Back to Journals » Journal of Inflammation Research » Volume 15
Lipocalin 2 Participates in the Epidermal Differentiation and Inflammatory Processes of Psoriasis
Received 14 January 2022
Accepted for publication 22 March 2022
Published 31 March 2022 Volume 2022:15 Pages 2157—2166
DOI https://doi.org/10.2147/JIR.S358492
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Kaixuan Ren, Yumin Xia
Department of Dermatology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710004, People’s Republic of China
Correspondence: Yumin Xia, Department of Dermatology, The Second Affiliated Hospital of Xi’an Jiaotong University, 157 Xiwu Road, Xi’an, 710004, People’s Republic of China, Tel/Fax +86-29-87679969, Email [email protected]
Abstract: As a multifunctional cytokine, lipocalin 2 is weakly expressed in skin and serum under normal conditions. However, it is over-expressed by neutrophils and keratinocytes in the skin lesions and sera in several skin diseases. Recent studies demonstrated that lipocalin 2 participates in the pathogenesis of psoriasis by exerting versatile effects on skin resident cells and infiltrating immune cells. Lipocalin 2 inhibits the synthesis of keratin, involucrin, and loricrin in keratinocytes, leading to epidermal parakeratosis via the Tcf7l1-lipocalin 2 signaling axis. It also recruits inflammatory cells such as T cells and neutrophils into skin lesions via the IL-23/IL17, p38-MAPK, and ERK-1/2 signaling pathways. Additionally, lipocalin 2 and other cytokines such as IL-17 have the synergetic effects on skin cells. The neutralization of lipocalin 2 or relevant cytokines can alleviate psoriasis, verifying that lipocalin 2 is an effective interfering target for psoriasis. In this review, we summarize the roles of lipocalin 2 in the processes of psoriatic inflammation and the promising therapeutic strategies based on lipocalin 2-related molecules.
Keywords: lipocalin 2, psoriasis, keratinocyte, inflammation, differentiation
Introduction
Psoriasis is a common, chronic, immune-mediated dermatosis affected by genetic and environmental factors that can be triggered by injuries, infections, or mechanical stimulation.1 The fundamental mechanism of psoriasis consists of three main aspects: immune and inflammatory cell infiltration, vascular proliferation and skin hyperplasia,2 and abnormal differentiation of keratinocytes.3,4 In terms of immune mediation, activated CD4+, CD8+ T lymphocytes, CD3 T cells, macrophages, mast cells,5 keratinocytes, dendritic cells, and neutrophils lead to innate and adaptive immune responses by the release of cytokines and enzymes in psoriasis.6–8 During the inflammation process, interleukin (IL)-23 and IL-12, secreted by dendritic cells, activate Th1 and Th17 cells to produce cytokines such as IL-17, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α. This leads to macrophage migration, keratinocyte proliferation,9 and neovascularization, subsequently resulting in the appearance of psoriatic scaly plaques.
Lipocalin 2 is a 25-kDa glycoprotein coded in a gene located at chromosome 9q34.11, and it is composed of an N-linked low-molecular weight polysaccharide and a protein backbone. With this kind of structure, lipocalin 2 can bind with three receptors including megalin,10 24p3R, and melanocortin-4. It possesses a lipophilic barrel-shaped tertiary structure and binds with matrix metalloproteinase (MMP)-9 in neutrophils for the inhibition of proteolysis of MMP-9.11,12 In addition to secretion by granulocytes, lipocalin 2 is mainly secreted by a variety of cells, including keratinocytes,13 adipocytes,14 CD4+ T cells,15 macrophages,16 and dendritic cells.17 It is also produced in different tissues such as the liver, lungs, and kidneys.18 Lipocalin 2 is widely expressed, although it was initially considered as an antimicrobial peptide that was increased in the plasma and tissues of patients with infectious diseases. In recent years, studies have shown that lipocalin 2 levels are also elevated in several inflammatory skin diseases such as psoriasis,19,20 eczema, and skin wounds,21 and its overexpression has even been detected in neoplasms for its potential role in cell proliferation in diseases such as keratoacanthoma and squamous cell carcinoma.22,23 Thus, lipocalin 2 functions in a versatile manner in antibiosis, inflammation, and cell differentiation or dysfunction.
Several studies have shown that lipocalin 2 levels are elevated in the skin lesions and serum of patients with psoriasis, and lipocalin 2 has been proved to be effective in regulating inflammation and cell proliferation in psoriasis.24 There has been a large number of articles highlighting lipocalin 2ʹs versatile functions, such as being an indicator for predicting the prognosis and mortality of neoplasms and psoriasis. However, there are few convincing pieces of evidence that highlight the diagnostic and treatment value of lipocalin 2. The relationship between lipocalin 2 and the onset or development of psoriasis remains unclear and should be elucidated. Here, we review the current findings regarding the effect of lipocalin 2 on the formation and development of psoriasis and suggest novel therapeutic strategies.
Lipocalin 2 Functions in Both Innate and Adaptive Immune Responses
As the body’s first line of defense, innate immunity plays a central role in antibacterial action.25 Lipocalin 2 plays a non-negligible role in the innate immunity process not only by inducing the infiltration, migration, and adhesion of inflammatory cells such as neutrophils into skin lesions, but also by directly binding with iron-containing siderophores of bacteria to prevent their uptake of iron and limiting bacterial growth.26 The depletion of lipocalin 2 in mice results in susceptibility to bacterial infection due to its function of maintaining microbiotic eubiosis and provoking inflammation.27,28 IL-10 plays an important role in the anti-inflammatory response. Lcn2 (coding lipocalin 2) deficiency can cause a significant decrease in IL-10 expression in cultured macrophages. The expression of IL-6, IL-1β, TNF-α, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein 2, reactive oxygen species, and nitric oxide decreases in Lcn2-deficient mice.29 Therefore, a lack of lipocalin 2 will impair the antibacterial effect of macrophages and neutrophils. In the antiviral response, lipocalin 2 inhibits the proliferation of T lymphocytes and exerts immune effects by regulating the antigen presentation activity of dendritic cells. Moreover, the expression of inflammatory cytokines such as IL-12, IFN-γ, interferon-inducible protein (IP)-10, IL-6, MCP-1, B cell activating factor, and IFN-α increased, and in lipocalin 2-deficient mice with influenza, high mortality resulted.30 Therefore, lipocalin 2 plays a significant role in balancing the proliferation of immune cells and the secretion of immune cytokines in the body’s antiviral effect. Lipocalin 2 also modulates adaptive immunity either by contacting with T cells as a self-antigen or by interacting with the IL-23/IL-17 axis.31 Obviously, the role of lipocalin 2 in innate and adaptive immune responses has not yet been clearly elucidated, and its interactions with various immune cells and inflammatory factors require further exploration.
Lipocalin 2 Regulates Epidermal Differentiation
In addition to neutrophils, keratinocytes are the second main source of lipocalin 2. Evidence shows that lipocalin 2 participates in keratinocyte differentiation, and the appearance of overexpressed lipocalin 2 leads to skin disorders such as epidermal parakeratosis.22 During skin development and cell differentiation in embryos, lipocalin 2 is initially weakly produced in the epidermis, then strongly expressed in granular and horny layers, and finally accumulates in the follicular epithelium. In contrast to lipocalin 2, filaggrin, as an indicator of cell differentiation, translocated from the perifollicular epithelium to the epidermis and was inhibited in psoriatic hyperplasia lesions where lipocalin 2 was overexpressed.32 Thus, lipocalin 2 potentially inhibits the expression of filaggrin and induces dysfunction in the differentiation and proliferation of keratinocytes.
As a lipocalin 2 inducer that always predicts a poor differentiation condition of keratinocytes,33 transcription factor 7-like 1 (Tcf7l1) facilitates the expression of lipocalin 2 in wounded skin repair by promoting the motility and differentiation of keratinocytes.21 In vitro, human foreskin keratinocytes infected with papillomavirus and SCC-13 cells exhibited upregulated Tcf7l1 and lipocalin 2, with downregulated expression of involucrin and loricrin.34 Moreover, inhibition of Tcf7l1 can eliminate the modulatory effect of lipocalin 2 on the keratinocyte differentiation process.35 This result proves that Tcf7l1 and lipocalin 2 confer a synergistic effect on the modulation of keratinocyte proliferation. In addition, lipocalin 2 levels increased in squamous cell carcinoma and keratoacanthoma, whereas they decreased in basal cell carcinoma, which indicates that lipocalin 2 contributes to cell terminal differentiation and keratin synthesis.22 Furthermore, lipocalin 2 dysregulates cell differentiation into a more immature phenotype by inhibiting the nuclear factor κB pathway,36 upregulating p-Met, and phosphorylating p-focal adhesion kinase (FAK) to activate the Met/FAK cascade, and finally downregulating the epidermal marker E-cadherin while upregulating the mesenchymal marker.37 Thus, these studies support the vital role of lipocalin 2 in regulating the differentiation and proliferation of keratinocytes.
Lipocalin 2 Mediates the Psoriatic Inflammatory Responses
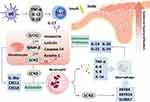
Several studies have elucidated the potential mechanisms of lipocalin 2 in the pathogenesis of psoriasis, which is related to the functions of manifold cells in a differential manner, as shown in Figure 1. One of them puts forward that psoriasis, as an inflammatory skin disorder, is mediated by the IL-23/IL-17 pathway. Lipocalin 2 is considered to participate in the genesis of psoriasis by modulating neutrophil functions and augmenting the Th17 cell response.13,38 It has been determined that lipocalin 2 is a chemoattractant of neutrophils, which are designated to be activated by lipocalin 2 via one of the receptors on it, 24p3R.39 Thereafter, neutrophils produced IL-6, IL-8, TNF-α, and IL-1α via the extracellular signal-regulated kinase (ERK)-1/2 and p38-mitogen-activated protein kinase (MAPK) signaling pathway.13 Moreover, keratinocytes play an indispensable role in producing psoriatic cytokines such as IL-17A, IL-22 and TNF-α, which enhance the formation and progression of psoriasis.13 Produced by CD4+ T cells and strongly associated with inflammation, IL-17 is transcriptionally stimulated by lipocalin 2,40 stimulates the expression and secretion of lipocalin 2, and both of them have the synergetic effect of activating Th17 cells, which thus leads to the accumulation and activation of macrophages.41,42 Additionally, injecting lipocalin 2 can stimulate dendritic cells to secrete IL-23p19, IL-12p40, and TNF-α. Neutralizing lipocalin 2 alleviates the hyperplasia of the psoriatic epidermis and decreases the expression of IL-17 and IL-23, which consequently decreases the infiltration of inflammatory cells into skin lesions. Moreover, lipocalin 2 also induced the secretion of multiple antimicrobial peptides or chemokines such as defensin beta (DEFB) 4, DEFB14, S100 calcium binding protein A7 (S100A7), and lipocalin 2 in imiquimod-induced psoriasis-like lesions accelerated the inflammatory process.38
In addition to inducing inflammation, previous research deduced that lipocalin 2 modulates cell differentiation and proliferation via the Tcf7l1-lipocalin 2 axis. Tcf7l1 is a pivotal protein that determines cell development and proliferation by regulating Wnt-β-catenin and c-Myc signaling in different cells.43–45 Tcf7l1 enhances the expression of MMP-2 from keratinocytes and then exerts its role in inducing lipocalin 2 upregulation in a circular feedback manner.34 Hence, the Tcf7l1-lipocalin 2 axis is a potential pathogenesis pathway associated with the abnormal proliferation and differentiation of keratinocytes in psoriasis.
Approximately 30% of psoriasis patients are affected by psoriatic arthritis (PsA). In recent years, there has been some evidence that lipocalin 2 is correlated with PsA.13 In PsA, neutrophils are indispensable for secreting lipocalin 2, which modulates Th17 cells secreting IL-17 and IL-22 and then leads to bone remodeling and the inflammation reaction.46–48 In addition, the IL-23/IL-17 axis stimulates the expression of chemokine (C-X-C motif) ligand (CXCL) 1, CXCL2, CXCL5, and CXCL8/IL-8 in the PsA synovial membrane, contributing to neutrophil infiltration and recruitment,49,50 as we have demonstrated in the pathogenesis of psoriasis. Hence, we deduced that lipocalin 2 also assumed an important role in the pathogenesis of PsA via the IL-23/IL-17 axis in order to induce inflammation and cell proliferation. Palmoplantar pustular psoriasis differs from psoriasis vulgaris in that it is derived from the eccrine gland.51 Lipocalin 2, which is also expressed in sebaceous gland cells,52 is thought to be induced by IL-1β in keratinocytes, which is indicated by serum lipocalin 2 levels that are positively related to the pustule score and Dermatology life quality index.39 As a consequence, lipocalin 2 may act as an indicator of severity and prognosis in PsA and palmoplantar pustular psoriasis.
Diagnostic and Therapeutic Strategies Based on Lipocalin 2 Signals
Psoriasis affects almost 1% of the worldwide population, and the diagnosis of psoriasis is primarily clinical, but the evaluation methods for prognosis require additional development.48 In the past, serum c-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels have been used to evaluate the treatment efficiency of psoriasis, but they have their own limitation of accuracy.53 Recently, lipocalin 2 has been widely investigated as a promising biomarker for evaluating disease status or even a potential target for the treatment of psoriasis. For example, the serum lipocalin 2 level is positively related to IL-17, which is one of the targets for treating psoriasis, but measuring it with the current technology results in inaccuracy. Therefore, researchers proposed that serum lipocalin 2 is the best proxy of serum IL-17, and this may assist in guiding therapy and evaluating prognosis.53 High levels of lipocalin 2 have been widely detected in skin, plasma, saliva, and other body fluids of patients and animals with psoriasis.13,39,54–56 Some researchers reported that, in psoriasis patients, serum lipocalin 2 levels were significantly higher compared to those in healthy controls.18,57 However, El-Hadidi et al did not show significant differences in lipocalin 2 sera levels compared to controls.58 Except for serum, lipocalin 2 was diminished in psoriasis patients’ saliva in contrast to healthy controls.59 Moreover, in psoriatic skin lesions, lipocalin 2 has been widely detected highly expressed in the granular layer and hair follicles of psoriatic lesions compare to healthy controls.36,60–63 In contrast to the level of lipocalin 2 in skin, the serum levels of lipocalin 2 in psoriasis patients are not always consistent with the high level in skin.18 For example, a study concluded that there was no significant difference in patient serum lipocalin 2 levels after treatment.57 These differences can be attributed to several factors. Firstly, lipocalin 2 is derived from manifold tissues, such as liver, lungs, and kidneys, adipocytes, macrophages, and epithelial cells. Moreover, the diversity of disease durations and severities, past medical history including complications and therapies, may also explain the contradictory results. Thus, lipocalin 2 may not fully reflect the severity degree of psoriasis.
Studies investigated the relationship between lipocalin 2 and the psoriasis area and severity index (PASI), pustule score, and dermatology life quality index, which are shown in Table 1.38,39,57,64,65 We noticed that there are conflicting results regarding the correlation between lipocalin 2 and PASI score. Romani et al reported a positive correlation between baseline serum lipocalin 2 and PASI score.57 Otherwise, in other studies, no correlation was found in several studies between serum or tissue lipocalin 2 and the disease duration, extent, treatment efficiency and PASI score.18,57,61,66 Moreover, as an adipokine, lipocalin 2 has been investigated in psoriasis patients with atherosclerosis, metabolic syndrome, obesity, or diabetes mellitus.57,58,62 While lipocalin 2 is an adipokine, researchers found no correlation between sera lipocalin 2 and Body Mass Index, lipid profile (high density lipoprotein-cholesterol (HDL-C), triglyceride and cholesterol) and a negative correlation between serum lipocalin 2, low density lipoprotein-cholesterol (LDL-C) and plasma glucose in psoriasis patients or controls.18 Moreover, a study also reported no correlation between lipocalin 2 and BMI in patients with metabolic syndrome.63 However, there still have different results reported the positive correlation between them.58 These arguments may indicate that tissue lipocalin 2 level is not related to the severity of the disease, but it may reflect a warning signal of excessive proliferation or metabolic disorders.
 |
Table 1 Predictive Values Involving Lipocalin 2 in Psoriasis |
The therapeutic options for psoriasis consist of local therapy, systematic therapy, phototherapy, and biologic therapy. New treatment methods have been consistently explored and applied, with the aim of finding a final resolution for psoriasis, and they are shown in Table 2. Lipocalin 2 is a potential target for psoriasis treatment, and it mediates Tcf7l1 function. Thus, blocking lipocalin 2 can inhibit the Tcf7l1-lipocalin 2 pathway and subsequently terminate the proliferation of keratinocytes.34 Moreover, the activation and infiltration of neutrophils also determine the progression of psoriasis. Because lipocalin 2 induces neutrophil chemotaxis and activation via the p38-MAPK and ERK-1/2 signaling pathways, inhibiting lipocalin 2 is also an effective method to modulate inflammation in psoriatic lesions.13 For example, it was recognized that phototherapy with narrowband ultraviolet B (NBUVB) exerts its therapeutic function by decreasing the expression of lipocalin 2 in psoriatic lesions.24 Recently, it was found that methyl 4-(adenin-9-yl)-2-hydroxybutanoate (DZ2002) inhibits psoriatic inflammation by regulating the DNA methylation of the lipocalin 2 promoter, which further confirmed the therapeutic role of lipocalin 2 in psoriasis.67 Moreover, it has been proved that neutralizing Tcf7l1 attenuates keratinocyte apoptosis and alleviates the effect of neutrophil aggregation and keratinocyte proliferation by decreasing lipocalin 2 and other downstream cytokines.34 Hence, lipocalin 2 is a promising target for the treatment of psoriasis.
 |
Table 2 Therapies Targeting Lipocalin 2 or Downstream Inflammatory Cytokines |
In biotherapy, mAbs such as anti-IL-17, IL-23, and TNF-α display high efficiency in psoriasis treatment.3 Because there are several mAbs that distinctively inhibit different key cytokines, it is often unclear as to which one is the most optimal for treating a specific condition. The serum TNF-α level is positively associated with lipocalin 2, which suggests that patients with high serum lipocalin 2 levels are sensitive to anti-TNF-α mAb treatment.52 Lipocalin 2 is also involved in the IL-23/IL-17 axis and plays an indispensable role.68 Blocking IL-23 or IL-17 could also decrease the expression of lipocalin 2 and terminate its function in aggravating psoriatic diseases.
Beside psoriasis, lipocalin 2 is also a survival factor that decreases invasion and metastasis and promotes the oncogenesis process. Lipocalin 2 infiltrates into differentiated lesions and induces cell apoptosis by facilitating the lipocalin 2-mediated export of iron and reduction of ferritin.69 In pityriasis rubra, lipocalin 2 is defined as a parakeratosis indicator that is absent in lichen planus, acute contact eczema, and basaloma.32 It also has been considered as a biomarker to determine the inflammatory activity in acne vulgaris and acne inversa.52,70 Furthermore, lipocalin 2 delivers its function in acne treatment with isotretinoin via facilitating the apoptosis of sebaceous gland cells.40 Lipocalin 2 also highly expressed in wound and improve healing by promoting neutrophils migration and angiogenesis in wound lesion.21,71,72 Obviously, lipocalin 2 participates in different skin diseases and exerts manifold functions, and they are shown in Table 3. Lipocalin 2 may become a promising treatment target of various skin diseases.
 |
Table 3 Features of Lipocalin 2 in Other Skin Diseases |
Conclusions and Prospective Views
Lipocalin 2 is highly expressed in the skin lesions and blood of patients with psoriasis and is closely related to the severity of psoriasis. It not only stimulates various immune cells involved in the innate and adaptive immune responses to provoke inflammation via the IL-23/IL-27, p38-MAPK, and ERK-1/2 signaling pathways, but it also inhibits keratinocyte differentiation, thus leading to skin parakeratosis via the Tcf7l1-lipocalin 2 signaling axis. Moreover, lipocalin 2 could be a promising biomarker for predicting psoriasis severity and prognosis, and lipocalin 2 mAb could be an effect target for treating psoriasis. Blocking lipocalin 2 and its relevant signaling pathway may relieve the symptoms of psoriasis. Nevertheless, the pathogenesis of psoriasis is complicated, and inhibiting a single pathway is prone to fail, while extensively blocking several pathways may lead to an enormous cascade of side effects. Consequently, the functions of lipocalin 2 and the curative effects of anti-lipocalin 2 reagents require further investigation so that they can be developed into novel therapies for patients with psoriasis.
Acknowledgments
This study was supported by the Key Research and Development Plan of Shaanxi Province (Project No. 2020ZDLSF02-08).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi:10.1016/S0140-6736(14)D61909-7
2. Cheng H, Xu M, Liu X, Zou X, Zhan N, Xia Y. TWEAK/Fn14 activation induces keratinocyte proliferation under psoriatic inflammation. Exp Dermatol. 2016;25(1):32–37. doi:10.1111/exd.12820
3. Rapalli VK, Waghule T, Gorantla S, Dubey SK, Saha RN, Singhvi G. Psoriasis: pathological mechanisms, current pharmacological therapies, and emerging drug delivery systems. Drug Discov Today. 2020;25(12):2212–2226. doi:10.1016/j.drudis.2020.09.023
4. Liu W, Zhang D, Luo M, et al. TNF-like weak inducer of apoptosis promotes angiogenesis, thereby exacerbating cutaneous psoriatic disease. J Invest Dermatol. 2021;141(5):1356–1360. doi:10.1016/j.jid.2020.09.023
5. Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9(9):504–513.
6. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–653. doi:10.1016/j.jaci.2017.07.004
7. Skrzeczynska-Moncznik J, Wlodarczyk A, Zabieglo K, et al. Secretory leukocyte proteinase inhibitor-competent DNA deposits are potent stimulators of plasmacytoid dendritic cells: implication for psoriasis. J Immunol. 2012;189(4):1611–1617. doi:10.4049/jimmunol.1103293
8. Wang H, Wang S, Li L, et al. Involvement of the cytokine TWEAK in the pathogenesis of psoriasis vulgaris, pustular psoriasis, and erythrodermic psoriasis. Cytokine. 2021;138:155391. doi:10.1016/j.cyto.2020.155391
9. Gu H, Zhang Y, Zeng W, Xia Y. Participation of interferons in psoriatic inflammation. Cytokine Growth Factor Rev. 2021. doi:10.1016/j.cytogfr.2021.12.002
10. Gilet A, Zou F, Boumenir M, et al. Aldosterone up-regulates MMP-9 and MMP-9/NGAL expression in human neutrophils through p38, ERK1/2 and PI3K pathways. Exp Cell Res. 2015;331(1):152–163. doi:10.1016/j.yexcr.2014.11.004
11. Hwang ST, Nijsten T, Elder JT. Recent highlights in psoriasis research. J Invest Dermatol. 2017;137(3):550–556. doi:10.1016/j.jid.2016.11.007
12. Buonafine M, Martinez-Martinez E, Jaisser F. More than a simple biomarker: the role of NGAL in cardiovascular and renal diseases. Clin Sci. 2018;132(9):909–923. doi:10.1042/CS20171592
13. Shao S, Cao T, Jin L, et al. Increased lipocalin-2 contributes to the pathogenesis of psoriasis by modulating neutrophil chemotaxis and cytokine secretion. J Invest Dermatol. 2016;136(7):1418–1428. doi:10.1016/j.jid.2016.03.002
14. Sun WY, Bai B, Luo C, et al. Lipocalin-2 derived from adipose tissue mediates aldosterone-induced renal injury. JCI Insight. 2018;3(17):e120196. doi:10.1172/jci.insight.120196
15. Lee SA, Noel S, Kurzhagen JT, et al. CD4(+) T cell-derived NGAL modifies the outcome of ischemic acute kidney injury. J Immunol. 2020;204(3):586–595. doi:10.4049/jimmunol.1900677
16. Eilenberg W, Stojkovic S, Piechota-Polanczyk A, et al. Neutrophil gelatinase-associated lipocalin (NGAL) is associated with symptomatic carotid atherosclerosis and drives pro-inflammatory state in vitro. Eur J Vasc Endovasc Surg. 2016;51(5):623–631. doi:10.1016/j.ejvs.2016.01.009
17. Floderer M, Prchal-Murphy M, Vizzardelli C. Dendritic cell-secreted lipocalin 2 induces CD8+ T-cell apoptosis, contributes to T-cell priming and leads to a TH1 phenotype. PLoS One. 2014;9(7):e101881. doi:10.1371/journal.pone.0101881
18. Kamata M, Tada Y, Tatsuta A, et al. Serum lipocalin-2 levels are increased in patients with psoriasis. Clin Exp Dermatol. 2012;37(3):296–299. doi:10.1111/j.1365-2230.2011.04265.x
19. Matsuura T, Sato M, Nagai K, et al. Serum peptides as putative modulators of inflammation in psoriasis. J Dermatol Sci. 2017;87(1):36–49. doi:10.1016/j.jdermsci.2017.03.014
20. Sokolova MV, Simon D, Nas K, et al. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of C-reactive protein and its link to clinical disease manifestations. Arthritis Res Ther. 2020;22(1):26. doi:10.1186/s13075-020-2111-8
21. Miao Q, Ku AT, Nishino Y, et al. Tcf3 promotes cell migration and wound repair through regulation of lipocalin 2. Nat Commun. 2014;5(1):4088. doi:10.1038/ncomms5088
22. Lee JH, Kye KC, Seo EY, et al. Expression of neutrophil gelatinase-associated lipocalin in calcium-induced keratinocyte differentiation. J Korean Med Sci. 2008;23(2):302–306. doi:10.3346/jkms.2008.23.2.302
23. Ku AT, Shaver TM, Rao AS, et al. TCF7L1 promotes skin tumorigenesis independently of β-catenin through induction of LCN2. eLife. 2017;6:e23242. doi:10.7554/eLife.23242
24. Abdel Hay R, Samir N, Safwat M, Rashed L, Soliman M. Tissue lipocalin-2 in psoriasis: is it a marker of metabolic disturbance or a possible marker of therapeutic efficacy after narrow band ultraviolet B? J Dermatolog Treat. 2020;31(5):519–523. doi:10.1080/09546634.2019.1605141
25. Wang D, Fang L, Pan G. Association of serum lipocalin-2 concentrations with psoriasis and psoriatic arthritis: an updated meta-analysis. Dis Markers. 2019;2019:7361826. doi:10.1155/2019/7361826
26. Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10(5):1033–1043. doi:10.1016/s1097-2765(02)00708-6
27. Klüber P, Meurer SK, Lambertz J, et al. Depletion of lipocalin 2 (LCN2) in mice leads to dysbiosis and persistent colonization with segmented filamentous bacteria. Int J Mol Sci. 2021;22(23):13156. doi:10.3390/ijms222313156
28. Qiu X, Macchietto MG, Liu X, et al. Identification of gut microbiota and microbial metabolites regulated by an antimicrobial peptide lipocalin 2 in high fat diet-induced obesity. Int J Obes. 2021;45(1):143–154. doi:10.1038/s41366-020-00712-2
29. Wang Q, Li S, Tang X, Liang L, Wang F, Du H. Lipocalin 2 protects against Escherichia coli infection by modulating neutrophil and macrophage function. Front Immunol. 2019;10:2594. doi:10.3389/fimmu.2019.02594
30. Watzenboeck ML, Drobits B, Zahalka S, et al. Lipocalin 2 modulates dendritic cell activity and shapes immunity to influenza in a microbiome dependent manner. PLoS Pathog. 2021;17(4):e1009487. doi:10.1371/journal.ppat.1009487
31. Lande R, Botti E, Jandus C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5(1):5621. doi:10.1038/ncomms6621
32. Mallbris L, O’Brien KP, Hulthén A, et al. Neutrophil gelatinase-associated lipocalin is a marker for dysregulated keratinocyte differentiation in human skin. Exp Dermatol. 2002;11(6):584–591. doi:10.1034/j.1600-0625.2002.110611.x
33. Howard JM, Nuguid JM, Ngole D, Nguyen H. Tcf3 expression marks both stem and progenitor cells in multiple epithelia. Development. 2014;141(16):3143–3152. doi:10.1242/dev.106989
34. Xu M, Zhang Y, Cheng H, et al. Transcription factor 7-like 1 dysregulates keratinocyte differentiation through upregulating lipocalin 2. Cell Death Discov. 2016;2(1):16028. doi:10.1038/cddiscovery.2016.28
35. Liu Y, Cheng H, Xiao S, Xia Y. A transcription factor 7-like 1-lipocalin 2 axis in the differentiation of keratinocytes. Cell Death Dis. 2016;7(6):e2241. doi:10.1038/cddis.2016.152
36. Kim HJ, Yoon HJ, Yoon KA, et al. Lipocalin-2 inhibits osteoclast formation by suppressing the proliferation and differentiation of osteoclast lineage cells. Exp Cell Res. 2015;334(2):301–309. doi:10.1016/j.yexcr.2015.03.008
37. Chung IH, Chen CY, Lin YH, et al. Thyroid hormone-mediated regulation of lipocalin 2 through the Met/FAK pathway in liver cancer. Oncotarget. 2015;6(17):15050–15064. doi:10.18632/oncotarget.3670
38. Hau CS, Kanda N, Tada Y, et al. Lipocalin-2 exacerbates psoriasiform skin inflammation by augmenting T-helper 17 response. J Dermatol. 2016;43(7):785–794. doi:10.1111/1346-8138.13227
39. Wolk K, Frambach Y, Jacobi A, et al. Increased levels of lipocalin 2 in palmoplantar pustular psoriasis. J Dermatol Sci. 2018;90(1):68–74. doi:10.1016/j.jdermsci.2017.12.018
40. Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest. 2008;118(4):1468–1478. doi:10.1172/JCI33869
41. Colak S, Omma A, Sandikci SC, Yucel C, Omma T, Turhan T. Vaspin, neutrophil gelatinase-associated lipocalin and apolipoprotein levels in patients with psoriatic arthritis. Bratisl Lek Listy. 2019;120(1):65–69. doi:10.4149/BLL_2019_010
42. Chiricozzi A, Suárez-Fariñas M, Fuentes-Duculan J, et al. Increased expression of interleukin-17 pathway genes in nonlesional skin of moderate-to-severe psoriasis vulgaris. Br J Dermatol. 2016;174(1):136–145. doi:10.1111/bjd.14034
43. Shah M, Rennoll SA, Raup-Konsavage WM, Yochum GS. A dynamic exchange of TCF3 and TCF4 transcription factors controls MYC expression in colorectal cancer cells. Cell Cycle. 2015;14(3):323–332. doi:10.4161/15384101.2014.980643
44. Zhang X, Gao Y, Lu L, et al. JmjC domain-containing protein 6 (Jmjd6) derepresses the transcriptional repressor transcription factor 7-like 1 (Tcf7l1) and is required for body axis patterning during xenopus embryogenesis. J Biol Chem. 2015;290(33):20273–20283. doi:10.1074/jbc.M115.646554
45. Kuwahara A, Sakai H, Xu Y, Itoh Y, Hirabayashi Y, Gotoh Y. Tcf3 represses Wnt-β-catenin signaling and maintains neural stem cell population during neocortical development. PLoS One. 2014;9(5):e94408. doi:10.1371/journal.pone.0094408
46. Lories RJ, de Vlam K. Is psoriatic arthritis a result of abnormalities in acquired or innate immunity? Curr Rheumatol Rep. 2012;14(4):375–382. doi:10.1007/s11926-012-0257-3
47. Sakkas LI, Bogdanos DP. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun Rev. 2017;16(1):10–15. doi:10.1016/j.autrev.2016.09.015
48. Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int J Mol Sci. 2018;19(2):530. doi:10.3390/ijms19020530
49. Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–390. doi:10.1007/s12016-018-8702-3
50. Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13(4–5):496–502. doi:10.1016/j.autrev.2014.01.050
51. Murakami M, Ohtake T, Horibe Y, et al. Acrosyringium is the main site of the vesicle/pustule formation in palmoplantar pustulosis. J Invest Dermatol. 2010;130(8):2010–2016. doi:10.1038/jid.2010.87
52. Wolk K, Wenzel J, Tsaousi A, et al. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br J Dermatol. 2017;177(5):1385–1393. doi:10.1111/bjd.15424
53. Turina MC, Landewé R, Baeten D. Lessons to be learned from serum biomarkers in psoriasis and IBD - The potential role in SpA. Expert Rev Clin Immunol. 2017;13(4):333–344. doi:10.1080/1744666X.2017.1244004
54. Ma JY, Shao S, Wang G. Antimicrobial peptides: bridging innate and adaptive immunity in the pathogenesis of psoriasis. Chin Med J. 2020;133(24):2966–2975. doi:10.1097/CM9.0000000000001240
55. Gul FC, Cicek D, Kaman D, Demir B, Nazik H. Changes of serum lipocalin-2 and retinol binding protein-4 levels in patients with psoriasis and Behçet’s disease. Eur J Dermatol. 2015;25(2):195–197. doi:10.1684/ejd.2014.2490
56. Baran A, Świderska M, Myśliwiec H, Flisiak I. Effect of psoriasis activity and topical treatment on serum lipocalin-2 levels. J Dermatolog Treat. 2017;28(2):136–140. doi:10.1080/09546634.2016.1180340
57. Romaní J, Caixàs A, Ceperuelo-Mallafré V, et al. Circulating levels of lipocalin-2 and retinol-binding protein-4 are increased in psoriatic patients and correlated with baseline PASI. Arch Dermatol Res. 2013;305(2):105–112. doi:10.1007/s00403-012-1306-5
58. El-Hadidi H, Samir N, Shaker OG, Otb S. Estimation of tissue and serum lipocalin-2 in psoriasis vulgaris and its relation to metabolic syndrome. Arch Dermatol Res. 2014;306(3):239–245. doi:10.1007/s00403-013-1414-x
59. Belstrøm D, Eiberg JM, Enevold C, et al. Salivary microbiota and inflammation-related proteins in patients with psoriasis. Oral Dis. 2020;26(3):677–687. doi:10.1111/odi.13277
60. Seo SJ, Ahn JY, Hong CK, et al. Expression of neutrophil gelatinase-associated lipocalin in skin epidermis. J Invest Dermatol. 2006;126(2):510–512. doi:10.1038/sj.jid.5700035
61. Ataseven A, Kesli R, Kurtipek GS, Ozturk P. Assessment of lipocalin 2, clusterin, soluble tumor necrosis factor receptor-1, interleukin-6, homocysteine, and uric acid levels in patients with psoriasis. Dis Markers. 2014;2014:541709. doi:10.1155/2014/541709
62. Wu G, Li H, Zhou M, et al. Mechanism and clinical evidence of lipocalin-2 and adipocyte fatty acid-binding protein linking obesity and atherosclerosis. Diabetes Metab Res Rev. 2014;30(6):447–456. doi:10.1002/dmrr.2493
63. Stejskal D, Karpísek M, Humenanska V, et al. Lipocalin-2: development, analytical characterization, and clinical testing of a new ELISA. Horm Metab Res. 2008;40(6):381–385. doi:10.1055/s-2008-1062746
64. El-Mesallamy HO, Hamdy NM, Sallam AA. Effect of obesity and glycemic control on serum lipocalins and insulin-like growth factor axis in type 2 diabetic patients. Acta Diabetol. 2013;50(5):679–685. doi:10.1007/s00592-012-0373-6
65. Aizawa N, Ishiuji Y, Tominaga M, et al. Relationship between the degrees of itch and serum lipocalin-2 levels in patients with psoriasis. J Immunol Res. 2019;2019:8171373. doi:10.1155/2019/8171373
66. Gulkesen A, Akgol G, Poyraz AK, et al. Lipocalin 2 as a clinical significance in rheumatoid arthritis. Cent Eur J Immunol. 2017;3(3):269–273. doi:10.5114/ceji.2017.70969
67. Chen L, Lin Z, Liu Y, et al. DZ2002 alleviates psoriasis-like skin lesions via differentially regulating methylation of GATA3 and LCN2 promoters. Int Immunopharmacol. 2021;91:107334. doi:10.1016/j.intimp.2020.107334
68. Baran A, Myśliwiec H, Kiluk P, Świderska M, Flisiak I. Serum irisin levels in patients with psoriasis. J Dermatolog Treat. 2017;28(4):304–308. doi:10.1080/09546634.2016.1254327
69. Xiao X, Yeoh BS, Vijay-Kumar M. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu Rev Nutr. 2017;37(1):103–130. doi:10.1146/annurev-nutr-071816-064559
70. Watanabe K, Yoshino T, Takahashi M, et al. Clinical significance of neutrophil gelatinase-associated lipocalin and galectin-7 in tape-stripped stratum corneum of acne vulgaris. J Dermatol. 2018;45(5):618–621. doi:10.1111/1346-8138.14261
71. Nguyen VT, Farman N, Palacios-Ramirez R, et al. Cutaneous wound healing in diabetic mice is improved by topical mineralocorticoid receptor blockade. J Invest Dermatol. 2020;140(1):223–234.e227. doi:10.1016/j.jid.2019.04.030
72. Abdollahi M, Ng TS, Rezaeizadeh A, et al. Insulin treatment prevents wounding associated changes in tissue and circulating neutrophil MMP-9 and NGAL in diabetic rats. PLoS One. 2017;12(2):e0170951. doi:10.1371/journal.pone.0170951
73. Navrazhina K, Renert-Yuval Y, Frew JW, et al. Large-scale serum analysis identifies unique systemic biomarkers in psoriasis and hidradenitis suppurativa. Br J Dermatol. 2021. doi:10.1111/bjd.20642
74. Wang H, Xu Y, Jin M, Li H, Li S. miR-383 reduces keratinocyte proliferation and induces the apoptosis in psoriasis via disruption of LCN2-dependent JAK/STAT pathway activation. Int Immunopharmacol. 2021;96:107587. doi:10.1016/j.intimp.2021.107587
75. Akgül B, Bauer B, Zigrino P, Storey A, Mauch C, Pfister H. Upregulation of lipocalin-2 in human papillomavirus-positive keratinocytes and cutaneous squamous cell carcinomas. J Gen Virol. 2011;92(2):395–401. doi:10.1099/vir.0.025064-0
76. Takahashi T, Asano Y, Noda S, et al. A possible contribution of lipocalin-2 to the development of dermal fibrosis, pulmonary vascular involvement and renal dysfunction in systemic sclerosis. Br J Dermatol. 2015;173(3):681–689. doi:10.1111/bjd.13779
77. Lumsden KR, Nelson AM, Dispenza MC, et al. Isotretinoin increases skin-surface levels of neutrophil gelatinase-associated lipocalin in patients treated for severe acne. Br J Dermatol. 2011;165(2):302–310. doi:10.1111/j.1365-2133.2011.10362.x
78. Navrazhina K, Garcet S, Gonzalez J, Grand D, Frew JW, Krueger JG. In-depth analysis of the hidradenitis suppurativa serum proteome identifies distinct inflammatory subtypes. J Invest Dermatol. 2021;141(9):2197–2207. doi:10.1016/j.jid.2021.02.742
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.