/mTORC1 autophagy signaling pathway in conditions such as neuronal damage and myocardial hypertrophy.11 Therefore, AMPK/mTORC1/TFEB autophagy signaling pathway plays a significant role in the pathological changes of liver tissues in T2DM with NAFLD.
Currently, there are no drugs approved by the FDA for the treatment of NAFLD. Therefore, the development of effective drugs for the treatment of NAFLD is an urgent task.12 Glucagon-like peptide-1 (GLP-1) is an endogenous peptide secreted by gastrointestinal L cells and pancreatic cells. Liraglutide, as a long-acting GLP-1 receptor agonist, closely resembles human GLP-1, is resistant to degradation, and can better exert its biological effects.13 Liraglutide can improve inflammation caused by lipotoxicity through the mTORC1 signaling pathway. Liraglutide can activate the cellular autophagic response in rats with T2DM through the AMPK-mTOR pathway.14 Meanwhile, liraglutide can provide protection for the liver of NAFLD patients.15 However, the specific mechanism of this protective effect is not yet fully understood, and it remains an issue to be resolved.
In this research, we want to investigate if liraglutide can provide protection for liver tissues in individuals with T2DM and NAFLD through the AMPK/mTORC1/TFEB autophagy signaling pathway. We established a rat model of T2DM combined with NAFLD as the research subjects. Additionally, an in vitro model of IR-fatty liver HepG2 cells was created using palmitic acid and high glucose. Liraglutide was used for intervention, and various assessments were conducted, including the examination of animal serum lipid levels and the observation of morphological changes in liver tissues and liver cells. We aimed to elucidate the potential mechanisms by which liraglutide offers liver protection in T2DM with comorbid NAFLD, providing a theoretical basis for drug therapy aimed at mitigating liver damage in individuals with T2DM and NAFLD.
Materials and Methods
Cell Culture
Hep G2 cells purchased from ATCC were used in this research. DMEM medium containing 10% FBS was used to culture cell. The cells were cultured in an incubator at 37 °C, containing 5% CO2. Culturing Hep G2 cells in a medium with a concentration of 200 µmol/L palmitic acid (PA) and 25 mmol/L glucose for 24 hours is used to simulate a cellular model of T2DM combined NAFLD. 100 nmol/L liraglutide, Compund C (Com-C, 20 μM), or PBS was used to treat cells for 24 hours, and the cells were used for following experiments.
Flow Cytometry
Following enzymatic digestion, cellular samples were subjected to centrifugation at 1500 g for 8 minutes, and the resulting supernatant was subsequently removed. To assess cellular apoptosis, the samples were treated with Propidium iodide and Annexin V-FITC (Beyotime, Beijing, China) and incubated in a light-protected environment for a duration of 15 minutes. Flow cytometry was employed as the analytical technique to evaluate and quantify cellular apoptosis.
Western Blotting
The proteins were extracted from liver tissues and cells using a protein lysis buffer, and protein concentration in the extracted samples was measured with BCA assay. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed to separate the proteins according to their molecular weight. The separated proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane, and the PVDF membrane was blocked by immersing it in 5% non-fat milk for 2 hours. The blocked PVDF membranes were incubated with a specific primary antibody (1:1000) overnight at 4°C, and washed with Tris-buffered saline with Tween (TBST) to remove excess primary antibody. The membranes were incubated with a secondary antibody at room temperature for 1 hour. The membranes were scanned using an ODYSSEY Infrared Imaging System. Image J software was used to measure protein band grey.
NAFLD Combined T2DM Animal Model
All animal experiments were approved by the animal care and welfare committee of Affiliated Nanping First Hospital of Fujian Medical University. All animal experiment followed the guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892–2018. The animal model was established as described previously.16,17 A total of 45 SD rats (Charles River) were randomly divided into two groups after being adaptively fed. Fifteen rats were given a basal diet, while 30 rats were fed a high-sugar and high-fat diet. After 4 weeks, both groups of rats were fasted (without water) for 13 hours, weighed, and had their blood glucose levels measured via tail vein sampling. The rats fed with high-sugar and high-fat diet were then subjected to a one-time intraperitoneal injection of 1% streptozotocin (STZ) at a dose of 35 mg/kg to induce the T2DM rat model, while rats fed with basal diet received an equivalent intraperitoneal injection of citrate buffer as a control. After the successful injection, blood glucose levels in the rats were monitored, and for those rats whose blood glucose levels did not meet the criteria within 2 days, an additional intraperitoneal injection of STZ at 25 mg/kg was administered. Blood glucose levels were monitored for an additional 2 weeks, and rats that achieved and maintained blood glucose levels ≥16.7 mmol/L were considered to be successfully modeled as T2DM rats. Two weeks later, all rats were divided into model and treatment groups. The treatment group received daily intraperitoneal injections of liraglutide at a dose of 0.6 mL/kg twice daily for 4 weeks, while the remaining rats received an equivalent volume of saline as a control. The diet for each group of rats remained the same as before. The modeling process was completed after 8 weeks, and all samples were collected for additional experiments. Blood was collected through the eye socket, and then the collected blood samples were centrifuged at 4°C at 2000 rpm for 10 minutes. The upper layer of serum was collected and stored in a −80°C freezer.
Oil Red Staining
After anesthesia, liver tissues were collected and subjected to freezing sectioning, which was later used for Hematoxylin and Eosin (HE) staining, Oil Red O staining, and immunohistochemistry. Prepare the working solution by mixing saturated Oil Red O stock solution with distilled water in a 3:2 ratio. The frozen sections were fixed in 10% formaldehyde for 10 minutes, rinsed slightly with distilled water. The sections were incubated in 60% isopropanol for 30 seconds. The sections were incubated with Oil Red O staining solution for 15 minutes. The sections were rinsed with 60% isopropanol. The nuclei were stained with Mayer’s hematoxylin for 1 minute, followed by a brief rinse with distilled water. Filter paper was used to remove excess moisture around the tissue and seal sections with glycerin gelatin.
HE Staining
The paraffin sections were incubated in Xylene I and Xylene II for 5 minutes each to deparaffinize them. The sections were dehydrated in anhydrous alcohol for 1 minute twice. The sections were hydrated using a gradient of alcohol concentrations: 95%, 90%, and 85% ethanol, for 1 minute each, rinsed in tap water for 1 minute, and soaked in distilled water for 1 minute. The sections were stained with Mayer’s hematoxylin for 5 minutes, and rinsed with tap water three times, each time for 1 minute. Differentiate the sections in 1% hydrochloric acid alcohol for 10 seconds, followed by a 1-minute rinse in tap water. The sections were incubated with 1% ammonia water for 30 seconds, followed by a 1-minute rinse in tap water. Eosin was used to incubate sections for 2 minutes, followed by a 1-minute rinse in tap water. The sections were dehydrated using a gradient of alcohol concentrations: 95%, 90%, and 85% ethanol, for 1 minute each. Then, dehydrate in anhydrous alcohol I and II for 2 minutes each. The sections were mounted with neutral gum.
Immunofluorescence Staining
To initiate the dewaxing process, the tissue sections were initially subjected to heating at 65 °C for a duration of 1 hour within an oven. This was followed by a series of immersion steps, involving xylene (5 minutes), 100% ethanol (10 minutes), 95% ethanol (10 minutes), 70% ethanol (10 minutes), and distilled water (5 minutes). Subsequently, endogenous peroxidase was quenched by immersing the sections in 3% hydrogen peroxide for 5 minutes. The sections were then exposed to primary antibody for an incubation period of 12 hours at 4 °C. Following a PBS wash, secondary antibodies were applied for an additional 2 hours. Lastly, DAB reagents were employed for slide incubation, and the resulting staining was observed under a fluorescence microscope.
RT-PCR
Total RNA was extracted from tissues or cells using RNAiso Plus (TaKaRa, 9109). Subsequently, the concentration and purity of the RNA samples were assessed utilizing the NanoDrop 2000C spectrophotometer. The extracted RNA was then subjected to reverse transcription, yielding complementary DNA (cDNA), through the application of the NovoScript® Plus All-in-one 1st Strand cDNA Synthesis SuperMix kit (Novoprotein, E047-01A). The cDNA was employed as a template for quantitative real-time polymerase-chain reaction (qRT-PCR) using the NovoStart® SYBR qPCR SuperMix Plus (Novoprotein, E096-01B). The qRT-PCR reaction was carried out under the following thermal cycling conditions: initial denaturation at 95°C for 35 seconds, annealing and extension at 60°C for 30 seconds, and final extension at 95°C for 10 seconds, followed by a 65°C step for 5 seconds. Subsequently, amplification and melt curve analyses were performed, and the 2−ΔΔ Ct method was employed to calculate the relative expression levels of the target mRNA.
Measurement of Glucose, Triglyceride, Total Cholesterol, SOD. GSH, and MDA
Levels of SOD, MDA, GSH, glucose, triglyceride, and total cholesterol were determined utilizing appropriate commercial kits according to the manufacturer’s instructions.
Transmission Electron Microscope (TEM)
The tissues were promptly immersed in a 3% glutaraldehyde solution for a 2-hour fixation, followed by a 1-hour fixation in 1% osmic acid. Subsequently, a gradient of acetone was employed for tissue dehydration. Afterward, the samples underwent epoxy propane replacement, embedding in Epon-812 resin, ultra-thin sectioning, and dual staining with uranium acetate and lead citrate. This process enabled the observation of tight junctions in intestinal epithelial cells using TEM.
Statistical Analysis
Statistical analysis was conducted using SPSS version 22.0. Analysis of Variance (ANOVA) was employed to assess data variability among multiple groups. A significance level of 0.05 was chosen for hypothesis testing, and any observed p-values less than 0.05 were considered to indicate a statistically significant difference.
Results
Liraglutide Significantly Improved the Liver Injury of NAFLD Combined T2DM Rats, but Com-C Reversed the Effect of Liraglutide
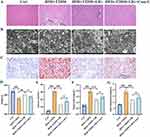
The NAFLD combined T2DM animals’ model was established. The pathology of the liver tissues in the group control of rats showed that the liver cells were evenly sized, the liver lobule structure was neat, and arranged radially around the central vein, without inflammatory cell infiltration or cell necrosis. The liver tissue pathology of model group rats showed that the structure of liver lobules was damaged, liver cells were swollen and disorderly arranged, and there were many fat vacuoles of varying sizes in the cytoplasm. Inflammatory cells infiltrated from the portal area, and some areas showed punctate or focal necrosis of liver cells (Figure 1A and B). However, liraglutide treatment significantly improved the tissue injury. Treatment with Com-C reversed the protection effect of liraglutide. Oil red staining indicates that liraglutide greatly inhibited lipid droplet, but after treatment with Com-C, the level of lipid droplet was remarkably elevated (Figure 1C). Some T2DM indicators were investigated. The decreased rat weight, increased glucose, TC, and TG were significantly reversed by liraglutide (Figure 1D-G). However, Com-C presented an injury promoting effect in the NAFLD combined T2DM animals’ model (Figure 1D-G).
The Decreased AMPK/mTOR Signaling Pathway in the NAFLD Combined T2DM Animals Was Greatly Activated by Liraglutide
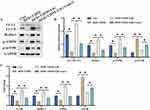
AMPK/mTOR signaling pathway and autophagy are believed to be closely linked with the development of NAFLD and T2DM. We found that the protein and mRNA levels of LC3 II/LC3 I, Beclin-1, AMPK/mTOR signaling pathway were remarkably inhibited in NAFLD combined T2DM animals (Figure 2A-C). However, treatment with liraglutide significantly promoted LC3 II/LC3 I, Beclin-1, and AMPK/mTOR signaling pathway in protein and mRNA levels (Figure 2A-C), indicating that liraglutide could activate autophagy process and AMPK/mTOR signaling pathway.
Com-C Reversed the Protection Effects of Liraglutide on Palmitic Acid+glucose Induced Cell Damage
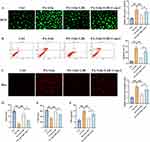
We further investigate if Com-C could affect the influence of liraglutide on palmitic acid+glucose induced cell damage, which is a cell model of NAFLD combined T2DM. Liraglutide is shown to have cell protection effects via suppressing ROS, cell apoptosis, and Bax expression (Figure 3A-C). Meanwhile, some oxidation-reduction indicators, SOD, GSH, MDA, were detected. Liraglutide also protects cells from palmitic acid+glucose induced cell via increasing SOD, GSH, but decreasing MDA (Figure 3D-F). However, treatment with Com-C, the inhibitor of AMPK/mTOR signaling pathway, greatly revered the protection effects of liraglutide.
The Decreased AMPK/mTOR Signaling Pathway in Palmitic Acid Combined Glucose Induced Cell Model Was Greatly Activated by Liraglutide
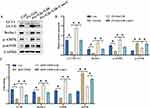
We also investigate the influence of liraglutide on AMPK/mTOR signaling pathway in cell level. The protein and mRNA levels of LC3 II/LC3 I, Beclin-1, AMPK/mTOR signaling pathway were greatly inhibited in palmitic acid combined glucose treated cells (Figure 4A-C). However, treatment with liraglutide significantly promoted LC3-II/LC3-I, Beclin-1, and AMPK/mTOR signaling pathway in protein and mRNA levels (Figure 4A-C).
Discussion
The progression of NAFLD primarily includes non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma.18 Consequently, NAFLD can lead to severe consequences such as liver fibrosis, liver cancer, and even liver failure. It also promotes the development of diseases like T2DM and atherosclerosis.19 NASH represents a critical step in the progression from simple fatty liver to cirrhosis and is of particular concern due to its association with diabetes, atherosclerosis, and malignancies related to metabolism.20
There is a complex and finely regulated mutual control mechanism between AMPK and mTORC1. In maintaining cellular material and energy balance, mTORC1 serves as an important substrate and regulatory node for AMPK.21 AMPK can inhibit mTORC1 through various pathways, including direct phosphorylation of TSC2 to inhibit mTORC1 activity and direct phosphorylation of RAPTOR on the mTORC1 complex, resulting in structural changes that inhibit the complex’s activity.22 Conversely, some research has shown that mTORC1 can also inhibit AMPK activity in its activated state.23 mTORC1 can, to some extent, regulate autophagy through the phosphorylation and inhibition of transcription factor EB (TFEB) and modulate its nuclear translocation. This process drives the expression of lysosome biogenesis genes and autophagy-related genes.24
As a once-daily injectable GLP-1 analog, liraglutide not only provides sustained and potent blood glucose-lowering effects without increasing the risk of hypoglycemia but also offers benefits such as weight reduction, improvement of pancreatic beta-cell function, blood pressure regulation, lipid lowering, delayed gastric emptying, appetite suppression, and a reduction in cardiovascular risk.23,25
Autophagy primarily functions to degrade oversized cellular structures, such as the ubiquitin-proteasome system, and to maintain cellular function by recycling amino acids, allowing cells to survive in conditions of nutrient scarcity.26 Autophagy is primarily regulated by essential genes, including ATG (autophagy-related genes).27 Several experiments have confirmed that chloroquine effectively inhibits the autophagy signal in cells, making it suitable for inhibiting autophagy in HepG2 cell experiments.
The rat model that involves high-fat and high-sugar feeding followed by STZ injection is currently a well-recognized and relatively mature T2DM rat model in diabetes research.28 High-fat and high-sugar diet feeding induces systemic obesity in rats, including elevated triglycerides and fat degeneration in the liver, metabolic disruption in hepatic cells, lipid accumulation, ultimately leading to the development of NAFLD.29 We demonstrated that liraglutide could inhibit the NAFLD in T2DM rats.
In the study, we explored the therapeutic effects of liraglutide on non-alcoholic fatty liver disease (NAFLD) combined with type 2 diabetes mellitus (T2DM) in a rat model. Treatment with liraglutide significantly improved liver injury by reducing lipid droplets and reversing T2DM indicators such as weight, glucose, cholesterol, and triglycerides. We also investigated the AMPK/mTOR signaling pathway, which is associated with NAFLD and T2DM development. Liraglutide treatment activated autophagy and the AMPK/mTOR pathway. Furthermore, the protective effects of liraglutide against cell damage induced by palmitic acid and glucose. However, the presence of Com-C, an inhibitor of the AMPK/mTOR pathway, reversed liraglutide’s protective effects. In a palmitic acid and glucose-induced cell model, liraglutide also promoted the AMPK/mTOR signaling pathway at both protein and mRNA levels, further confirming its role in activating this pathway.
Conclusion
We demonstrated that liraglutide might alleviate the damage caused by NAFLD+T2DM in vivo and palmitic acid+glucose in vitro. However, the protection effects of liraglutide were partly restored by suppressing AMPK/mTOR signaling pathway, indicating that liraglutide might provide the protection function through targeting AMPK/mTOR signaling pathway. This research might provide a novel therapeutic strategy for the prevention and treatment of NAFLD combined T2DM disease.
Abbreviations
T2DM, Type 2 diabetes; NAFLD, nonalcoholic fatty liver disease; IDF, International Diabetes Federation; mTOR, Mammalian target of rapamycin; AMPK, Adenosine monophosphate-activated protein kinase; mTORC1, Rapamycin complex 1; GLP-1, Glucagon-like peptide-1; PA, Palmitic acid; Com-C, Compund C; SDS-PAGE, Sodium dodecyl sulfate-polyacrylamide gel electrophoresis; PVDF, Polyvinylidene difluoride; TBST, Tris-buffered saline with Tween; STZ, Streptozotocin; HE, Hematoxylin and eosin; TEM, Transmission electron microscope; NAFL, Non-alcoholic fatty liver; NASH, Non-alcoholic steatohepatitis; TFEB, Transcription factor EB.
Data Sharing Statement
The data and material used to support the findings of this study are included within the manuscript.
Ethical Approval and Consent to Participate
The experimental protocol was approved by the animal care and welfare committee of Affiliated Nanping First Hospital of Fujian Medical University. All animal experiment followed the guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018.
Funding
This study was funded by Fujian natural science foundation project (2021J011423) and Fujian Provincial Health Commission Science and Technology Plan Project (2021ZD01004).
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Younossi Z, Tacke F, Arrese M, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69(6):2672–2682. doi:10.1002/hep.30251
2. Tanase DM, Gosav EM, Costea CF, et al. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196. doi:10.1155/2020/3920196
3. Xie J, Huang H, Liu Z, et al. The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatology. 2023;77(3):949–964. doi:10.1002/hep.32728
4. Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):484–495. doi:10.1038/s41574-021-00507-z
5. Davuluri G, Welch N, Sekar J, et al. Activated Protein Phosphatase 2A Disrupts Nutrient Sensing Balance Between Mechanistic Target of Rapamycin Complex 1 and Adenosine Monophosphate-Activated Protein Kinase, Causing Sarcopenia in Alcohol-Associated Liver Disease. Hepatology. 2021;73(5):1892–1908. doi:10.1002/hep.31524
6. Xiao H, Zha C, Shao F, Wang L, Tan B. Amino acids regulate energy utilization through mammalian target of rapamycin complex 1 and adenosine monophosphate activated protein kinase pathway in porcine enterocytes. Anim Nutr. 2020;6(1):98–106. doi:10.1016/j.aninu.2019.12.001
7. Sun X, Li S, Gan X, et al. Wild-type p53-induced phosphatase 1 promotes vascular smooth muscle cell proliferation and neointima hyperplasia after vascular injury via p-adenosine 5’-monophosphate-activated protein kinase/mammalian target of rapamycin complex 1 pathway. J Hypertens. 2019;37(11):2256–2268. doi:10.1097/HJH.0000000000002159
8. Wang MZ, Wang J, Cao DW, et al. Fucoidan Alleviates Renal Fibrosis in Diabetic Kidney Disease via Inhibition of NLRP3 Inflammasome-Mediated Podocyte Pyroptosis. Front Pharmacol. 2022;13:790937. doi:10.3389/fphar.2022.790937
9. Ferri N. AMP-activated protein kinase and the control of smooth muscle cell hyperproliferation in vascular disease. Vascul Pharmacol. 2012;56(1–2):9–13. doi:10.1016/j.vph.2011.10.003
10. Benito-Cuesta I, Ordonez-Gutierrez L, Wandosell F. AMPK activation does not enhance autophagy in neurons in contrast to MTORC1 inhibition: different impact on beta-amyloid clearance. Autophagy. 2021;17(3):656–671. doi:10.1080/15548627.2020.1728095
11. Jing Z, He X, Jia Z, Sa Y, Yang B, Liu P. NCAPD2 inhibits autophagy by regulating Ca(2+)/CAMKK2/AMPK/mTORC1 pathway and PARP-1/SIRT1 axis to promote colorectal cancer. Cancer Lett. 2021;520:26–37. doi:10.1016/j.canlet.2021.06.029
12. Ren Q, Sun Q, Fu J. Dysfunction of autophagy in high-fat diet-induced nonalcoholic fatty liver disease. Autophagy. 2023;1–21. doi:10.1080/15548627.2023.2254191
13. Reis-Barbosa PH, Marcondes-de-Castro IA, Marinho TS, Aguila MB, Mandarim-de-Lacerda CA. The mTORC1/AMPK pathway plays a role in the beneficial effects of semaglutide (GLP-1 receptor agonist) on the liver of obese mice. Clin Res Hepatol Gastroenterol. 2022;46(6):101922. doi:10.1016/j.clinre.2022.101922
14. Fang Y, Ji L, Zhu C, et al. Liraglutide Alleviates Hepatic Steatosis by Activating the TFEB-Regulated Autophagy-Lysosomal Pathway. Front Cell Dev Biol. 2020;8:602574. doi:10.3389/fcell.2020.602574
15. Yuan P, Ma D, Gao X, et al. Liraglutide Ameliorates Erectile Dysfunction via Regulating Oxidative Stress, the RhoA/ROCK Pathway and Autophagy in Diabetes Mellitus. Front Pharmacol. 2020;11:1257. doi:10.3389/fphar.2020.01257
16. Kim DY, Chung KS, Park JY, Gee HY. Preventive effect of empagliflozin and ezetimibe on hepatic steatosis in adults and murine models. Biomed Pharmacother. 2023;161:114445. doi:10.1016/j.biopha.2023.114445
17. Dohmen J, Praktiknjo M, Rudeloff A, et al. Impact of sleeve gastrectomy and dietary change on metabolic and hepatic function in an obesity rat model - Experimental research. Int J Surg. 2020;75:139–147. doi:10.1016/j.ijsu.2020.01.139
18. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi:10.1016/j.jhep.2019.06.021
19. Muzica CM, Sfarti C, Trifan A, et al. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: a Bidirectional Relationship. Can J Gastroenterol Hepatol. 2020;2020:6638306. doi:10.1155/2020/6638306
20. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. doi:10.1016/j.jhep.2014.12.012
21. Vilchinskaya NA, Krivoi II, Shenkman BS. AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling. Int J Mol Sci. 2018;19(11). doi:10.3390/ijms19113558
22. Wilson JL, Mayr HK, Weichhart T. Metabolic Programming of Macrophages: implications in the Pathogenesis of Granulomatous Disease. Front Immunol. 2019;10:2265. doi:10.3389/fimmu.2019.02265
23. Wang D, Jiang L, Feng B, He N, Zhang Y, Ye H. Protective effects of glucagon-like peptide-1 on cardiac remodeling by inhibiting oxidative stress through mammalian target of rapamycin complex 1/p70 ribosomal protein S6 kinase pathway in diabetes mellitus. J Diabetes Investig. 2020;11(1):39–51. doi:10.1111/jdi.13098
24. Zhu YP, Brown JR, Sag D, Zhang L, Suttles J. Adenosine 5’-monophosphate-activated protein kinase regulates IL-10-mediated anti-inflammatory signaling pathways in macrophages. J Immunol. 2015;194(2):584–594. doi:10.4049/jimmunol.1401024
25. Shi A, Li T, Zheng Y, et al. Chlorogenic Acid Improves NAFLD by Regulating gut Microbiota and GLP-1. Front Pharmacol. 2021;12:693048. doi:10.3389/fphar.2021.693048
26. Song S, Guo R, Mehmood A, et al. Liraglutide attenuate central nervous inflammation and demyelination through AMPK and pyroptosis-related NLRP3 pathway. CNS Neurosci Ther. 2022;28(3):422–434. doi:10.1111/cns.13791
27. Zhang Y, Wang S, Chen X, et al. Liraglutide prevents high glucose induced HUVECs dysfunction via inhibition of PINK1/Parkin-dependent mitophagy. Mol Cell Endocrinol. 2022;545:111560. doi:10.1016/j.mce.2022.111560
28. Yan J, Yao B, Kuang H, et al. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: the Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69(6):2414–2426. doi:10.1002/hep.30320
29. Xu X, Wang W, Lin L, Chen P. Liraglutide in combination with human umbilical cord mesenchymal stem cell could improve liver lesions by modulating TLR4/NF-kB inflammatory pathway and oxidative stress in T2DM/NAFLD rats. Tissue Cell. 2020;66:101382. doi:10.1016/j.tice.2020.101382
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.