Back to Journals » Drug Design, Development and Therapy » Volume 12
Linezolid: a review of its properties, function, and use in critical care
Authors Hashemian SMR , Farhadi T, Ganjparvar M
Received 3 February 2018
Accepted for publication 24 April 2018
Published 18 June 2018 Volume 2018:12 Pages 1759—1767
DOI https://doi.org/10.2147/DDDT.S164515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Seyed MohammadReza Hashemian,1,2 Tayebeh Farhadi,1 Mojdeh Ganjparvar3
1Chronic Respiratory Disease Research Center (CRDRC), National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2Clinical Tuberculosis and Epidemiology Research Center, National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran; 3Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran
Abstract: Linezolid can be considered as the first member of the class of oxazolidinone antibiotics. The compound is a synthetic antibiotic that inhibits bacterial protein synthesis through binding to rRNA. It also inhibits the creation of the initiation complex during protein synthesis which can reduce the length of the developed peptide chains, and decrease the rate of reaction of translation elongation. Linezolid has been approved for the treatment of infections caused by vancomycin-resistant Enterococcus faecium, hospital-acquired pneumonia caused by Staphylococcus aureus, complicated skin and skin structure infections (SSSIs), uncomplicated SSSIs caused by methicillin-susceptible S. aureus or Streptococcus pyogenes, and community-acquired pneumonia caused by Streptococcus pneumoniae. Analysis of high-resolution structures of linezolid has demonstrated that it binds a deep cleft of the 50S ribosomal subunit that is surrounded by 23S rRNA nucleotides. Mutation of 23S rRNA was shown to be a linezolid resistance mechanism. Besides, mutations in specific regions of ribosomal proteins uL3 and uL4 are increasingly associated with linezolid resistance. However, these proteins are located further away from the bound drug. The methicillin-resistant S. aureus and vancomycin-resistant enterococci are considered the most common Gram-positive bacteria found in intensive care units (ICUs), and linezolid, as an antimicrobial drug, is commonly utilized to treat infected ICU patients. The drug has favorable in vitro and in vivo activity against the mentioned organisms and is considered as a useful antibiotic to treat infections in the ICU.
Keywords: linezolid, intensive care unit, MRSA, VRE, antibacterial drugs
Introduction
Linezolid can be considered as the first member of the class of oxazolidinone antibiotics. Oxazolidinones were first introduced in 1978 for their effectiveness in the control of plant diseases. Six years later, their antibacterial characteristics, with significantly improved antibacterial properties relative to their progenitor compounds, were documented.1 These oxazolidinone compounds are referred to as the first real lead compounds in the oxazolidinone family.
A lead compound can be defined as a compound that displays pharmacological parameters proposing the compound’s value as a starting point for therapeutics development.2 Further structural discussion led to the development of piperazine derivatives using lead compounds, as shown in studies by Barbachyn et al.2 Such compounds were selected for further modification as they possessed outstanding antibacterial activity with a suitable safety profile. Linezolid was introduced in 1996 following intensive examinations at Pharmacia3 and has since been identified as a lead compound. Linezolid was approved by the US Food and Drug Administration in 2000. During the last 40 years, oxazolidinones have been considered a truly new class of antibiotics which are currently used in clinics.4
Mechanism of action of linezolid
Linezolid is a synthetic antibiotic which prevents the synthesis of bacterial protein via binding to rRNA on both the 30S and 50S ribosomal subunits.5 It inhibits the formation of initiation complex which can reduce the length of the developed peptide chains and decrease the rate of translation reaction.5 However, the initiation process at the site of inhibition takes place prior to that of other protein synthesis inhibitors that prevent the elongation procedure. Because of the unique site of inhibition, cross-resistance to other protein synthesis inhibitors has not yet been demonstrated.6 Linezolid may also prevent the expression of virulence elements leading to decreased toxins produced by Gram-positive pathogens.7 The chemical structure of linezolid is shown in Figure 1. The activity of the compound is increased by the morpholino group in the first ring (from the left) and the fluoride atom in the second ring.4
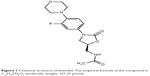  | Figure 1 Chemical structure of linezolid. The empirical formula of the compound is C16H20FN3O4 (molecular weight: 337.35 g/mol). |
Structure–activity relationship of linezolid
The structure–activity relationship studies on oxazolidinones revealed that the N-aryl group and 5-S configuration are essential for the activity.8 The 5-acylaminomethyl group is responsible for the activity. The electron-withdrawing group in the aryl ring has been shown to increase the activity. Extra substituents on the proximal aromatic ring do not affect the antibacterial activity but can change the solubility and pharmacokinetics.8
Applications of linezolid
Summary of antibacterial activity
Linezolid has been approved by the Food and Drug Administration for the treatment of the following: (a) hospital-acquired pneumonia caused by Staphylococcus aureus, including methicillin-susceptible (MSSA) and methicillin-resistant S. aureus (MRSA) strains, or Streptococcus pneumoniae including multidrug-resistant strains; (b) vancomycin-resistant Enterococcus faecium (VREF) infections, including cases with concurrent bacteremia; (c) complicated skin and skin structure infections (SSSIs), including diabetic foot infections (DFIs) without concomitant osteomyelitis, caused by S. aureus (MSSA and MRSA), Streptococcus pyogenes, or Streptococcus agalactiae; (d) uncomplicated SSSIs caused by MSSA or S. pyogenes; (e) community-acquired pneumonia caused by S. pneumoniae, including cases with simultaneous bacteremia, or MSSA;9 and (f) pneumococcal meningitis caused by penicillin-resistant S. pneumoniae.10
Recent advances in the use of linezolid
Linezolid-containing regimens have been suggested as promising potential alternatives to treat patients with multidrug-resistant tuberculosis (MDR-TB) or extensively drug-resistant TB (XDR-TB). Therefore, linezolid could be considered as an effective choice for treating MDR-TB/XDR-TB.11
In the recent published guidelines for the treatment of MRSA pneumonia, linezolid has been considered as a first-line antibiotic.12 Moreover, several studies including the recent ZEPHyR trial13 have reported higher treatment effectiveness for linezolid compared to vancomycin. However, it remains debatable whether linezolid is better than vancomycin for its approved indications such as SSSIs and nosocomial pneumonia or not. Several recent studies have supported the clinical use of linezolid in complicated MRSA-SSSIs, including DFIs without osteomyelitis.14 Figure 2 shows the pictorial representation of linezolid applications.
  | Figure 2 Pictorial representation of the linezolid applications. |
Examples of treatment effects
In a case report, linezolid was used in addition to penicillin and clindamycin for suppression of toxic shock syndrome (TSS). In the study, the patient had right upper extremity necrotizing fasciitis and group A streptococcus TSS that were treated by adding linezolid as the patient showed no improvement on penicillin and clindamycin.15
Studies have suggested that linezolid can be efficient in treating MDR-TB and XTR-TB.16–27
In a case report, a patient with subarachnoid hemorrhage, who underwent ventriculostomy and embolization of cerebral aneurysms, was affected by multiple infections after her operation. The patient was successfully treated with linezolid.28
In another study, ten patients with central nervous system infections (infections in three cases were caused by mycobacteria) were treated by linezolid because of its good cerebrospinal fluid penetration.29
In a study on 80 patients with MRSA and vancomycin-intermediate S. aureus endocarditis, linezolid alone was administered to 66.7% of patients, while the rest received a combination therapy.30
Linezolid has been reported to be very effective in the treatment of ventilator-associated pneumonia and catheter-related bacteremia, and hence, it is reasonable to use linezolid in the intensive care unit (ICU).31
Pharmacokinetics
Linezolid is very well absorbed orally with a bioavailability of 100%.1,32 The presence of food does not affect its absorption.5 Therefore, the administration route of antibiotic can be changed from intravenous (IV) to oral (per os [PO]) in clinically stable patients.6 Moreover, co-administration with antacids like magnesium hydroxide and aluminum hydroxide had no effect on the oral absorption.33
Plasma protein-binding level of the molecule is approximately 31%. The volume of distribution approximates to the whole-body water content of 40–50 L, and the plasma half-life ranges from 3.4 to 7.4 h. The compound is metabolized to inactive metabolites including hydroxyethyl glycine and aminoethoxy-acetic acid.33 The clearance rate is 80±29 mL/min through both nonrenal and renal mechanisms, and renal tubular reabsorption may take place. A fraction of the dose is excreted in unaltered form in urine.34 The pharmacokinetics of linezolid in different groups of patients with dissimilar doses has been studied in detail.33 A low degree of nonlinearity has been found, with a 30% reduction in clearance, after a fivefold increase in the dose. The nonlinearity is not related to the therapeutic dosage range. Plasma concentrations of linezolid in elderly patients, and patients with mild-to-moderate hepatic damage or mild-to-chronic renal failure were similar to those obtained in healthy or young volunteers. It has been reported that a dose adjustment is not necessary when females have a higher concentration compared to males. Patients with severe renal impairment with the requirement for hemodialysis are reported to have seven- to eightfold higher exposures to the drug metabolites than patients with normal renal function. Hence, recommendation should be carefully evaluated. Clearance of linezolid is shown to be higher in children compared to adults. This can lead to higher requirement of daily doses of drug per kilogram of body weight in children.33
Adverse effects
Some of the adverse effects associated with linezolid are as follows: (a) peripheral35 and ocular36–39 neuropathy; (b) anemia that occurs by direct effect of linezolid on red cell population of bone marrow;40 (c) thrombocytopenia;41–43 (d) hyperlactatemia (lactic acidosis with plasma lactate level >2 mmol/L);40,44–46 (e) diarrhea, nausea, and headache;47,48 (f) hypoglycemia;49 and (g) reticulocytopenia.50
Drug interactions
When a drug is administered along with a second drug, a change in the drug’s effectiveness on the body may occur. Such interaction may delay, diminish, or increase the absorption of either or both the drugs or cause adverse effects.51–53 Linezolid can be safely co-administered with aztreonam; however, there is no enough evidence about the interaction between linezolid and rifampin.40 Co-administration with Gram-negative antibiotics, ceftazidime, ciprofloxacin, meropenem, and gentamicin had no adverse effect. Besides, using linezolid with antifungal drugs such as amphotericin B and azoles, aminoglycosides, antivirals, fluoroquinolones, or β-lactams did not affect their sufficiency. It therefore seems that linezolid can be used with other antimicrobials with no interaction.40
Linezolid can cause life-threatening serotonin toxicity when combined with serotonin reuptake inhibitors since it is a nonspecific inhibitor of monoamine oxidase.54,55 While clinicians must be aware of this potentially severe interaction and carefully monitor patients who are given linezolid in combination with serotonergic therapeutics, findings indicate that linezolid is not contraindicated in this condition.56
Patents of linezolid
There are a number of patents as inventions to provide better forms of the linezolid in oral dosage forms. Some examples are discussed here. An invention (publication number: US6451345B1) provided taste-masked microcapsules of linezolid, as oral dosage forms in which the bitter taste of linezolid included therein is covered by a mixture of microencapsulation. These oral dosage forms are suitable for the oral administration.57 Another invention (publication number: US9492459B2) aims to provide a novel pharmaceutical composition comprising linezolid form III along with pharmaceutically satisfactory excipients and a procedure to make such composition.58 An invention (publication number: US20170066728A1) relates to a process of preparation of enantiomeric pure linezolid form I. The process comprised converting an enantiomerically pure linezolid hydroxide compound of formula II to linezolid form I compound of formula I.59
Comparison with other antibiotics
Vancomycin has been considered the “gold standard” drug against serious MRSA infections. Although, the weak clinical effects, appearance of less susceptible strains, and enhanced nephrotoxicity with high-dose treatment are challenging its current role as a first-line treatment. Linezolid is suggested for PO or IV treatment of SSSIs and pneumonia caused by MRSA. Daptomycin (IV) should be used in complicated SSSIs and in patients with right-sided endocarditis as well as MRSA bacteremia but should not be used to treat MRSA pneumonia. Both telavancin and tigecycline can be used as alternative (IV) treatments for SSSIs caused by MRSA; however, safety concerns have restricted the usage of these drugs. Ceftaroline is the latest approved parenteral drug for the treatment of SSSIs caused by MRSA. A number of investigational factors for the treatment of infections caused by drug-resistant Gram-positive bacteria are being developed originally to treat MRSA infections. These factors include tedizolid, dalbavancin, and oritavancin.14 The linezolid vs daptomycin and vancomycin comparison is discussed below with some examples.
Linezolid vs daptomycin
A report of the treatment of vancomycin-resistant enterococcal (VRE) bacteremia showed that 30-day mortality of daptomycin was higher than that of linezolid. Moreover, the infection-related mortality and the in-hospital mortality were higher in the daptomycin group compared to the linezolid group. A study showed that treatment with daptomycin led to abnormal liver function test results, although adverse effects had no significant difference.60–63
In another study, among 212 patients with VRE, 141 received daptomycin and 71 received linezolid. All-cause 14-day mortality was higher in the daptomycin group (36.9% vs 21.1%; p=0.03), and higher-dose daptomycin led to enhanced survival than lower-dose daptomycin. Compared to lower-dose daptomycin, higher-dose daptomycin and linezolid independently predicted lower mortality. However, in terms of mortality, linezolid was not superior to higher-dose daptomycin and higher-dose daptomycin had more survival benefits than linezolid, and the findings revealed that the recommended daptomycin dose is suboptimal for treating VRE bacteremia.64
Linezolid vs vancomycin
The results of a systematic review and meta-analysis indicated that there is no difference between linezolid and vancomycin in the treatment of nosocomial pneumonia. Besides, the results showed that there were no statistically significant differences between linezolid and comparator vancomycin or teicoplanin in analysis of microbiological eradication.65 Similar results were obtained when the analysis was stratified according to the comparator vancomycin or teicoplanin. In the main analysis, there were statistically significant higher rate of gastrointestinal events with linezolid compared to glycopeptides. In addition, compared to vancomycin, there was a significantly higher rate of thrombocytopenia with linezolid. In the incidence of renal failure, there was no statistically significant variance between the treatments.65
Some studies demonstrated that administration of linezolid is more cost-effective than vancomycin in the treatment of MRSA infection because of earlier discharge from the hospital.66,67 Generally, linezolid may reduce mortality of patients compared to vancomycin.68,69
In a report, linezolid was compared with vancomycin for the treatment of patients with suspected or confirmed skin and soft tissue infections caused by MRSA. Skin and soft tissue infections are common causes of mortality in both hospital and community settings. The study included patients with MRSA infections involving substantial areas of skin or deeper soft tissues, such as abscesses, cellulitis, infected ulcers, or burns. In the intent-to-treat population, 92.2% and 88.5% of patients were treated with linezolid and vancomycin, respectively. Linezolid outcomes were superior to those of vancomycin, and drug-related adverse effects were similar in both linezolid and vancomycin group. The results of this study showed that linezolid therapy is superior to vancomycin for the treatment of skin and soft tissue infections due to MRSA.70–72
Mechanism of resistance
Analysis of high-resolution structures of linezolid showed that it binds to a deep cleft of 50S ribosomal subunit that is surrounded by 23S rRNA nucleotides.73 Accordingly, mutation of 23S rRNA has been established as one of the linezolid resistance mechanisms. Moreover, mutations in particular regions of ribosomal proteins uL3 and uL4 are increasingly being associated with linezolid resistance, although these proteins are placed further away from the bound drug. Furthermore, based on new findings on the Cfr methyltransferase, the transferable modification of 23S rRNA can cause high resistance to linezolid. Such precise information of the linezolid-binding site has enabled the design of a novel generation of oxazolidinones that display enhanced characteristics against the identified resistance mechanisms.74
Recently, a novel oxazolidinone resistance gene, optrA, has been identified, and the extent to which this gene is expressed in E. faecalis and E. faecium has been investigated.75 OptrA is an adenosine triphosphate-binding cassette (ABC) transporter. A common mechanism by which bacteria develop antibiotic resistance is by using the ABC transporters to strongly pump the drugs from the cell. The ABC-F family contains proteins conferring resistance to a diversity of clinically significant ribosome-targeting antibiotics. It has been reported that these proteins use ribosome protection mechanisms by interacting with the ribosome and shifting the drug from its binding site.76
Use of linezolid in critical care
Severe infections in critically ill patients exhibit high rates of morbidity and mortality, and approximately half of the bloodstream infections in such patients are caused by Gram-positive bacteria.77 A major part of the Gram-positive infections are caused by multidrug-resistant strains including MRSA and VRE that are extremely common in the ICUs.78 Linezolid is an antimicrobial drug that is commonly utilized by the ICU patients. The drug has favorable in vitro and in vivo activity against the mentioned organisms and is considered a useful antibiotic to treat infections in the ICU.79,80,91 Some recent clinical trial findings on the use of linezolid in ICU are summarized in Table 1.
Conclusion
The present treatment options for infections caused by common pathogens in the ICU are limited. Inclusion of linezolid in multiple clinical practice guidelines demonstrates that the drug can be a beneficial addition to the antibiotic armamentarium against MRSA and VRE.4 Outcomes from the current clinical trials on linezolid for MRSA pneumonia and VRE infections are worthwhile for clinicians and give certainty that the drug is efficient. Although linezolid is efficient against multidrug-resistant strains, researchers must strive and optimize infection-control measures to inhibit their spread. While most patients tolerate linezolid well, the ongoing surveillance is important to find potential and serious adverse reactions including thrombocytopenia and anemia as well as permanent adverse effects such as optic neuritis and peripheral neuropathy. Further clinical investigations are required to clarify the role of linezolid in various patient populations and treating particular conditions. Cost-control issues affect antimicrobial stewardship plans; therefore, more investigation is required to evaluate the cost-effectiveness of linezolid, and the resulting measurements are essential to help reduce health care costs in resource-restricted settings.
Disclosure
The authors report no conflicts of interest in this work.
References
Ford CW, Zurenko GE, Barbachyn MR. The discovery of linezolid, the first oxazolidinone antibacterial agent. Curr Drug Targets Infect Disord. 2001;1(2):181–199. | ||
Barbachyn MR, Toops DS, Grega KC, et al. Synthesis and antibacterial activity of new tropone-substituted phenyloxazolidinone antibacterial agents 2. Modification of the phenyl ring – the potentiating effect of fluorine substitution on in vivo activity. Bioorg Med Chem Lett. 1996;6(9):1009–1014. | ||
Zyvox [package insert]. Kalamazoo, MI: Pharmacia-Upjohn; 2004. | ||
Watkins RR, Lemonovich TL, File TM Jr. An evidence-based review of linezolid for the treatment of methicillin-resistant Staphylococcus aureus (MRSA): place in therapy. Core Evid. 2012;7:131–143. | ||
Batts DH. Linezolid-a new option for treating Gram-positive infections. Oncology. 2000;14(8 Suppl 6):23–29. | ||
Ament PW, Jamshed N, Horne JP. Linezolid: its role in the treatment of Gram-positive, drug-resistant bacterial infections. Am Fam Phys. 2002;65(4):663–670. | ||
Zurenko GE, Gibson JK, Shinabarger DL, Aristoff PA, Ford CW, Tarpley WG. Oxazolidinones: a new class of antibacterials. Curr Opin Pharmacol. 2001;1:470–476. | ||
Poce G, Cocozza M, Consalvi S, Biava M. SAR analysis of new anti-TB drugs currently in pre-clinical and clinical development. Eur J Med Chem. 2014;86:335–351. | ||
Birmingham MC, Rayner CR, Meagher AK, Flavin SM, Batts DH, Schentag JJ. Linezolid for the treatment of multidrug-resistant, Gram-positive infections: experience from a compassionate-use program. Clin Infect Dis. 2003;36(2):159–168. | ||
Faella F, Pagliano P, Fusco U, Attanasio V, Conte M. Combined treatment with ceftriaxone and linezolid of pneumococcal meningitis: a case series including penicillin-resistant strains. Clin Microbiol Infect. 2006;12(4):391–394. | ||
Zhang X, Falagas ME, Vardakas KZ, et al. Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J Thorac Dis. 2015;7(4):603–615. | ||
Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. | ||
Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621–629. | ||
Rodvold KA, Kevin W, McConeghy KW. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis. 2014;58 Suppl 1:S20–S27. | ||
Rac H, Bojikian KD, Lucar J, Barber KE. Successful treatment of necrotizing fasciitis and streptococcal toxic shock syndrome with the addition of linezolid. Case Rep Infect Dis. 2017;2017:5720708. | ||
Cox H, Ford N. Linezolid for the treatment of complicated drug resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2012;16(4):447–454. | ||
Tang S, Yao L, Hao X, et al. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J. 2015;45(1):161–170. | ||
Brown AN, Drusano GL, Adams JR, et al. Preclinical evaluations to identify optimal linezolid regimens for tuberculosis therapy. mBio. 2015;6(6):e01741-15. | ||
Agyeman AA, Ofori-Asenso R. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2016;15(1):41. | ||
Maartens G, Benson CA. Linezolid for treating tuberculosis: a delicate balancing act. EBioMedicine. 2015;2(11):1568–1569. | ||
Ramírez-Lapausa M, Pascual Pareja JF, Carrillo Gómez R, et al. Retrospective study of tolerability and efficacy of linezolid in patients with multidrug-resistant tuberculosis (1998–2014). Enferm Infecc Microbiol Clin. 2016;34(2):85–90. | ||
Bhuniya S, Mohapatra PR, Panigrahi MK, et al. Linezolid in drug-resistant tuberculosis: haste makes waste. Eur Respir J. 2015;46(6):1843–1844. | ||
Wasserman S, Meintjes G, Maartens G. Linezolid in the treatment of drug-resistant tuberculosis: the challenge of its narrow therapeutic index. Expert Rev Anti Infect Ther. 2016;14(10):901–915. | ||
Lee M, Song T, Kim Y, et al. Linezolid for XDR-TB – final study outcomes. N Engl J Med. 2015;373(3):290–291. | ||
Sotgiu G, Pontali E, Migliori GB. Linezolid to treat MDR-/XDR-tuberculosis: available evidence and future scenarios. Eur Respir J. 2015;45(1):25–29. | ||
Bolhuis MS, Tiberi S, Sotgiu G, et al. Linezolid tolerability in multidrug-resistant tuberculosis: a retrospective study. Eur Respir J. 2015;46(4):1205–1207. | ||
Liu Y, Bao P, Wang D, et al. Clinical outcomes of linezolid treatment for extensively drug-resistant tuberculosis in Beijing, China: a hospital-based retrospective study. Jpn J Infect Dis. 2015;68(3):244–247. | ||
Shaikh ZH, Peloquin CA, Ericsson CD. Successful treatment of vancomycin-resistant Enterococcus faecium meningitis with linezolid: case report and literature review. Scand J Infect Dis. 2001;33(5):375–379. | ||
Rupprecht TA, Pfister HW. Clinical experience with linezolid for the treatment of central nervous system infections. Eur J Neurol. 2005;12(7):536–542. | ||
Falagas ME, Manta KG, Ntziora F, et al. Linezolid for the treatment of patients with endocarditis: a systematic review of the published evidence. J Antimicrob Chemother. 2006;58(2):273–280. | ||
Rodríguez O, Alvarez F, Oltra R, et al. Use of linezolid in critically ill patients admitted to intensive care units. Rev Esp Quimioter. 2009;22(2):68–75. | ||
Otter JA, French GL. Molecular epidemiology of community-associated methicillin-resistant Staphylococcus aureus in Europe. Lancet Infect Dis. 2010;10(4):227–239. | ||
Dryden MS. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J Antimicrob Chemother. 2011;66(4):iv7–iv15. | ||
Macgowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J Antimicrob Chemother. 2003;51 Suppl 2:ii17–ii25. | ||
Rho JP, Sia IG, Crum BA, Dekutoski MB, Trousdale RT. Linezolid-associated peripheral neuropathy. Mayo Clin Proc. 2004;79(7):927–930. | ||
Karuppannasamy D, Raghuram A, Sundar D. Linezolid-induced optic neuropathy. Indian J Ophthalmol. 2014;62(4):497–500. | ||
Xerri O, Lemaire B, Nasser G, Rousseau-Huvey B, Labetoulle M, Rousseau A. Severe linezolid-induced toxic optic neuropathy. J Fr Ophtalmol. 2015;38(3):e55–e58. | ||
Mehta S, Das M, Laxmeshwar C, Jonckheere S, Thi SS, Isaakidis P. Linezolid-associated optic neuropathy in drug-resistant tuberculosis patients in Mumbai, India. PLoS One. 2016;11(9):e0162138. | ||
Ishii N, Kinouchi R, Inoue M, Yoshida A. Linezolid-induced optic neuropathy with a rare pathological change in the inner retina. Int Ophthalmol. 2016;36(6):761–766. | ||
Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. J Infect. 2009;59 Suppl 1:S59–S74. | ||
Waldrep TW, Skiest DJ. Linezolid-induced anemia and thrombocytopenia. Pharmacotherapy. 2002;22(1):109–112. | ||
Moraza L, Leache L, Aquerreta I, Ortega A. Linezolid-induced haematological toxicity. Farm Hosp. 2015;39(6):320–326. | ||
Tessier JM, Puzio T, Young A, Wolfe L, Han J, Duane TM. Thrombocytopenia associated with linezolid therapy in solid organ transplant recipients: a retrospective cohort study. Surg Infect (Larchmt). 2015;16(4):361–367. | ||
Im JH, Baek JH, Kwon HY, Lee JS. Incidence and risk factors of linezolid-induced lactic acidosis. Int J Infect Dis. 2015;31:47–52. | ||
Sawyer AJ, Haley HL, Baty SR, McGuffey GE, Eiland EH 3rd. Linezolid-induced lactic acidosis corrected with sustained low-efficiency dialysis: a case report. Am J Kidney Dis. 2014;64(3):457–459. | ||
Hsu SN, Shih MF, Yang CW, Wu CC, Chen CC. Severe linezolid-induced lactic acidosis in a cirrhosis patient. Nephrology (Carlton). 2015;20(1):47–48. | ||
French G. Safety and tolerability of linezolid. J Antimicrob Chemother. 2003;51(2):45–53. | ||
Kamal K, Dhasmana N, Kamble SS, Sahu RK. Linezolid induced adverse drug reactions – an update. Curr Drug Metab. 2015;16(7):553–559. | ||
Viswanathan P, Iarikov D, Wassel R, Davidson A, Nambiar S. Hypoglycemia in patients treated with linezolid. Clin Infect Dis. 2014;59(8):e93–e95. | ||
Oh DH, Motorna O, Kong JB, Brown S, Gilbertson M. Linezolid-associated reticulocytopenia. Ann Hematol. 2016;95(12):2095–2097. | ||
Farhadi T. In silico designing of peptide inhibitors against pregnane X receptor: the novel candidates to control drug metabolism. Int J Pept Res Ther. Epub 2017 Aug 31. | ||
Farhadi T, Fakharian A, Ovchinnikov RS. Virtual screening for potential inhibitors of CTX-M-15 protein of Klebsiella pneumoniae. Interdiscip Sci. Epub 2017 Apr 3. | ||
Wankhade G, Kamble S, Deshmukh S, Jena L, Waghmare P, Harinath BC. Inhibition of mycobacterial CYP125 enzyme by sesamin and β-sitosterol: an in silico and in vitro study. Biomed Biotechnol Res J. 2017;1:49–54. | ||
Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth. 2005;95(4):434–441. | ||
Frykberg RG, Gordon S, Tierney E, Banks J. Linezolid-associated serotonin syndrome. A report of two cases. J Am Podiatr Med Assoc. 2015;105(3):244–248. | ||
Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother. 2013;47(3):388–397. | ||
Percel PJ, Vishnupad KS, Venkatesh GM, inventors; Aptalis Pharma Ltd, assignee. Functional coating of linezolid microcapsules for taste-masking and associated formulation for oral administration. United States patent US 6451345B1. 2000 Jan 20. | ||
Panandikar A, Bambolkar S, Inamdar K, Ramesh S, Burkul A, Shaikh N, inventors; Indoco Remedies Ltd, assignee. Pharmaceutical composition of linezolid. United States patent US 9492459B2. 2012 Aug 10. | ||
Biswas S, Panda AK, Gupta AK, et al; Jubilant Life Science Limited, assignee. Process for the preparation of stable crystalline form-i of linezolid, substantially free of residual solvent. United States patent US 20170066728A1. 2017 Mar 9. | ||
Mansouri D, Mahdaviani SA, Khalilzadeh S, et al. IL-2-inducible T-Cell kinase deficiency with pulmonary manifestations due to disseminated Epstein-Barr virus infection. Int Arch Allergy Immunol. 2012;158:418–422. | ||
Whang DW, Miller LG, Partain NM, McKinnell JA. Systematic review and meta-analysis of linezolid and daptomycin for treatment of vancomycin-resistant enterococcal bloodstream infections. Antimicrob Agents Chemother. 2013;57(10):5013–5018. | ||
McKinnell JA, Arias CA. Editorial commentary: linezolid vs daptomycin for vancomycin-resistant enterococci: the evidence gap between trials and clinical experience. Clin Infect Dis. 2015;61(6):879–882. | ||
Zhao M, Liang L, Ji L, et al. Similar efficacy and safety of daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bloodstream infections: a meta-analysis. Int J Antimicrob Agents. 2016;48(3):231–238. | ||
Chuang YC, Lin HY, Chen PY, et al. Daptomycin versus linezolid for the treatment of vancomycin-resistant enterococcal bacteraemia: implications of daptomycin dose. Clin Microbiol Infect. 2016;22(10):890.e1–890.e7. | ||
Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med. 2010;38(9):1802–1808. | ||
von Dach E, Morel CM, Murthy A, et al. Comparing the cost-effectiveness of linezolid to trimethoprim/sulfamethoxazole plus rifampicin for the treatment of MRSA infection: a health-care system perspective. Clin Microbiol Infect. 2017;23(9):659–666. | ||
Collins CD, Schwemm AK. Linezolid versus vancomycin in the empiric treatment of nosocomial pneumonia: a cost-utility analysis incorporating results from the ZEPHyR trial. Value Health. 2015;18(5):614–621. | ||
Reveles KR, Mortensen EM, Attridge RT, Frei CR. Comparative-effectiveness of vancomycin and linezolid as part of guideline-recommended empiric therapy for healthcare-associated pneumonia. BMC Res Notes. 2015;8:450. | ||
Niederman MS, Chastre J, Solem CT, et al. Health economic evaluation of patients treated for nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus: secondary analysis of a multicenter randomized clinical trial of vancomycin and linezolid. Clin Ther. 2014;36(9):1233.e1–1243.e1. | ||
Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch C; Linezolid CSSTI Study Group. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother. 2005;49(6):2260–2266. | ||
Fu J, Ye X, Chen C, Chen S. The efficacy and safety of linezolid and glycopeptides in the treatment of Staphylococcus aureus infections. PLoS One. 2013;8(3):e58240. | ||
Yue J, Dong BR, Yang M, Chen X, Wu T, Liu GJ. Linezolid versus vancomycin for skin and soft tissue infections. Cochrane Database Syst Rev. 2016;(1):CD008056. | ||
Belousoff MJ, Eyal Z, Radjainia M, et al. Structural basis for linezolid binding site rearrangement in the Staphylococcus aureus ribosome. mBio. 2017;8:e00395-17. | ||
Long KS, Vester B. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother. 2012;56(2):603–612. | ||
Wang Y, Lv Y, Cai J, et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother. 2015;70(8):2182–2190. | ||
Sharkey LK, Edwards TA, O’Neill AJ. ABC-F proteins mediate antibiotic resistance through ribosomal protection. mBio. 2016;7:e01975-15. | ||
Gales AC, Sader HS, Ribeiro J, Zoccoli C, Barth A, Pignatari AC. Antimicrobial susceptibility of gram-positive bacteria isolated in Brazilian hospitals participating in the SENTRY Program (2005–2008). Braz J Infect Dis. 2009;13:90–98. | ||
Zoller M, Maier B, Hornuss C, et al. Variability of linezolid concentrations after standard dosing in critically ill patients: a prospective observational study. Crit Care. 2014;18:R148. | ||
Cepeda JA, Whitehouse T, Cooper B, et al. Linezolid versus teicoplanin in the treatment of Gram-positive infections in the critically ill: a randomized, double-blind, multicentre study. Antimicrob Chemother. 2004;53:345–355. | ||
McKenzie C. Antibiotic dosing in critical illness. J Antimicrob Chemother. 2011;66:25–31. | ||
Peyrani P, Wiemken TL, Kelley R, Zervos MJ, Kett DH, File TM Jr. Higher clinical success in patients with ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus treated with linezolid compared with vancomycin: results from the IMPACT-HAP study. Crit Care. 2014;18:R118. | ||
Jiang H, Tang RN, Wang J. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: meta-analysis of randomised controlled trials. Eur J Clin Microbiol Infect Dis. 2013;32:1121–1128. | ||
Awad SS, Rodriguez AH, Chuang YC, et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin Infect Dis. 2014;59(1):51–61. | ||
Wang Y, Zou Y, Xie J, et al. Linezolid versus vancomycin for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a systematic review employing meta-analysis. Eur J Clin Pharmacol. 2015;71:107–115. | ||
Whang DW, Miller LG, Partain NM, McKinnell JA. Systematic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant enterococcal blood stream infections. Antimicrob Agents Chemother. 2013;57(10):5013–5018. | ||
Balli EP, Venetis CA, Miyakis S. Systematic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant enterococcal bacteremia. Antimicrob Agents Chemother. 2014;58(2):734–739. | ||
Chuang YC, Wang JT, Lin HY, Chang SC. Daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bacteremia: systematic review and meta-analysis. BMC Infect Dis. 2014;14:687. | ||
Britt NS, Potter EM, Patel N, Steed ME. Comparison of the effectiveness and safety of linezolid and daptomycin in vancomycin-resistant enterococcal bloodstream infection: a national cohort study of veterans affairs patients. Clin Infect Dis. 2015;61(6):871–878. | ||
Crank CW, Scheetz MH, Brielmaier B, et al. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study. Clin Ther. 2010;32(10):1713–1719. | ||
Erlandson KM, Sun J, Iwen PC, Rupp ME. Impact of the more-potent antibiotics quinupristin-dalfopristin and linezolid on outcome measure of patients with vancomycin-resistant Enterococcus bacteremia. Clin Infect Dis. 2008;46:30–36. | ||
Khamesipour F, Hashemian SM, Tabarsi P, Velayati AA. A review of teicoplanin used in the prevention and treatment of serious infections caused by Gram-positive bacteria and compared its effects with some other antibiotics. Biomed Pharmacol J. 2015;8(1):513–521. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

