Back to Journals » International Journal of Nanomedicine » Volume 18
Lipid Nanocarrier-Based Drug Delivery Systems: Therapeutic Advances in the Treatment of Lung Cancer
Authors Kim SJ, Puranik N, Yadav D, Jin JO , Lee PC
Received 30 January 2023
Accepted for publication 6 May 2023
Published 18 May 2023 Volume 2023:18 Pages 2659—2676
DOI https://doi.org/10.2147/IJN.S406415
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
So-Jung Kim,1,* Nidhi Puranik,2,* Dhananjay Yadav,3,* Jun-O Jin,1 Peter CW Lee4
1Department of Microbiology, University of Ulsan College of Medicine, Seoul, 05505, South Korea; 2Department of Biochemistry & Genetics, Barkatullah University, Bhopal, Madhya Pradesh, 462026, India; 3Department of Life Science, Yeungnam University, Gyeongsan, 38541, Korea; 4Department of Biochemistry and Molecular Biology, University of Ulsan College of Medicine, ASAN Medical Center, Seoul, 05505, South Korea
*These authors contributed equally to this work
Correspondence: Peter CW Lee, Department of Biochemistry and Molecular Biology, University of Ulsan College of Medicine, ASAN Medical Center, Seoul, 05505, Korea, Email [email protected] Jun-O Jin, Department of Microbiology, University of Ulsan College of Medicine, Seoul, 05505, Korea, Email [email protected]
Abstract: Although various treatments are currently being developed, lung cancer still has a very high mortality rate. Moreover, while various strategies for the diagnosis and treatment of lung cancer are being used in clinical settings, in many cases, lung cancer does not respond to treatment and presents reducing survival rates. Cancer nanotechnology, also known as nanotechnology in cancer, is a relatively new topic of study that brings together scientists from a variety of fields, including chemistry, biology, engineering, and medicine. The use of lipid-based nanocarriers to aid drug distribution has already had a significant impact in several scientific fields. Lipid-based nanocarriers have been demonstrated to help stabilize therapeutic compounds, overcome barriers to cellular and tissue absorption, and improve in vivo drug delivery to specific target areas. For this reason, lipid-based nanocarriers are being actively researched and used for lung cancer treatment and vaccine development. This review discusses the improvements in drug delivery achieved with lipid-based nanocarriers, the obstacles that still exist with in vivo applications, and the current clinical and experimental applications of lipid-based nanocarriers in lung cancer treatment and management.
Keywords: lung cancer, lipid-based nanocarriers, liposome, drug delivery system
Introduction
Lung cancer (LC) is a global health issue affecting approximately 2.1 million people and causing 1.8 million deaths per year. While LC rates continue to rise worldwide, they are decreasing among men in several Western countries. The morphological, etiological, and molecular aspects of LC have been extensively studied.1,2 The most common cases of LC are usually of two types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), comprising 85% and 15% of patients, respectively.
Histological studies, immunological studies, and molecular analyses are used to make a pathological diagnosis, while the eighth edition of the Tumor, Node, Metastasis (TNM) classification for LC is currently used to stage LC. Awareness of the tumor stage is crucial for diagnosis and treatment. The five-year survival rate is approximately 15–20%. Non-small cell lung cancer has four stages: stage I, which is most common in the lung but does not extend to lymph nodes; stage II, which spreads to lymph nodes close to the lungs; stage three, which spreads further into your lymph nodes and the middle of your chest; and stage four, in which the cancer has spread broadly throughout the body. These rates approach 90% for patients with stage 1A1 NSCLC, but fall to below 10% for those with stage 4.3 Patients with SCLC have a 30% chance of developing a modest illness and a 10% chance of developing severe disease. Surgery, radiation, systemic therapy (targeted therapies, chemotherapy, and immune checkpoint inhibitors), supportive care, and hospice care are used in the management of patients with LC. Treatment is determined by tumor features, tumor stage, and other patient-related factors. Nevertheless, for many countries, obtaining and reimbursing innovative pharmaceuticals is becoming increasingly difficult.4
Various attempts have been made to treat LC.5,6 Among these, targeted therapy has recently attracted some attention. Targeted therapy induces the selective death of cancer cells by detecting specific substances, such as hormone receptors, expressed in these cells.5 Therefore, in many cases, anticancer drugs are delivered together with a target substance to improve the efficiency of the targeted therapy. Recently, nanocarriers have been actively used to deliver anticancer drugs and their target substances. Various materials have been researched and developed as nanocarriers after considering their biostability.7,8 Lipid-based nanoparticles are one of the more promising molecules for carriers of anticancer drugs because they are easy to manufacture, have high stability in vivo, and have a low potential for side effects.9 Recently, lipid nanoparticles (LNPs) have been used in the development of a COVID-19 vaccine as an mRNA delivery system, and have been recognized for their stability and ability to deliver drugs.10 Therefore, it is necessary to analyze and understand the research trends related to LC treatment using lipid-based nanocarriers.
In this review, current technologies for anticancer, drug-loaded, lipid-based nanocarriers for LC treatments are discussed (Figure 1). In addition, the potential function, benefits, and limitations of using lipid-based nanocarriers for the delivery of anticancer medicines in treating LC are also discussed. Furthermore, improvements in the use of inhalable lipid-based nanocarriers in the treatment of LC, as well as their evaluation in vitro, in vivo, and in clinical trials, are discussed.
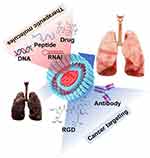 |
Figure 1 Schematic illustration of liposome-mediated lung cancer (LC) treatment. |
Challenges of Conventional Drug Therapy in LC Treatment
While a variety of drug therapies for LC treatment are currently being developed, chemotherapy is still common. The medicines for chemotherapy are usually administered intravenously, which then circulate throughout the body and act as the first-line of treatment for advanced stages of LC.11 Platinum-based medicines such as cisplatin and carboplatin are commonly used as first-line treatments for LC.12 However, chemotherapy’s indiscriminate cytotoxicity can have unfavorable side effects by inhibiting fast-developing cells and tissues, such as hair follicles, bone marrow, and cells of the gastrointestinal tract.13 Moreover, chemotherapy can potentially cause multidrug resistance. The non-specificity and heterogeneity of the distribution of the cytotoxic chemical agents employed in chemotherapy contribute to multidrug resistance in the therapy process.14 This non-specificity reduces chemotherapeutic efficacy while hindering tumor growth, metastasis, and suppression of recurrence.15 A lack of selectivity, cell toxicity, short half-life, multidrug resistance, poor solubility, and stem-like cell proliferation are some of the issues faced using current chemotherapy. Therefore, to overcome these shortcomings, a recently developed method of combining various treatments with chemotherapy is being studied. These treatments include molecular approaches, apoptosis regulation, immunotherapy, nucleic acid-based therapy, and anti-angiogenesis therapy.
Immunotherapy has been the most popular treatment in recent years.16,17 Compared with chemotherapy, it has the advantage of fewer side effects as it uses the activity of immune cells in our body to remove cancer cells. However, it is not completely free from side effects, which include inflammation. The most widely used form of immunotherapy utilizes immune checkpoint blockade (ICB) antibodies to treat LC.16 However, the ICB antibodies have limited use as they can be used only for LC cells expressing checkpoint protein, and there are symptoms of ineffectiveness for reasons unknown.18 For this reason, attempts are being made to treat LC using the single use of ICB antibodies combined with the use of existing anticancer drugs. The Food and Drug Administration (FDA)-approved drugs used in the treatment of different stages of LC are listed in Table 1.
 |
Table 1 FDA-Approved Drugs Used in Different Stages and Types of Lung Cancer (LC) Treatments |
The Need for Nanotechnology and Nanomedicine in Therapeutics
Many traditional medicines are associated with poor pharmacokinetics and bioavailability, and toxicity, all of which limit the extent of their application.19 The emerging fields of nanotechnology and nanomedicine have made substantial progress in the detection, diagnosis, and treatment of various diseases at the clinical level to overcome these challenges and increase the therapeutic indices of drugs.20 Paclitaxel (PTX) is a drug commonly used for LC; however, it has severe limitations, which include the development of peripheral neurotoxicity, which may lead to treatment suspension and therapy failure.21 While PTX has severe side effects, there have been reports that these side effects can be significantly reduced by the treatment method using nanocarriers.22–24 Nanotechnology can not only lower the toxicity of anticancer drugs but can also confer various functions to cancer drug delivery systems (DDSs). Such functions include a) targeting, which entails increasing drug concentrations at desired areas of action while lowering systemic drug levels and their harmful consequences in healthy tissues;25 b) increased solubility, thereby making parenteral medication delivery easier;26 c) maintaining a constant therapeutic dosage at the site of action with a constant rate of drug delivery and zero-order release kinetics;25 d) reduced clearance to extend the half-life of the medication;27 e) improved drug stability, resulting in less drug breakdown and better drug activity;27 and f) drug transport across the blood-brain and blood-cochlear barriers.28
Lipid-Based Nanocarriers
The most promising colloidal DDSs currently available involve the use of NPs produced from natural polymers, such as polysaccharides, phospholipids, and proteins.29 With regard to drug loading volume, biocompatibility, and reticuloendothelial system (RES) opsonization, such systems have been recognized as more effective than synthetic polymers.30 Furthermore, natural polymers have shown superiority to synthetic polymers in terms of their ability to be absorbed by the human body and produce less harmful end products following destruction.31 Thus, NPs made from naturally isolated polymers may be the more appropriate choice for the colloidal drug delivery method for human use, as they are reasonably safe and easy to prepare. An effective option for overcoming drug delivery problems is to use a nanotherapeutic approach to administer hydrophobic chemotherapeutic drugs.32
Lipid-based nanocarriers have the advantage of being easily absorbed into cells and have fewer side effects, as they are similar to the membrane components of cells.33–35 The hydrophobic chemotherapeutics can be loaded inside the lipid-based nanocarriers, and the hydrophilic cancer targeting reagent can be attached to the outside, which is the biggest advantage of using lipid-based nanocarriers for cancer treatment. Effective delivery of anticancer drugs to cancer cells depends on which target material is attached to the lipid-based nanocarriers. Lipid-based nanocarriers use various functional groups on their surface to facilitate the attachment of various substances, including antibodies, polysaccharides, and drugs, to enhance the efficacy of their delivery to cancer cells (Figure 2). In addition, lipid-based nanocarriers can be administered via a variety of routes for drug delivery, including oral, parenteral, ophthalmic, intranasal, and dermal/transdermal routes.34 The oral route is the most favored route because of its non-invasiveness, lower cost, and lower risk of adverse effects, such as injection-site reactions.36 The nasal administration method is thought to be the most effective for drug delivery for LC treatment, but it has not yet shown any remarkable results with regard to its efficiency. Lipid-based nanocarriers can form various structures depending on the formulation method. These formulation methods may exhibit differences in drug loading and delivery efficiencies. The most common lipid-based nanocarriers used as DDS in LC are listed in Table 2.
 |
Table 2 Lipid-Based Nanocarriers Used in Drug Delivery Systems for LC Treatment |
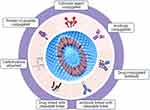 |
Figure 2 Various types of modifications and conjugations on lipid-based nanocarriers for drug delivery. |
Characteristics of Liposomes as DDSs
Liposomes have been used for a long time for drug administration owing to their distinctive properties. A liposome is a hydrophobic membrane that surrounds a patch of aqueous solution and prevents the dissolution of hydrophilic solutes from increasing lipids. Liposomes may accept both hydrophobic and hydrophilic molecules because hydrophobic substances can dissolve into the membrane.33 Lipofection is the process of employing liposomes to convert or transfect DNA into the host cell. In addition to transporting genes and medications, liposomes might be used as carriers. The liposome design for DDS as a drug carrier is far more sophisticated than it was 30 years ago when they were first established.38 Approximately 12 liposome-based medications have been approved for medical use in various phases of clinical studies.44
Liposomes are mostly composed of natural and/or manufactured phospho- and sphingolipids with other membrane bilayer ingredients, such as cholesterol and hydrophilic polymer-conjugated lipids, randomly scattered around each liposomal vesicle.45 Amphiphilic lipids are composed of a glycerol molecule coupled to a phosphate group and two saturated or unsaturated fatty acid chains.46 Another organic molecule can be linked to the phosphate group. Natural phospholipids are classified as phosphatidic acid, phosphatidylcholine (PC; also known as lecithin), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylglycerol (PG), and phosphatidylserine (PS) according to their chemical groups.47 Glycerophospholipids, which are responsible for liposome formation, are separated into two types: natural and synthetic. PC and PE, abundant phosphatides in plants and animals, are the most natural phospholipids used to prepare liposomes. However, liposomes and other lipid-based DDSs normally have PC membranes with very little PE. PE can form non-bilayer structures that destabilize membranes and cause membrane fusion under biological conditions. Based on the desired liposome characteristics, other phospholipids such as PS, PG, and PI can be used to prepare liposomes.48
Liposomal Dry Powder as DDSs in LC Therapeutics
Anticancer medications delivered through the lungs in the form of dry nanoparticulate powders are considered a promising treatment option for LC.49 Zhu et al worked on a liposomal dry powder (LDP) of docetaxel (DTX) as LC therapeutics. DTX encapsulated in folic acid-conjugated liposomes demonstrated significant cytotoxicity and excellent tumor targeting properties.49 In both pharmacological and pharmacokinetic terms, the re-dispersed liposomes obtained after the redispersion of inhaled dry powders differ from the initial liposomes. A study by Gandhi et al showed that by using the lyophilization process, gemcitabine HCl could be effectively integrated into LDP.50 The potential application of formulated LDP was observed in human adenocarcinoma cell line A549, as well as in in vivo experiments. These experiments concluded that LDP formulations can be deposited all over the lung and can be used in the nontoxic treatment of LC.
Liposome-Mediated Targeting of LC
Because various functional groups can be added to liposomes, they can be used in cancer-targeted treatments. Triptolide encapsulation in liposomes modifies the distribution behavior of triptolide, and, according to Congcong Lina et al, dual-ligand modified triptolide-loaded liposomes elicited the highest anticancer response in the trial, with no clear systemic harm.51 This shows that treating triptolide-loaded liposomes with anti-CA IX antibodies and lineage-homing cell-penetrating peptides simultaneously improves the therapeutic benefits of these liposomes.
A recent study used RGD (arginine, glycine, and aspartic acid) tripeptide-modified PTX for LC therapeutics and found that RGD-modified co-loaded liposomes are effective candidates for antitumor drug delivery.52 Transporting PTX in large cationic liposomes could prevent negative effect of PTX such as tissue toxicity, thereby improving the patient’s prognosis. Cationic liposomes are used to encapsulate PTX. High biocompatibility, enhanced internalization, and anticancer efficacy of PTX was observed in human and mouse LC cells in culture, multicellular spheroids, and cancer stem cells (CSCs).53
The therapeutic effect of quercetin (QR) in the treatment of LC through the targeting of transferrin receptors is known to be high in tumor cells. T7 (HAIYPRH) surface-functionalized liposomes with various T7 peptide densities and nontargeted quercetin-loaded liposomes, with an encapsulation efficiency of 95%, were successfully produced in this study.54 Using manufactured liposomes, anticancer activity was examined in LC cells (A549 cells) and normal lung cells (MRC-5 cells). T7 surface-functionalized liposomes (2% T7-QR-lip) increased cytotoxicity, cellular uptake, S-phase cell-cycle arrest, and mortality in A549 cells. This contrasts with what is observed with nontargeted QR-lip due to receptor-mediated endocytosis, although in MRC-5 cells no significant differences in cytotoxicity or cellular uptake were found between T7-QR-lip and QR-lip. Furthermore, in 3D lung tumor spheroids, the 2% T7-Cou6-lip presented much deeper penetration. Using in vivo experiments, Riaz et al revealed the beneficial effects of T7-QR-lip after pulmonary administration in BALB/c nude mice with orthotopic lung tumor implantation.54
Characteristics of Lipid Micelles as DDSs
Lipid micelles refer to the form in which nano-sized particles are made in aqueous solution via self-aggregation with lipid as the main component.55 Lipid micelles are made of lipids with amphiphilic properties contributed by the lipid-containing polar head groups (hydrophilic) and the long hydrophobic chains. The polar part of the lipid faces water and the hydrophobic chain is aggregated to form a micelle.55 Because of their amphiphilic properties, micelles are suitable for loading both hydrophilic and hydrophobic drugs. The lipid micelle structure controls the sustained and controlled release of anticancer drugs, and provides chemical and physical stability to the drug. In addition, it is being used as a drug delivery agent because it can improve drug pharmacokinetics, is advantageous for intra-tissue delivery, and can improve drug bioavailability.39
Lipid Micelle-Mediated Treatment of LC
Compared to liposomes, micelles are small in size and simple to manufacture; consequently, they are used in treatments by loading various anticancer drugs that have been previously used. The epidermal growth factor receptor (EGFR) is widely used as a drug delivery target for the treatment of LC.56 Although it is common to use antibodies to target the EGFR, the use of aptamers based on nucleic acids has recently become popular. Aptamers can be easy to manufacture, and have the advantage of low cost when compared with that of monoclonal antibodies. The micelles with EGFR aptamer on the surface effectively deliver drugs to LC. In this study, salinomycin was introduced into micelles as a drug capable of killing cancer stem cells in LC. The micelles containing the EGFR aptamer and salinomycin were of size 24 nm, and were confirmed to effectively target LC in mice and inhibit cancer growth.57
Although EGFR is targeted for the delivery of LC treatment drugs, EGFR is often mutated in many patients.56 For this reason, a method of blocking the signaling system, induced by EGFR, is sometimes used. Afatinib is an inhibitor that blocks the tyrosine kinase of the EFGR signaling system that exists inside the cell, and is being used as a treatment for EGFR-mutant NSCLC. For the effective delivery of afatinib to LC, a micelle with transferrin decorated on the surface was fabricated, as transferrin receptors are overexpressed in LC.58 For LC, the delivery efficiency of afatinib by micelles was four times higher than that of afatinib alone, and as a result, it was also shown to effectively inhibit LC growth in the mice in vivo.59
In addition, the overexpression of transferrin receptors in LC has been used for the delivery of other drugs. PTX, which is the most commonly used anticancer drug, is known to have severe side effects. PTX was also loaded into micelles and used in LC treatment via targeting of transferrin receptors. PTX@FT-NB, a micelle loaded with PTX and having transferrin on the surface, was shown to be delivered to LC with high efficiency, and also inhibited cancer growth in mice in vivo.60
Characteristics of Solid Lipid Nanoparticles (SLNs) as DDSs
SLNs are an emerging drug delivery method that is an alternative to the traditional colloidal delivery system.30 The SLN is a fusion of polymeric nanoparticles and liposomes, and the resulting advantages can be applied to therapy.30 Because the surface of the SLN is lipid, its biocompatibility is very high, and it is highly tolerable in the lungs and in the body. Therefore, it is suitable as a drug delivery agent for the treatment of LC. SLNs are mainly prepared using physiological solid lipids, which are in a solid state at room temperature and body temperature. In water, it disperses and the inner lipid fuses to form a structure. Polyvinyl, compritol, glyceride, and steroids are commonly used for the synthesis of SLNs.61
SLN-Mediated Treatment of LC
SLN is also suitable for loading hydrophobic drugs inside, similar to other lipid-based nanocarriers. DTX is a hydrophobic anticancer drug, which is used for breast cancer, head and neck cancer, and NSCLC. Common side effects of DTX include hair loss, cytopenia, and vomiting. These side effects appear to be due to the decreased delivery efficiency of DTX to cancer cells, and research is being conducted to overcome them using the targeted effect of nanostructures. DTX was loaded onto SLN made with Compritol® 888 ATO as the main raw material. Compritol-based SLN, which was named SLN-DOX, was able to support DTX with 86% efficiency. In the in vitro assay, the SLN-DOX efficiently promoted apoptosis of the cancer cells, as well as the arrest of the G2/M phase of the cell cycle. In addition, administration of SLN-DOX prevented metastatic LC in mice in vivo.62
Transferrin was also introduced into SLN for the LC-specific delivery of DOX, similar to lipid micelles. SLNs were prepared with stearic acid and injectable soy lecithin. Together with DOX, plasmids of enhanced green fluorescent protein (pEGFP) were co-delivered to cancer cells, and delivery efficiency was evaluated using EGFP expression. In the in vitro assay, the transfection efficiencies of the EGFP plasmid using SLN were higher than 80%, and the released DOX concentration was also higher than 80% in the A549 cells. As indicated by the specific targets of the transferrin receptor by transferrin decoration in SLN, it promoted a dramatically increased DOX-mediated anticancer effect in the A549 xenograft tumor model in mice.63
Hydrogenated soybean phospholipids and polyvinyl pyrrolidone k15-based SLNs have also been applied in the treatment of LC. In this SLN, PTX and curcumin were incorporated, which was then referred to as PC-SLN. PC-SLN prolonged the residence time and half-life when compared with the combination of PTX and curcumin, which improved circulation time. PC-SLNs showed a much greater inhibitory effect of tumor growth than the combined treatment of PTX and curcumin in the xenograft tumor model.64
SLN has also been used for the delivery of traditional herbal medicine. As a Chinese tradition, Yuxingcao has been used for the treatment of lung diseases such as pneumonia and respiratory infections. However, due to the indiscriminate administration of Yuxingcao, a number of adverse drug reactions (ADRs) occurred, some of which led to death.65 This side effect of Yuxingcao administration also appeared because effective drug delivery did not occur inside the diseased lung, and efforts were made to overcome this using SLN. Polyvinyl acetate- and glyceryl behenate-based SLN were synthesized, and Yuxingcao oil was loaded inside the SLN. The loaded Yuxingcao was fully released in the cultured medium within 50 h. In addition, the intratracheal administration of Yuxingcao-loaded SLN effectively delivered Yuxingcao to the lung.66 Although this study did not show in vivo results for LC treatment in mice, it suggested that because SLN could deliver herbal medicine to the lungs, it may also be useful in LC treatment.
Characteristics of Lipid Nanoparticles (LNPs) as DDSs
LNP is a term that can collectively refer to all nanostructures containing lipids, but the meaning has changed slightly as it has also been used in the development of a COVID-19 vaccine.67 The LNP is synthesized using microfluidics, and unlike liposomes, the inside is filled with lipids. Microfluidic-mediated synthesis of LNPs is a promising new method for the mass production of LNPs.68 This method is well established and can be scaled up to Good Manufacturing Practice (GMP) production in the currently preferred research lab for use in clinical trials.68 In addition, the use of microfluidics has the advantage of ensuring qualitative control, which cannot be maintained by conventional methods. LNPs are most actively used as carriers for delivering mRNA that is capable of producing antigenic proteins and thus have several special compositions. Such conditions include a) ionized or cationic lipids having tertiary or quaternary amine heads for facilitating loading inside LNPs using the negative conversion of nucleic acid molecules; b) phospholipids that can form hydrophilic membranes such as DSPC; c) cholesterol capable of stabilizing the lipid bilayer of LNP; and d) PEGylated-lipids to block the degradation of LNP in the blood and increase the stability and blood circulation time.69
LNP-Mediated Treatment of LC
Unlike other lipid-based nanocarriers, LNPs are often used as carriers to deliver nucleic acids.68 The reason for this is most likely the effectiveness of the COVID-19 vaccine, in which an mRNA was inserted into the LNP. In LC treatment using LNPs, the research on delivering nucleic acids is more active than that on delivering anticancer drugs. In addition, the delivery of nucleic acids via LNPs is used for immunotherapy by targeting immune cells. Similar to COVID-19 vaccine, where mRNA capable of translating the antigen protein of SARS-CoV-2 was inserted into the LNP, cancer treatment was attempted by introducing the cancer antigen into the LNP.68 Experimental antigen ovalbumin-contained LNPs as well as LNPs containing melanoma antigen TRP2 mRNA promoted effective immunity against antigen-expressing tumors, as these treatments suppressed tumor growth.70
Dendritic cells (DCs) are central cells of immunity that can induce the activation of T cells.71 T cells activated by dendritic cells can find and eliminate antigen-expressing cells. DCs activate themselves by recognizing the pattern of pathogens, and substances that can stimulate this pattern are used as immune enhancers.72 In addition, activated DCs express CD40 on their surface, and show stronger activity via the action of the CD40 ligand of T cells.72 To take advantage of this DC activity, pattern receptor stimulant R848 (toll-like receptor 7/8 agonist) and CD40 mRNA were loaded onto LNPs, referred to as RAL2 CD40-LNP, to induce DC activity. The RAL2 CD40-LNP-induced activity of DCs was notably improved by the additional treatment of the anti-CD40 antibody. Consequently, the combined treatment of RAL2 CD40-LNP and the anti-CD40 antibody almost completely inhibited tumor growth in mice in vivo.73
There are a variety of nucleic acid components of immune stimulatory substances that can activate the pattern recognition receptors of immune cells. Among them, cyclic dinucleotide (CDN) molecules, which are STING ligands, have recently been shown to have excellent immune stimulatory effects. In the mouse lung metastasis model, STING-LNP treatment elicits the infiltration of T cells and NK cells in the lung, as well as the expression of PD-1 and interferon (IFN)-γ. In addition, the combined treatment of STING-LNP and anti-PD-1 exerted a synergistic anticancer immunity in the lung metastasis model.74
Nanostructured Lipid Carrier (NLC)
Lipid nanoparticles, such as SLN and LNP have long been thought of as potential carrier systems with promising therapeutic uses however have some limitations. The NLCs can be used as a cutting-edge drug delivery method for a variety of medication classes due to having some unique properties of intelligent, adaptable systems that can increase drug loading, control release, and production of final dosage forms such creams, pills, capsules, and injectables.75 NLCs are lipid-based formulations and contain a blend of both solid and liquid lipid.76 The binary lipid-based nanocarriers known as NLCs allow for the trapping of lipophilic actives, preventing their deterioration and enhancing their stability.77 It was demonstrated that NLCs had some advantages over conventional carriers for medication therapy, including enhanced permeability, improved bioavailability, fewer side effects, prolonged half-life, and tissue-targeted delivery. Recent years have seen an increase in interest in NLCs.78 These nanocarriers can be used to deliver medications that are both lipophilic and hydrophilic. For the administration of medications via oral, parenteral, ophthalmic, pulmonary, topical, and transdermal routes, NLCs have emerged as a viable carrier system. Recently, NLCs have also been used in the administration of cosmeceuticals and nutraceuticals as well as chemotherapy, gene therapy, and brain targeting.79 Due to their increased stability during storage and simplicity of scalability without the need for sterile conditions, NLCs are a promising oral delivery medium and both hydrophilic and lipophilic medicines can be captured by these systems.80 Numerous studies have been conducted on NLC-based medication delivery systems for different kind of cancer treatment, and many scientists are analyzing the features of NLCs to enhance their functionality.81,82 In 2014, Yiqun Han and group form an NLC based formulation “EGFP-loaded NLC” and transfected in human alveolar adenocarcinoma cell line (A549 cells) and in mice having A549 cell line using a modified vector. Result of in vitro and in vivo experiment shown that NLC-based gene delivery system offers an effective strategy for LC gene therapy due to high transfection rate.83 In 2019, NLC based drug delivery of doxorubicin (DOX) and β-elemene (ELE) (DOX/ELE Hyd NLCs) was studied in both in vitro and in vivo for LC therapeutics. The formulation inhibits the lung tumor cells and tumor growth effectively by synergistic effect.84 The NLC based one more study was performed by Shenghu Guo and group for the treatment of LC. They have loaded the cetuximab (CET), paclitaxel (PTX) and 5-Demethylnobiletin (DMN) drug in NLCs to form CET-PTX/DMN-NLCs formulation. The formulation was checked both in vitro (A549 cell line) and in vivo (Lung tumor xenografts mice) and found that the drug with NLC synergistically decreases the viability of the cancer cells with low toxicity and which could be used as a promising system for the synergistic combination therapy of LC.85 In a recent study Cisplatin (CDDP) and etoposide (Etp) drug was conjugated with NLC to form a new formulation “EtpP–CDDP-NLCs” for the treatment of LC. The formulation was checked both in vitro and in vivo and resulted in improved the tumor-cell uptake, cytotoxicity, and tumor-inhibition efficiency.86
Although NLCs have a lot of potential as drug delivery vehicles, there are still not enough preclinical and clinical research. As a result, it is necessary to broaden the range of their applications to incorporate clinical trials under proper ethical guidelines.
Inhalation of Lipid-Based Nanocarriers for LC Treatment
One of the strategies for the treatment of LC is the inhalation of drugs. The lungs are readily accessible tissues after passing the respiratory track, and the inhalation of drugs can induce the direct cancer-targeting effects of drugs. However, because the respiratory track is a mucosal tissue, drugs do not pass through it in the usual manner. In addition, mucosal damage from drugs may cause serious side effects.87 Therefore, a stable and effective DDS is required when using inhalation as a route for the treatment of LC. As mentioned above, a liposome is a delivery vehicle of a vesicle based on lipids. The lipids used in liposomes are natural and non-toxic to the body. In addition, liposomes are transporters that can easily pass through mucous and stably carry drugs to tissues. Based on these advantages, liposomes are being studied as inhaled drug delivery agents for the treatment of LC (Figure 3).
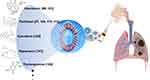 |
Figure 3 LC treatment via nasal inhalation of anticancer drug-loaded liposome. |
In a recent study on drug development for LC done by Khushaboo et al,88 they demonstrated the possibility of using a sorafenib tosylate-loaded LDP inhaler as a vehicle for the direct targeting of NSCLC via a pulmonary DDS. The LDP inhaler showed greater lung deposition than the standard formulation in the in vitro aerosol experiments. In vitro experiments showed that the developed dry powder inhaler had long-term release characteristics. As a result, gradual systemic dilution following an LDP inhaler injection is likely to result in a tailored distribution of sorafenib tosylate, thereby lowering dose frequency and drug-related side effects.89 Zhang et al, formulated a liposomal curcumin dry-powder (LCDP) inhaler for primary LC inhalation treatment.90 After freeze-drying the curcumin liposomes, LCDPs were obtained. Curcumin liposomes were more readily absorbed by human A549 LC cells than free curcumin. These liposomes caused significant cytotoxicity in A549 cells but low cytotoxicity in normal human bronchial BEAS-2B epithelial cells. Curcumin powder, LCDPs, and gemcitabine were sprayed directly into the lungs of LC-affected rats via the trachea. Concerning the pathology and expression of various cancer-related indicators, LCDPs have a stronger anticancer effect than the other two drugs.90 The curcumin-liposomal formulated drug was further studied by Adel et al in 2021. They used a nano-spray dryer to create spray-dried curcumin-loaded proliposomes for inhalation. When matched with curcumin powder and plain formulations, using an MTT assay, cytotoxicity tests revealed better growth inhibitory properties on lung tumor A549 cancer cells and low IC50 values.91 A study by Parvathaneni et al, concluded that inhalable pirfenidone (PFD)-loaded liposomes could be a potential treatment strategy for NSCLC.92 Chen et al, studied the role of nanocarriers in DDSs for LC and reported that lycobetaine (LBT)-oleic acid (OA)-PEG liposomes could entrap more LBT and displayed a slower drug release and prolonged circulation time in pharmacokinetic studies when compared with LBT-OA-PEG-nanoemulsions (NEs). In vitro studies on lung carcinoma cells showed a boosted antitumor effect of LBT-OA-PEG liposomes compared with that of free LBT. Additionally, when nRGD was used as a therapeutic adjuvant, LBT-OA-PEG liposomes with nRGD demonstrated competence in the microenvironment-depletion of tumors, as well as robust anti-lung cancer activity. Hence, LBT-OA-PEG liposomes with nRGD could be beneficial for clinical cancer therapy.93 The various liposome-based bioformulations for the treatment and management of LC are presented in Table 3.
 |
Table 3 Recent Studies Presenting Different Liposome-Based Bioformulations for Drug Delivery in LC Treatment |
Discussion
LC is a diverse molecular disease and understanding its cause is essential for the development of successful treatments. The discovery and application of nanotechnology in the detection, diagnosis, prognosis, and treatment of cancer has made huge strides in the previous decade, resulting in the emergence of a new discipline of “cancer nanomedicine”.110 According to the National Institutes of Health, a nanoparticle is any material used in the formulation of a medicine, which results in a final product that is less than one micron in size. Owing to their ability to penetrate biological barriers, efficiently deliver hydrophobic treatments, and preferentially target areas of illness, nanoparticle-based therapeutic systems have grown in popularity. Currently, lipid-based, polymeric, branching polymeric, metal-based, magnetic, and mesoporous silica nanocarrier formulations are used. Moreover, novel methodologies have been applied to use multicomponent, three-dimensional constructions that provide multifunctional capabilities.12 Using such designs, chemotherapeutics and anticancer gene treatments can be simultaneously delivered to precise targets. Nanoparticle-based medicines are paving the way for primary and metastatic LC diagnoses, as well as their imaging, screening, and treatment. However, problems in the fields of pharmacology, medicine, immunology, large-scale production, and regulation have impeded the translation of such discoveries from the laboratory to the patients. Therefore, this study covers the current developments and obstacles in DDSs using lipid-based nanocarriers using recent LC therapy cases as examples.
Medication formulations of lipid-based nanocarriers have long been promoted as promising DDSs; however, there are various limitations and challenges with DDSs using lipid-based nanocarriers, as shown in Figure 4. DDSs using lipid-based nanocarriers are costly to manufacture, resulting in high production costs due to the high price of raw materials, as well as require costly equipment to enhance manufacturing capacity. Other than cost, further challenges are also observed with DDSs using lipid-based nanocarriers. These include the sterilization process (as liposomes are temperature-sensitive), short half-life and low stability (one of the major challenges as liposomes can be easily oxidized and hydrolyzed by reagents), toxicity at increased doses (some lipids, especially charged lipids, used in liposome synthesis become toxic when drug doses are increased), and rapid clearance from the bloodstream (phagocytic cells of the RES rapidly remove the liposomes from the body).37,38,108 In addition to these, challenges also lie in vesicular destabilization as DDSs using lipid-based nanocarriers require interaction with plasma proteins which play a significant role in nanocarrier biodistribution and in lipid nanoparticle clearance by the RES via opsonization, as well as in vesicular destabilization. Additionally, pseudoallergies are an issue as some lipid-based nanocarrier systems activate the innate immune response, which then activates the complement system, causing an acute hypersensitivity condition, also called complement activation-related pseudoallergies.109
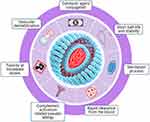 |
Figure 4 Limitations and challenges with DDSs using lipid-based nanocarriers. |
The pharmaceutical industry made enormous strides in the second half of the twentieth century, with biopharmaceutics and improved pharmacokinetics receiving considerable attention. Within this period the concept of a controlled and targeted medication delivery system was introduced for the first time. As nanotechnology has become increasingly prevalent in the medical industry, a delivery mechanism has become possible in the form of submicron-sized particles.48,111,112 The use of lipid-based nanocarriers to aid drug distribution has already had a significant influence in several scientific fields. Lipid-based nanocarriers were the first nanotechnology-centered DDS for clinical use, owing to their biocompatibility and biodegradability.113,114 Understanding the advancements in lipid-based nanocarrier technology, as well as the hurdles that remain, will enable forthcoming research to upgrade existing platforms and discourse on the translational and regulatory limits. Professionals participating in all stages of development for lipid-based nanocarrier technology need to communicate and collaborate to ensure further translational success in manufacturing, pharmaceutical design, cellular interfaces, and toxicology for clinical evaluation studies.109
Conclusion
Lipid-based nanocarriers have an extensive range of applications as DDSs, owing to their versatility. They are particularly appealing because of the ease with which they can be chemically modified, their capacity to transport a variety of drugs/genes, and their ability to be applied through a variety of methods. Furthermore, the safe transport of medications to their target sites and the ability to use a trigger-based release are additional highly valuable characteristics of lipid-based nanocarriers as DDSs. The possible cytotoxic effects of lipid-based nanocarriers are a hindrance to their successful market development. Nevertheless, given the recent technological breakthroughs, the high expectations for the sustained development of lipid-based nanomedicines as drug delivery methods are justified.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; agree to be accountable for all aspects of the work.
Funding
This research was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) funded by MSIT (RS-2023-00207868).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schottenfeld D, Fraumeni JF. Cancer Epidemiology and Prevention. Oxford University Press; 2006.
2. Sherman ME, Troester MA, Hoadley KA, Anderson WF. Morphological and molecular classification of human cancer. In: Cancer Epidemiology and Prevention. Oxford, UK: Oxford University Press; 2017.
3. Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9):170070. doi:10.1098/rsob.170070
4. Pirker R. Conquering lung cancer: current status and prospects for the future. Pulmonology. 2020;26(5):283–290. doi:10.1016/j.pulmoe.2020.02.005
5. Norouzi M, Hardy P. Clinical applications of nanomedicines in lung cancer treatment. Acta biomaterialia. 2021;121:134–142. doi:10.1016/j.actbio.2020.12.009
6. Xu K, Zhang C, Du T, et al. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother. 2021;134:111111. doi:10.1016/j.biopha.2020.111111
7. Carrasco-Esteban E, Domínguez-Rullán JA, Barrionuevo-Castillo P, et al. Current role of nanoparticles in the treatment of lung cancer. J Clin Transl Res. 2021;7(2):140.
8. Razak SA, Mohd Gazzali A, Fisol FA, et al. Advances in nanocarriers for effective delivery of docetaxel in the treatment of lung cancer: an overview. Cancers. 2021;13(3):400.
9. Dhiman N, Awasthi R, Sharma B, Kharkwal H, Kulkarni GT. Lipid nanoparticles as carriers for bioactive delivery. Front Chem. 2021;9:580118. doi:10.3389/fchem.2021.580118
10. Le TT, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306.
11. El-Hussein A, Manoto SL, Ombinda-Lemboumba S, Alrowaili ZA, Mthunzi-Kufa P. A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anti-Cancer Agents Med Chem. 2021;21(2):149–161. doi:10.2174/1871520620666200403144945
12. Babu A, Templeton AK, Munshi A, Ramesh R. Nanoparticle-based drug delivery for therapy of lung cancer: progress and challenges. J Nanomater. 2013;2013:863951. doi:10.1155/2013/863951
13. Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347(2):159–166. doi:10.1016/j.canlet.2014.03.013
14. Catalano A, Iacopetta D, Ceramella J, et al. Multidrug resistance (MDR): a widespread phenomenon in pharmacological therapies. Molecules. 2022;27(3):616. doi:10.3390/molecules27030616
15. Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21(9):3233. doi:10.3390/ijms21093233
16. Yadav D, Kwak M, Chauhan PS, Puranik N, Lee PC, Jin J-O. Cancer Immunotherapy by Immune Checkpoint Blockade and Its Advanced Application Using Bio-Nanomaterials. Elsevier; 2022.
17. Wang Y, Wang M, Wu HX, Xu RH. Advancing to the era of cancer immunotherapy. Cancer Commun. 2021;41(9):803–829. doi:10.1002/cac2.12178
18. Taefehshokr S, Parhizkar A, Hayati S, et al. Cancer immunotherapy: challenges and limitations. Pathol Res Pract. 2022;229:153723. doi:10.1016/j.prp.2021.153723
19. Hodroj K, Barthelemy D, Lega J-C, et al. Issues and limitations of available biomarkers for fluoropyrimidine-based chemotherapy toxicity, a narrative review of the literature. ESMO Open. 2021;6(3):100125. doi:10.1016/j.esmoop.2021.100125
20. Gavas S, Quazi S, Karpiński TM. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res Lett. 2021;16(1):1–21.
21. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121.
22. Feng B, Niu Z, Hou B, Zhou L, Li Y, Yu H. Enhancing triple negative breast cancer immunotherapy by ICG‐templated self‐assembly of paclitaxel nanoparticles. Adv Funct Mater. 2020;30(6):1906605.
23. Chowdhury P, Nagesh PK, Hatami E, et al. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J Coll Interface Sci. 2019;535:133–148. doi:10.1016/j.jcis.2018.09.072
24. Massey AE, Sikander M, Chauhan N, et al. Next-generation paclitaxel-nanoparticle formulation for pancreatic cancer treatment. Nanomedicine. 2019;20:102027. doi:10.1016/j.nano.2019.102027
25. Cojocaru F-D, Botezat D, Gardikiotis I, et al. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics. 2020;12(2):171. doi:10.3390/pharmaceutics12020171
26. Yadav HK, Almokdad AA, Sumia I, Debe MS. Polymer-based nanomaterials for drug-delivery carriers. In: Nanocarriers for Drug Delivery. Elsevier; 2019:531–556.
27. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):1–27.
28. Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30(11):592–599. doi:10.1016/j.tips.2009.08.004
29. Abarca-Cabrera L, Fraga-García P, Berensmeier S. Bio-nano interactions: binding proteins, polysaccharides, lipids and nucleic acids onto magnetic nanoparticles. Biomater Res. 2021;25(1):12. doi:10.1186/s40824-021-00212-y
30. Duan Y, Dhar A, Patel C, et al. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10(45):26777–26791. doi:10.1039/d0ra03491f
31. Reddy MSB, Ponnamma D, Choudhary R, Sadasivuni KK. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers. 2021;13(7):1105. doi:10.3390/polym13071105
32. Din FU, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine. 2017;12:7291–7309. doi:10.2147/IJN.S146315
33. Alavi M, Karimi N, Safaei M. Application of various types of liposomes in drug delivery systems. Adv Pharma Bull. 2017;7(1):3–9. doi:10.15171/apb.2017.002
34. Attama AA, Momoh MA, Builders PF. Lipid nanoparticulate drug delivery systems: a revolution in dosage form design and development. Recent Adv Novel Drug Carrier Syst. 2012;5:107–140.
35. Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal Formulations in Clinical Use: an Updated Review. Pharmaceutics. 2017;9(2):12. doi:10.3390/pharmaceutics9020012
36. Shrestha H, Bala R, Arora S. Lipid-Based Drug Delivery Systems. J Pharma. 2014;2014:801820. doi:10.1155/2014/801820
37. Yadav D, Sandeep K, Pandey D, Dutta RK. Liposomes for drug delivery. J Biotechnol Biomater. 2017;7(4):276. doi:10.4172/2155-952X.1000276
38. Rommasi F, Esfandiari N. Liposomal nanomedicine: applications for drug delivery in cancer therapy. Nanoscale Res Lett. 2021;16(1):95. doi:10.1186/s11671-021-03553-8
39. Wakaskar RR. General overview of lipid-polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J Drug Target. 2018;26(4):311–318. doi:10.1080/1061186X.2017.1367006
40. Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288–303. doi:10.4103/1735-5362.235156
41. Yoon G, Park JW, Yoon I-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. J Pharma Investig. 2013;43(5):353–362. doi:10.1007/s40005-013-0087-y
42. Shiraishi K, Yokoyama M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: a review. Sci Technol Adv Mater. 2019;20(1):324–336. doi:10.1080/14686996.2019.1590126
43. Chen B-M, Cheng T-L, Roffler SR. Polyethylene glycol immunogenicity: theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano. 2021;15(9):14022–14048. doi:10.1021/acsnano.1c05922
44. Chang H-I, Yeh M-K. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49–60. doi:10.2147/IJN.S26766
45. Nakhaei P, Margiana R, Bokov DO, et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioengine Biotechnol. 2021;9. doi:10.3389/fbioe.2021.705886
46. Pinot M, Vanni S, Pagnotta S, et al. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science. 2014;345(6197):693–697. doi:10.1126/science.1255288
47. van Hoogevest P, Wendel A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur J Lipid Sci Technol. 2014;116(9):1088–1107. doi:10.1002/ejlt.201400219
48. Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev. 2016;68(3):701–787. doi:10.1124/pr.115.012070
49. Zhu X, Kong Y, Liu Q, et al. Inhalable dry powder prepared from folic acid-conjugated docetaxel liposomes alters pharmacodynamic and pharmacokinetic properties relevant to lung cancer chemotherapy. Pulm Pharmacol Ther. 2019;55:50–61. doi:10.1016/j.pupt.2019.02.001
50. Gandhi M, Pandya T, Gandhi R, et al. Inhalable liposomal dry powder of gemcitabine-HCl: formulation, in vitro characterization and in vivo studies. Int J Pharm. 2015;496(2):886–895. doi:10.1016/j.ijpharm.2015.10.020
51. Lin C, Wong BCK, Chen H, et al. Pulmonary delivery of triptolide-loaded liposomes decorated with anti-carbonic anhydrase IX antibody for lung cancer therapy. Sci Rep. 2017;7(1):1–12. doi:10.1038/s41598-016-0028-x
52. Jiang K, Shen M, Xu W. Arginine, glycine, aspartic acid peptide-modified paclitaxel and curcumin co-loaded liposome for the treatment of lung cancer: in vitro/vivo evaluation. Int J Nanomedicine. 2018;13:2561. doi:10.2147/IJN.S157746
53. Jiménez-López J, Bravo-Caparrós I, Cabeza L, et al. Paclitaxel antitumor effect improvement in lung cancer and prevention of the painful neuropathy using large pegylated cationic liposomes. Biomed Pharmacother. 2021;133:111059. doi:10.1016/j.biopha.2020.111059
54. Riaz MK, Zhang X, Wong KH, et al. Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy. Int J Nanomedicine. 2019;14:2879. doi:10.2147/IJN.S192219
55. Ghosh B, Biswas S. Polymeric micelles in cancer therapy: state of the art. J Control Release. 2021;332:127–147. doi:10.1016/j.jconrel.2021.02.016
56. Tumbrink HL, Heimsoeth A, Sos ML. The next tier of EGFR resistance mutations in lung cancer. Oncogene. 2021;40(1):1–11. doi:10.1038/s41388-020-01510-w
57. Leng D, Hu J, Huang X, et al. Promoted delivery of salinomycin to lung cancer through epidermal growth factor receptor aptamers coupled DSPE-PEG2000 nanomicelles. J Nanosci Nanotechnol. 2018;18(8):5242–5251. doi:10.1166/jnn.2018.15424
58. Shen Y, Li X, Dong D, Zhang B, Xue Y, Shang P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am J Cancer Res. 2018;8(6):916.
59. Wang J, Su G, Yin X, et al. Non-small cell lung cancer-targeted, redox-sensitive lipid-polymer hybrid nanoparticles for the delivery of a second-generation irreversible epidermal growth factor inhibitor—Afatinib: in vitro and in vivo evaluation. Biomed Pharmacother. 2019;120:109493. doi:10.1016/j.biopha.2019.109493
60. Chan M-H, Chan Y-C, Liu R-S, Hsiao MJN. A selective drug delivery system based on phospholipid-type nanobubbles for lung cancer therapy. Nanomedicine. 2020;15(27):2689–2705. doi:10.2217/nnm-2020-0273
61. Paliwal R, Paliwal SR, Kenwat R, Kurmi BD, Sahu MK. Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin Therap Patents. 2020;30(3):179–194. doi:10.1080/13543776.2020.1720649
62. da Rocha MCO, da Silva PB, Radicchi MA, et al. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J Nanobiotechnol. 2020;18(1):1–20.
63. Han Y, Zhang P, Chen Y, Sun J, Kong F. Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. Int J Mol Med. 2014;34(1):191–196. doi:10.3892/ijmm.2014.1770
64. Pi C, Zhao W, Zeng M, et al. Anti-lung cancer effect of paclitaxel solid lipid nanoparticles delivery system with curcumin as co-loading partner in vitro and in vivo. Drug Delivery. 2022;29(1):1878–1891. doi:10.1080/10717544.2022.2086938
65. Yi Y, Liang A-H, Liu T, Zhao Y, Cao C-Y. Analysis of causes for adverse reaction of Yuxingcao injection. Zhongguo Zhong Yao Za Zhi. 2008;33(21):2439–2442.
66. Zhao Y, Chang Y-X, Hu X, Liu C-Y, Quan L-H, Liao Y. Solid lipid nanoparticles for sustained pulmonary delivery of Yuxingcao essential oil: preparation, characterization and in vivo evaluation. Int J Pharma. 2017;516(1–2):364–371. doi:10.1016/j.ijpharm.2016.11.046
67. Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev. 2021;6(12):1078–1094. doi:10.1038/s41578-021-00358-0
68. Schoenmaker L, Witzigmann D, Kulkarni JA, et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharma. 2021;601:120586. doi:10.1016/j.ijpharm.2021.120586
69. Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27(4):710–728. doi:10.1016/j.ymthe.2019.02.012
70. Chen J, Ye Z, Huang C, et al. Lipid nanoparticle-mediated lymph node–targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc Natl Acad Sci USA. 2022;119(34):e2207841119. doi:10.1073/pnas.2207841119
71. Hwang J, An E-K, Kim S-J, Zhang W, Jin J. Escherichia coli mimetic gold nanorod-mediated photo-and immunotherapy for treating cancer and its metastasis. ACS Nano. 2022;16(5):8472–8483. doi:10.1021/acsnano.2c03379
72. Zhang W, Xu L, Park H-B, et al. Escherichia coli adhesion portion FimH functions as an adjuvant for cancer immunotherapy. Protein Cell. 2020;11(1):1–14. doi:10.1007/s13238-019-0623-2
73. Yan J, Zhang Y, Du S, et al. Nanomaterials mediated co‐stimulation of toll‐like receptors and CD40 for antitumor immunity. Adv Mater. 2022;34:2207486. doi:10.1002/adma.202207486
74. Nakamura T, Sato T, Endo R, et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J Immunother Cancer. 2021;9(7):154.
75. Jaiswal P, Gidwani B, Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):27–40. doi:10.3109/21691401.2014.909822
76. Haider M, Abdin SM, Kamal L, Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics. 2020;12(3):288. doi:10.3390/pharmaceutics12030288
77. Elmowafy M, Al-Sanea MM. Nanostructured lipid carriers (NLCs) as drug delivery platform: advances in formulation and delivery strategies. Saudi Pharm J. 2021;29(9):999–1012. doi:10.1016/j.jsps.2021.07.015
78. Fang C-L, Al-Suwayeh S, Fang J-Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol. 2013;7(1):41–55. doi:10.2174/187221013804484827
79. Chauhan I, Yasir M, Verma M, Singh AP. Nanostructured lipid carriers: a groundbreaking approach for transdermal drug delivery. Adv Pharma Bull. 2020;10(2):150. doi:10.34172/apb.2020.021
80. Poonia N, Kharb R, Lather V, Pandita D. Nanostructured lipid carriers: versatile oral delivery vehicle. Future Sci OA. 2016;2(3):FSO135. doi:10.4155/fsoa-2016-0030
81. Salvi VR, Pawar P. Nanostructured lipid carriers (NLC) system: a novel drug targeting carrier. J Drug Deliv Sci Technol. 2019;51:255–267. doi:10.1016/j.jddst.2019.02.017
82. Izza NM, Suga K, Okamoto Y, et al. Systematic characterization of nanostructured lipid carriers from cetyl palmitate/caprylic triglyceride/tween 80 mixtures in an aqueous environment. Langmuir. 2021;37(14):4284–4293. doi:10.1021/acs.langmuir.1c00270
83. Han Y, Li Y, Zhang P, et al. Nanostructured lipid carriers as novel drug delivery system for lung cancer gene therapy. Pharm Dev Technol. 2016;21(3):277–281. doi:10.3109/10837450.2014.996900
84. Cao C, Wang Q, Liu Y. Lung cancer combination therapy: doxorubicin and β-elemene co-loaded, pH-sensitive nanostructured lipid carriers. In: Drug Design, Development and Therapy. Taylor & Francis; 2019:1087–1098.
85. Guo S, Zhang Y, Wu Z, et al. Synergistic combination therapy of lung cancer: cetuximab functionalized nanostructured lipid carriers for the co-delivery of paclitaxel and 5-demethylnobiletin. Biomed Pharmacother. 2019;118:109225. doi:10.1016/j.biopha.2019.109225
86. Du M, Yin J. Dual-drug nanosystem: etoposide prodrug and cisplatin coloaded nanostructured lipid carriers for lung cancer therapy. In: Drug Design, Development and Therapy. Taylor & Francis; 2022:4139–4149.
87. Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol. 2019;32(8):1469–1486. doi:10.1021/acs.chemrestox.9b00204
88. Patel K, Bothiraja C, Mali A, Kamble R. Investigation of sorafenib tosylate loaded liposomal dry powder inhaler for the treatment of non-small cell lung cancer. Particulate Sci Technol. 2021;39(8):990–999. doi:10.1080/02726351.2021.1906367
89. Berkenfeld K, Lamprecht A, McConville JT. Devices for dry powder drug delivery to the lung. AAPS PharmSciTech. 2015;16(3):479–490. doi:10.1208/s12249-015-0317-x
90. Zhang T, Chen Y, Ge Y, Hu Y, Li M, Jin Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm Sin B. 2018;8(3):440–448. doi:10.1016/j.apsb.2018.03.004
91. Adel IM, ElMeligy MF, Abdelrahim MEA, et al. Design and characterization of spray-dried proliposomes for the pulmonary delivery of curcumin. Int J Nanomedicine. 2021;16:2667–2687. doi:10.2147/IJN.S306831
92. Parvathaneni V, Kulkarni NS, Shukla SK, et al. Systematic development and optimization of inhalable pirfenidone liposomes for non-small cell lung cancer treatment. Pharmaceutics. 2020;12(3):206. doi:10.3390/pharmaceutics12030206
93. Chen T, Gong T, Zhao T, Fu Y, Zhang Z, Gong T. A comparison study between lycobetaine-loaded nanoemulsion and liposome using nRGD as therapeutic adjuvant for lung cancer therapy. Eur J Pharm Sci. 2018;111:293–302. doi:10.1016/j.ejps.2017.09.041
94. Naik H, Sonju JJ, Singh S, et al. Lipidated peptidomimetic ligand-functionalized HER2 targeted liposome as nano-carrier designed for doxorubicin delivery in cancer therapy. Pharmaceuticals. 2021;14(3):221. doi:10.3390/ph14030221
95. Miao YQ, Chen MS, Zhou X, et al. Chitosan oligosaccharide modified liposomes enhance lung cancer delivery of paclitaxel. Acta Pharmacol Sin. 2021;42(10):1714–1722. doi:10.1038/s41401-020-00594-0
96. Fu S, Zhao Y, Sun J, et al. Integrin α(v)β(3)-targeted liposomal drug delivery system for enhanced lung cancer therapy. Colloids Surf B Biointerfaces. 2021;201:111623. doi:10.1016/j.colsurfb.2021.111623
97. Ying X. Dequalinium-mediated mitochondria-targeting drug liposomes for the treatment of drug-resistant lung cancer. In: Liposome-Based Drug Delivery Systems. Springer; 2021:345–364.
98. Onodera R, Morioka S, Unida S, Motoyama K, Tahara K, Takeuchi H. Design and evaluation of folate-modified liposomes for pulmonary administration in lung cancer therapy. Eur J Pharma Sci. 2022;168:106081. doi:10.1016/j.ejps.2021.106081
99. Khan A, Alsahli MA, Aljasir MA, et al. Experimental and theoretical insights on chemopreventive effect of the liposomal thymoquinone against benzo [a] pyrene-induced lung cancer in Swiss albino mice. J Inflamm Res. 2022;15:2263. doi:10.2147/JIR.S358632
100. Almurshedi AS, Radwan M, Omar S, et al. A novel pH-sensitive liposome to trigger delivery of Afatinib to cancer cells: impact on lung cancer therapy. J Mol Liq. 2018;259:154–166. doi:10.1016/j.molliq.2018.03.024
101. Jin X, Yang Q, Cai N, Zhang Z. A cocktail of betulinic acid, parthenolide, honokiol and ginsenoside Rh2 in liposome systems for lung cancer treatment. Nanomedicine. 2020;15(1):41–54. doi:10.2217/nnm-2018-0479
102. Wang Y, Fu M, Liu J, et al. Inhibition of tumor metastasis by targeted daunorubicin and dioscin codelivery liposomes modified with PFV for the treatment of non-small-cell lung cancer. Int J Nanomedicine. 2019;14:4071. doi:10.2147/IJN.S194304
103. Ma J, Zhuang H, Zhuang Z, et al. Development of docetaxel liposome surface modified with CD133 aptamers for lung cancer targeting. Artif Cells, Nanomed Biotechnol. 2018;46(8):1864–1871. doi:10.1080/21691401.2017.1394874
104. Gai C, Liu C, Wu X, et al. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death Dis. 2020;11(9):1–11. doi:10.1038/s41419-020-02939-3
105. Tie Y, Zheng H, He Z, et al. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct Target Ther. 2020;5(1):1–15. doi:10.1038/s41392-020-0115-0
106. Zhang C, Zhang S, Zhi D, Zhao Y, Cui S, Cui J. Co-delivery of paclitaxel and survivin siRNA with cationic liposome for lung cancer therapy. Colloids Surf A. 2020;585:124054. doi:10.1016/j.colsurfa.2019.124054
107. Zhang M, Li M, Du L, Zeng J, Yao T, Jin Y. Paclitaxel-in-liposome-in-bacteria for inhalation treatment of primary lung cancer. Int J Pharm. 2020;578:119177. doi:10.1016/j.ijpharm.2020.119177
108. Moosavian SA, Bianconi V, Pirro M, Sahebkar A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin Cancer Biol. 2021;69:337–348. doi:10.1016/j.semcancer.2019.09.025
109. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi:10.3389/fphar.2015.00286
110. Lei Y, Li X, Huang Q, Zheng X, Liu M. Progress and challenges of predictive biomarkers for immune checkpoint blockade. Front Oncol. 2021;11. doi:10.3389/fonc.2021.617335
111. Yao Y, Zhou Y, Liu L, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7. doi:10.3389/fmolb.2020.00193
112. Subhan MA, Choudhury KP, Neogi N. Advances with molecular nanomaterials in industrial manufacturing applications. Nanomanufacturing. 2021;1(2):75–97. doi:10.3390/nanomanufacturing1020008
113. Pandey H, Rani R, Agarwal V. Liposome and their applications in cancer therapy. Braz Archiv Biol Technol. 2016;59. doi:10.1590/1678-4324-2016150477
114. Gagliardi A, Giuliano E, Venkateswararao E, et al. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.601626
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
