Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Lipoxin A4 Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis via Promoting the Regression of Inflammation
Authors Hu F, Qu Z , Chen K, Zhang P, Wang B, Jiang R, Zuo Y, Xia P, Chen H
Received 23 May 2023
Accepted for publication 3 August 2023
Published 8 August 2023 Volume 2023:16 Pages 2103—2111
DOI https://doi.org/10.2147/CCID.S418467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Feng Hu,1,2,* Zilu Qu,1,2,* Kai Chen,1,2 Ping Zhang,1 Bei Wang,1 Ruili Jiang,1 Yuyue Zuo,1 Ping Xia,1,* Hongxiang Chen3,4,*
1Department of Dermatology, Wuhan No. 1 Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Hubei Province & Key Laboratory of Skin Infection and Immunity, Wuhan No. 1 Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 3Department of Dermatology, Huazhong University of Science and Technology Union Shenzhen Hospital, Shenzhen, 518052, People’s Republic of China; 4Department of Dermatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongxiang Chen, Huazhong University of Science and Technology Union Shenzhen Hospital, No. 89, Taoyuan Road, Nanshan District, Shenzhen, 518052, People’s Republic of China, Tel +86 13871022932, Email [email protected] Ping Xia, Email [email protected]
Introduction: As a mediator of inflammation resolution, lipoxin A4 (LXA4) mainly plays an anti-inflammatory role and promotes inflammation resolution. LXA4 plays an inhibiting inflammatory role in a variety of diseases, tissues and cells, including keratinocytes. Psoriasis is a chronic inflammatory skin disease mediated by dysregulation of inflammation of immune cells and keratinocytes. However, the expression and role of LXA4 in psoriasis-like mouse models are still unclear.
Methods: Imiquimod (IMQ) topical treatment of dorsal skin induces psoriasis-like dermatitis in BALB/c mice, pretreated intraperitoneally with or without LXA4 prior to IMQ application. Severity of dorsal lesions is assessed by using a modified human scoring system and histopathology. The concentration of LXA4 and the expression of ALOX15 (a key gene in LXA4 metabolic synthesis) in lesional skins were detected by ELISA and Western blot. Quantitative PCR and ELISA were conducted to detect the mRNA and secretion levels of inflammatory cytokines. The proportion of IL-17A-producing γδT cells in skin and skin draining cervical lymph nodes and helper (Th) 17 cells in spleens was evaluated by flow cytometry. Western blotting was used to analyze the expressions of p-STAT3 and TRAF6.
Results: The concentration of LXA4 and the expression of ALOX15 were decreased in IMQ-induced lesional skin. LXA4 significantly relieved psoriasis-like lesions in IMQ-induced mouse models. Furthermore, LXA4 decreased IMQ-induced systemic inflammation, including reduced the proportion of IL-17A-producing gdT cells in skin and skin draining cervical lymph nodes and Th17 cells in spleens, the secretion and expression of CCL20, IL-17A, IL-1β, and TNF-α in skin and serum. LXA4 markedly inhibited IMQ-induced expression of TRAF6 and p-STAT3.
Conclusion: LXA4 significantly ameliorates IMQ-induced psoriasis-like inflammation, and LXA4 can be used as a target for psoriasis treatment.
Keywords: lipoxin A4, psoriasiform dermatitis, imiquimod, γδT cells, Th17, inflammation
Introduction
Psoriasis is manifested by excessive hyperplasia of keratinocytes of the epidermis, accompanied by extensive infiltration of inflammatory cells into the epidermis and dermis. Typical histologic features include hyperkeratosis, parakeratosis, and formation of neutrophil microabscesses (also known as Munro microabscesses) in the areas of parakeratosis; the granular layer is significantly reduced or disappeared, the muscular layer above the dermal papilla becomes thinner, with elongated capillaries, and clinical manifestations of erythema, scaling, and thickening.1 The IL-23/IL-17 cytokine axis plays a central role in the development and progression of psoriasis.2,3 Biologics and small molecule drugs targeting IL-23/IL-17 cytokine axis have made great progress in the treatment of psoriasis in recent years.4
Imiquimod (IMQ) is a small molecule immunomodulator, an antagonist of Toll-like receptor (TLR) 7/8, and is clinically used to treat genital warts caused by human papillomavirus infection. IMQ activates monocytes, macrophages and dendritic cells to produce multiple cytokines including IFN-α, TNF-α, IL-2, IL-6, which mediate antiviral effects. IMQ application to mouse skin induces the similar clinical and histological features to human psoriasis and has been widely used to explore the pathogenesis of psoriasis.5,6 It was reported that IMQ-induce psoriasis-like dermatitis mirrors the cytokine profile of human psoriasis, especially the IL-17 cytokine production. IL-17 is a characteristic cytokine of T helper (Th) 17 cells and is a key factor in the pathogenesis of psoriasis.7,8 Recent studies have highlighted the novel role of IL-17-producing γδT cells in psoriasis.6,9–11
The inflammatory response is an important response mechanism in the body against injury and infection. Previous studies have found that endogenous pro-resolving mediators can be activated during the body’s defense response, which can negatively regulate inflammation by rapidly removing inflammatory cells and inflammatory factors, promote inflammation resolution, and prevent excessive inflammation from damaging the body.12 The development of endogenous agonists based on pro-resolving mediators has potential to treat inflammation-related diseases. Lipoxin A4 (LXA4), as a typical representative member of the pro-resolving mediator, is a metabolite for arachidonic acid and a 15-lipoxygenase (ALOX15)-dependent lipid mediator that naturally exists in our body. LXA4 is involved in regulating a variety of inflammatory signaling pathways and has significant anti-inflammatory and pro-regression effects. LXA4 inhibits the recruitment of neutrophils at the site of inflammation, promotes the chemotaxis of monocytes and the clearance of apoptotic neutrophils by macrophages, and inhibits the production of inflammatory mediators.13–15 In our previous research, Lipoxin A4 inhibits the production of inflammatory cytokines/chemokines of human epidermal keratinocytes (NHEK) and inhibits cell proliferation.16 However, the effect of LXA4 on psoriasis-like mouse model has not been investigated. In this study, we aim to investigate the effect and mechanism of LXA4 on inflammation of psoriasis-like skin lesions and T cell differentiation in IMQ-induced mouse models, and examine the potential molecular mechanisms underlying this effects.
Materials and Methods
Ethics Statement
All mice were maintained in microisolator cages, and housed in the animal colony at the animal center of Tongji Medical College of Huazhong University of Science and Technology, and were fed a standard laboratory diet and water. All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals proposed by National Institutes of Health (NIH Publication No.85–23, revised 1996). All animal protocols were approved by the Institutional Animal Care and Use Committee of the Institute of Model Animals of Tongji Medical College of Huazhong University of Science and Technology.
Animals and Treatment
BALB/c mice (male, ages between 6–8 weeks) were purchased from Beijing Huafukang Biological Technology Co., Ltd. Mice were removed hairs of approximately 2.5 cm×2.5 cm in the central area of the back, and were treated as follows (n = 6 per group): (1) Control group, vaseline cream (Fagron, Rotterdam, the Netherlands) was applied for 7 consecutive days; (2) IMQ group, 62.5 mg daily dose of IMQ (5%) cream (Mingxin Pharmaceuticals, Sichuan, China) was applied for 7 consecutive days. (3) IMQ + LXA4 group, mice received intraperitoneal (i.p.) injection of LXA4 (Cayman chemical, USA) dissolved in ethanol at a dose of 10 mg/kg/day followed by continuous treatment with IMQ for 7 days. Mice were sacrificed on day 8, serum and skin sample were collected for analysis.
Evaluation of the Severity of Skin Inflammation
The disease severity of psoriasis-like lesion in mice was assessed by using a scoring system based on the clinical Psoriasis Area and Severity Index (PASI).5 Precisely, it includes three indicators: erythema, scaling, and thickening, with independent scores ranging from 0 to 4 (0, none; 1, slight; 2, medium; 3, marked; 4, severe). The sum of the three indicators scores represents the severity of the disease (scale 0–12).
Histopathological Analysis
The sections of skin samples from the treated site were fixed in 4% paraformaldehyde and 5 mm-thick pathological sections were stained with hematoxylin and eosin (H&E), and pathological results were observed under a light microscope (Olympus, Tokyo, Japan).
ELISA
Mice serum was obtained and detected the content of inflammatory cytokines. ELISA was performed to detect the concentrations of IL-1β, IL-17A, TNF-α and CCL20 in the serum of mice and the concentration of LXA4 in skin lesions according to the manufacturer’s instructions (IL-1β and IL-17 ELISA kit: eBioscience, USA; TNF-α ELISA kit: R&D Systems, USA; CCL20 ELISA kit: Invitrogen, USA; LXA4 ELISA kit: Cayman, USA).
Real-Time Quantitative PCR
Total mRNA was extracted from the dorsal skin samples using TRIzol Reagent (Life Technologies Corp, USA). The SynScript III RT SuperMix Kit (Tsingke Biotechnology, China) was used according to the manufacturer’s instructions to synthesize cDNA from total RNA sample. The RT-PCR reactions were run on an ABI StepOnePlus™ (Applied Biosystems). The primers were showed as follows: IL-17A forward primer:5’-TTTAACTCCCTTGGCGCAAAA-3’, IL-17A reverse forward: 5’- CTTTCCCTCCGCATTGACAC-3’; TNF-α forward primer: 5’-GACGTGGAACTGGCAGAAGAG-3’, TNF-α reverse primer: 5’- TTGGTGGTTTGTGAGTGTGAG-3’; IL-1β forward primer: 5’-CTTCAGGCAGGCAGTATC-3’, IL-1β reverse primer: 5’- CAGCAGGTTATCATCATCATC-3’; CCL20 forward primer: 5’- AACTGGGTGAAAAGGGCTGT-3’, CCL20 reverse primer: 5’-GTCCAATTCCATCCCAAAAA-3’; GAPDH forward primer: 5’- AGGTCGGTGTGAACGGATTTG-3’, GAPDH reverse primer: 5’- TGTAGACCATGTAGTTGAGGTCA-3’. the comparative cycle threshold (CT) method (2−ΔΔCT method) used to calculate relative mRNA levels.
Flow Cytometry
Mouse skin tissues were cut into small pieces and digested for 2 hours in RPMI1640 medium containing 1% FBS, 1 mg/mL collagenase type 1A, 50 µg/mL DNaseI and 400 µg/mL Hyaluronidase (all from Sigma) at 37°C with rotation, and then filtered through 40 mm cell strainers. Cervical lymph nodes and spleens from mice were produced by gentle grinding with a 200-mesh iron sand mesh. Red blood cells were removed using ACK Lysis Buffer (Beyotime, China). For the analysis of IL-17A production, mouse antibodies including: Zombie Aqua™ dye-live/dead, CD3-PerCP/Cyanine5.5, γδTCR-PE, CD4-FITC, IL17A-PE/Cyanine7, were purchased from Biolegend. Samples were harvested with BD FACS Canto and analyzed with FlowJo software.
Western Blot Analysis
The skin tissues were lysed using a RIPA lysis buffer (Yeasen, China) according to the manufacturer’s instructions. The proteins were subjected to immunoblot analysis with indicated antibodies. The target antibodies included ALOX15 (Genetex, USA), TRAF6 (Cell Signaling Technologies, USA), p-STAT3 (Abcam, USA), b-actin (Cell Signaling Technologies, USA). The immunoreactive bands were visualized using UltraSignal ECL substrate (4Abio, China). Image J software was used to quantify protein band density.
Statistical Analysis
The data were shown as the means ± standard deviation, and analyzed with GraphPad Prism v. 8. 0 software. Student’s t-test or one-way analysis of variance (ANOVA) was used to determine the statistical significance of differences. P values less than 0.05 was considered statistically significant.
Results
LXA4 and ALOX15 Were Reduced in Psoriasis-Like Mouse Skin
The concentration of LXA4 in IMQ-induced psoriasis-like skin lesions in mice was significantly reduced compared to the control group (2.125 ± 1.262 ng/g vs 5.765 ± 1.729 ng/g) (Figure 1A). Also, the expression of ALOX15 protein was reduced in psoriasis-like skin lesions (Figure 1B and C).
LXA4 Alleviates the Pathological Features of Psoriasiform Dermatitis in Mice
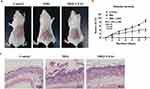
Based on the phenotypic manifestations of the dorsal skin of mice, the mice in the IMQ group showed erythema, scaling and thickened lesions, compared with the control group. After LXA4 pretreatment, the degree of skin lesions was significantly reduced, which showed less erythema, thinned scales, reduced thickening, smooth skin (Figure 2A). The scores of disease severity were consistent with the skin lesions. The score was no change with the increasing of treatment duration in the control group; The disease severity scores of the IMQ group were increased with processing time, while was significantly reduced after pretreatment with LAX4 (Figure 2B). The results of HE staining showed that IMQ induced the formation of skin lesions characteristic of psoriasis in mice, which showed epidermal hyperplasia, formation of munro microabscesses and the infiltration of inflammatory cells into the dermis layer. LXA4 significantly alleviated IMQ-induced histological injuries in the IMQ + LXA4 group (Figure 2C). These results suggest that LXA4 treatment can improve psoriasis-like skin lesions in IMQ-induced mouse models.
LXA4 Suppressed IL-17A, IL-1β, TNF-α and CCL20 Production in the Skin Lesions and Serum
The mechanism of IMQ-induced psoriasis-like dermatitis mouse model has been shown to be similar to the pathogenesis of human psoriasis, relying on the IL-17/IL-23 inflammatory axis, while LXA4 significantly alleviates skin inflammation, thus we infer that LXA4 may suppress inflammation by reducing the concentration of relevant inflammatory factors. We found that the concentrations of IL-17A, TNF-α, IL-1β and CCL20 in serum (Figure 3A–D) and its mRNA expressions in skin lesions (Figure 3E–H) were decreased significantly in pretreated with LXA4 prior to IMQ application (LXA4 + IMQ group) as compared to the IMQ group.
LXA4 Reduces the Proportion of IL-17A-Producing γδT Cells and Th17 Cells in IMQ-Induced Psoriasis Mouse Models
γδT and Th17 cells are a key driver of psoriasis pathogenesis, thus we suspect that LXA4 alleviates psoriasis-like skin inflammation by inhibiting the proportion of IL-17A-producing cells in IMQ-induced mouse model. Using flow cytometry to detect the proportion of IL-17A-producing γδT cells in skin and skin draining cervical lymph nodes and Th17 cells in spleens. The gating strategy for the skin tissues (Supplementary Figure 1A) and LNs (Supplementary Figure 1B) and spleens (Supplementary Figure 1C) is shown in Supplementary Figure 1. It was found that the IL-17A-producing gdT and Th17 cells of IMQ group showed a significant increase compared to the control group (Figure 3I-K). Importantly, LXA4 suppressed the percentage of the IL-17A-producing γδT and Th17 cells in IMQ-induced psoriatic mouse. Taken together, LXA4 decreased IMQ-induced systemic inflammation by reducing the proportion of IL-17A-producing γδT cells in skin and skin draining cervical lymph nodes and Th17 cells in spleens, the secretion and expression of CCL20, IL-17A, IL-1β, and TNF-α in skin and serum.
LXA4 Inhibits TRAF6 Expression and STAT3 Phosphorylation in Psoriasis-Like Mouse Skin Lesions
Previous studies have reported that TRAF6 is involved in TLR7/8-mediated activation of inflammatory signaling pathways and the phosphorylation of STAT3 (p-STAT3), which is crucial in the regulation of Th17 cell differentiation. Therefore, we next verified whether TRAF6 and p-STAT3 are involved in LXA4-mediated remission of IMQ-induced psoriasis like lesions. As shown in Figure 4A and B, the expression of TRAF6 and p-STAT3 proteins were increased in psoriasis-like skin lesions of IMQ-induced mouse models, and were reduced by LXA4 pretreatment.
Discussion
Previous studies have found that LXA4 can extensively inhibit the inflammatory response of a variety of diseases. In animal experiments such as acute lung injury, atherosclerosis, cerebral ischemia-reperfusion injury and cognitive impairment in the elderly, LXA4 demonstrated significant anti-inflammatory protection.17–20 ALOX15 also has a powerful anti-inflammatory and pro-regressive effect in vitro and vivo, and this effect is related to its mediated pro-inflammatory regression lipids production.21,22 Moreover, LXA4 binds with its receptors lipoxin A4 receptor (ALXR) and aromatic hydrocarbon receptor (AhR) to exerts its anti-inflammatory and pro-regressive biological effects,23 and immunotargeted drugs targeting AhR receptor have been approved for psoriasis treatment.24 We found that the content of LXA4 and its key synthetic molecule ALOX15 in the IMQ group was significantly lower than that in the control group, indicating that IMQ-induced psoriasis-like dermatitis may be caused by insufficient LXA4 content in vivo. In IMQ-induced psoriasis-like mouse model prior to pretreatment with LXA4 by intraperitoneal injection, the improvement of morphological and histopathological characteristics confirmed that LXA4 can alleviate the psoriasis-like lesions.
In psoriasis, TNF-α plays a key role in the pathogenesis of psoriasis by enhancing the function of T lymphocytes, activating myeloid dendritic cells (mDCs) to produce IL-12/IL-23 and stimulating keratinocytes to release a variety of inflammatory factors.25 IL-17A acts together with other inflammatory factors on keratinocytes or innate immune cells, inducing the production of various proinflammatory cytokines, chemokines and antimicrobial peptides/proteins (AMPs), which further recruit and activate immune cells to amplify skin inflammation, thus forming a positive feedback inflammatory circuit. Therapeutics that specifically target IL-17 is approved for the clinical treatment of psoriasis and have shown excellent efficacy.26 IL-1β is critical in mediating the differentiation and activation of IL-17-producing T cells. IL-1β stimulates mouse and human epidermal keratinocytes to secrete chemokines in psoriasis lesion skin. In addition, the expression of CCL20 is significantly increased in psoriatic lesions.27 CCL20 can chemotaxis more neutrophils or IL-17‒producing cells to the site of skin inflammation.28 Our study clarified that the expression and concentration of the above key inflammatory factors and chemokines was significantly down-regulated in skin lesions and serum of psoriasis-like mice after pretreatment with LXA4.
Th17 cells, as dominant cells in the pathogenesis of psoriasis, have been shown to be involved in mediating IMQ-induced mouse models of psoriasis. Recent studies have shown that dermal γδ T cells are mainly IL-17-producing cells in IMQ-induced psoriasis mouse model. The IL-17-producing γδT cells expand in the draining lymph nodes through the blood.29,30 In our study, a greater number of IL-17-producing γδ T cells were identified in IMQ-induced mouse skin and skin draining cervical lymph nodes, and the percentage of Th17 cells were identified in IMQ-induced mouse spleens, while LXA4 inhibits the proportion of IL-17-producing γδT and Th17 cells and its inflammation response. These data demonstrate that LXA4 ameliorates IMQ-induced psoriasis-like dermatitis by decreasing IMQ-induced systemic inflammation. This may be related to administered intraperitoneally of LAX4 treatment in mouse models, which inhibits the progression of skin lesions by modulating systemic immunity.
TRAF6 is involved in TLR7/8-mediated activation of inflammatory signaling pathways, and LXA4 binds to its receptor AHR can mediate the degradation of TRAF6 through a proteasome-dependent pathway.31 Consistently, our previous study suggested that LXA4 inhibits the production of TNF-α and IL-1β induced by LPS in NHEKs via downregulating TRAF6 expression.32 A large number of previous studies confirmed that the STAT3 signaling pathway is upregulated in psoriasis lesions and IMQ-induced psoriasis-like lesions, and plays an important role in mediating inflammation production and TH17 cell differentiation.33 We found that LXA4 downregulated the expression of TRAF6 and the phosphorylation levels of STAT3 in psoriasis-like lesions, thereby inhibiting imiquimod-mediated inflammatory signaling and the proportion of γδT cells in skin. The above results suggest that the alleviation of psoriasis lesions by LXA4 may be achieved by inhibiting TRAF6 and p-STAT3, but the regulatory mechanisms of inhibiting IL-17-producing γδT and Th7 cells differentiation deserve further exploration.
Conclusion
In summary, our present study manifested that LXA4 alleviates IMQ-induced psoriasis-like dermatitis by decreasing the systemic inflammation, including reduces the proportion of IL-17A-producing γδT cells in skin and skin draining cervical lymph nodes and the proportion of Th17 cells in spleens, and downregulates the expression of TRAF6 and p-STAT3 in psoriasis-like lesions. Moreover, the pro-resolving mediators, including LXA4, are synthesized and play a role only in the pathological process of inflammation, have no harmful side effects on the body.34 Thus, LXA4 may be potential used as a target for psoriasis treatment.
Data Sharing Statement
All data are available upon request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by National Natural Science Foundation of China (No 81974474, 82173423, 32200150, 82203926), Shenzhen Nanshan District Science and Technology Project / Key Project, China (No.2019003).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi:10.1001/jama.2020.4006
2. Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–1350. doi:10.1038/jid.2009.59
3. Blauvelt A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J Invest Dermatol. 2008;128(5):1064–1067. doi:10.1038/jid.2008.85
4. Griffiths CEMC, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi:10.1016/S0140-6736(20)32549-6
5. van der Fits L, Mourits S, Voerman JSA, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. doi:10.4049/jimmunol.0802999
6. Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596–610. doi:10.1016/j.immuni.2011.08.001
7. Fujiyama T, Ito T, Umayahara T, et al. Topical application of a vitamin D3 analogue and corticosteroid to psoriasis plaques decreases skin infiltration of TH17 cells and their ex vivo expansion. J Allergy Clin Immunol. 2016;138(2):517–528. doi:10.1016/j.jaci.2016.03.048
8. Zhao J, Di T, Wang Y, et al. Paeoniflorin inhibits imiquimod-induced psoriasis in mice by regulating Th17 cell response and cytokine secretion. Eur J Pharmacol. 2016;772:131–143. doi:10.1016/j.ejphar.2015.12.040
9. Shibata S, Tada Y, Hau CS, et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat Commun. 2015;6:7687. doi:10.1038/ncomms8687
10. Ramírez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci U S A. 2015;112(26):8046–8051. doi:10.1073/pnas.1508990112
11. Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol. 2011;187(10):5026–5031. doi:10.4049/jimmunol.1101817
12. Gilbert NC, Newcomer ME, Werz O. Untangling the web of 5-lipoxygenase-derived products from a molecular and structural perspective: the battle between pro- and anti-inflammatory lipid mediators. Biochem Pharmacol. 2021;193:114759. doi:10.1016/j.bcp.2021.114759
13. Mei HX, Ye Y, Xu HR, et al. LXA4 inhibits lipopolysaccharide-induced inflammatory cell accumulation by resident macrophages in mice. J Inflamm Res. 2021;14:1375–1385. doi:10.2147/JIR.S301292
14. Pan WH, Hu X, Chen B, Xu QC, Mei HX. The effect and mechanism of lipoxin A4 on neutrophil function in LPS-induced lung injury. Inflammation. 2022;45(5):1950–1967. doi:10.1007/s10753-022-01666-5
15. Chen Y, Zheng Y, Xin L, et al. 15-epi-lipoxin A4 inhibits TNF-α-induced tissue factor expression via the PI3K/AKT/NF-κB axis in human umbilical vein endothelial cells. Biomed Pharmacother. 2019;117:109099. doi:10.1016/j.biopha.2019.109099
16. Hu F, Liu X, Wang X, et al. Lipoxin A4 inhibits proliferation and inflammatory cytokine/chemokine production of human epidermal keratinocytes associated with the ERK1/2 and NF-κB pathways. J Dermatol Sci. 2015;78(3):181–188. doi:10.1016/j.jdermsci.2015.03.009
17. Sekheri M, El Kebir D, Edner N, Filep JG. 15-Epi-LXA4 and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc Natl Acad Sci U S A. 2020;117(14):7971–7980. doi:10.1073/pnas.1920193117
18. Petri MH, Laguna-Fernandez A, Arnardottir H, et al. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E−/− mice. Br J Pharmacol. 2017;174(22):4043–4054. doi:10.1111/bph.13707
19. Guo Y, Dong C, Tang J, et al. Lipoxin A4 alleviates cerebral ischemia-reperfusion injury through up-regulating Nrf2. J Neurosurg Sci. 2018;62(2):225–226. doi:10.23736/S0390-5616.17.04164-9
20. Wang X, Miao Z, Xu X, Schultzberg M, Zhao Y. Reduced levels of plasma lipoxin A4 are associated with post-stroke cognitive impairment. J Alzheimers Dis. 2021;79(2):607–613. doi:10.3233/JAD-201050
21. Queck A, Fink AF, Sirait-Fischer E, et al. Alox12/15 deficiency exacerbates, while lipoxin A4 ameliorates hepatic inflammation in murine alcoholic hepatitis. Front Immunol. 2020;11:1447. doi:10.3389/fimmu.2020.01447
22. Kim SN, Akindehin S, Kwon HJ, et al. Anti-inflammatory role of 15-lipoxygenase contributes to the maintenance of skin integrity in mice. Sci Rep. 2018;8(1):8856. doi:10.1038/s41598-018-27221-7
23. Wu RF, Huang ZX, Ran J, et al. Lipoxin A4 suppresses estrogen-induced epithelial-mesenchymal transition via ALXR-dependent manner in endometriosis. Reprod Sci. 2018;25(4):566–578. doi:10.1177/1933719117718271
24. Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinar of cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87(4):800–806. doi:10.1016/j.jaad.2022.06.1171
25. Campanati A, Paolinelli M, Diotallevi F, Martin E, Molinelli E, Offidani A. Pharmacodynamics of TNF-α inhibitors for the treatment of psoriasis. Expert Opin Drug Metab Toxicol. 2019;15(11):913–925. doi:10.1080/17425255.2019.1681969
26. Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397(10275):754–766. doi:10.1016/S0140-6736(21)00184-7
27. Cai Y, Xue F, Quan C, et al. A critical role of the IL-1β-IL-1R signaling pathway in skin inflammation and psoriasis pathogenesis. J Invest Dermatol. 2019;139(1):146–156. doi:10.1016/j.jid.2018.07.025
28. Wang A, Bai Y. Dendritic cells: the driver of psoriasis. J Dermatol. 2020;47(2):104–113. doi:10.1111/1346-8138.15184
29. Gray EE, Suzuki K, Cyster JG. Cutting edge: identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186(11):6091–6095. doi:10.4049/jimmunol.1100427
30. Liu N, Qin H, Cai Y, et al. Dynamic trafficking patterns of IL-17-producing γδ T cells are linked to the recurrence of skin inflammation in psoriasis-like dermatitis. EBioMedicine. 2022;82:104136. doi:10.1016/j.ebiom.2022.104136
31. Santos MRG, Queiroz-Junior CM, Madeira MFM, Machado FS. Suppressors of cytokine signaling (SOCS) proteins in inflammatory bone disorders. Bone. 2020;140:115538. doi:10.1016/j.bone.2020.115538
32. Hu F, Feng AP, Liu XX, et al. Lipoxin A4 inhibits lipopolysaccharide-induced production of inflammatory cytokines in keratinocytes by up-regulating SOCS2 and down-regulating TRAF6. J Huazhong Univ Sci Technolog Med Sci. 2015;35(3):426–431. doi:10.1007/s11596-015-1448-8
33. Zhang M, Li N, Cai R, et al. Rosmarinic acid protects mice from imiquimod induced psoriasis-like skin lesions by inhibiting the IL-23/Th17 axis via regulating Jak2/Stat3 signaling pathway. Phytother Res. 2021;35(8):4526–4537. doi:10.1002/ptr.7155
34. Fu T, Mohan M, Brennan EP, et al. Therapeutic potential of lipoxin A4 in chronic inflammation: focus on cardiometabolic disease. ACS Pharmacol Transl Sci. 2020;3(1):43–55. doi:10.1021/acsptsci.9b00097
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.