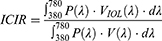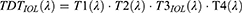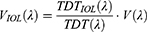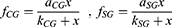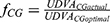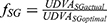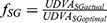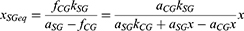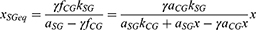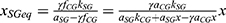Back to Journals » Clinical Ophthalmology » Volume 15
Lighting Standards Revisited: Introduction of a Mathematical Model for the Assessment of the Impact of Illuminance on Visual Acuity
Authors Labiris G , Panagiotopoulou EK , Taliantzis S, Perente A, Delibasis K , Doulos LT
Received 1 July 2021
Accepted for publication 27 August 2021
Published 28 November 2021 Volume 2021:15 Pages 4553—4564
DOI https://doi.org/10.2147/OPTH.S326139
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Georgios Labiris,1 Eirini-Kanella Panagiotopoulou,1 Sergios Taliantzis,1 Asli Perente,1 Konstantinos Delibasis,2 Lambros T Doulos3
1Department of Ophthalmology, University Hospital of Alexandroupolis, Dragana, 68131, Alexandroupolis, Greece; 2Department of Computer Science and Biomedical Informatics, University of Thessaly, Lamia, 35131, Greece; 3School of Applied Arts, Lighting Design, Hellenic Open University, Patras, 26335, Greece
Correspondence: Georgios Labiris
Department of Ophthalmology, University Hospital of Alexandroupolis, Dragana, 68131, Alexandroupolis, Greece
Tel +30 697 745 5027
Fax +30 255 103 0405
Email [email protected]
Purpose: Primary objective of present study is to introduce a contemporary methodology for the lighting standards update addressing both normophakic and pseudophakic patients.
Methods: For the sake of our study, we theoretically estimated the intraocular-to-crystalline lens iIluminance ratio (ICIR) and the intraocular lens (IOL) luminous efficiency function VIOL(λ) as a new lighting benefit metric. Then, in a sample of 24 pseudophakic patients (38 eyes) implanted with the trifocal diffractive IOL Panoptix (SG) and in a control group (CG) of 28 normophakic participants (50 eyes), uncorrected distance visual acuity (UDVA) was measured at illuminance of 550lx (optimal UDVA). Following dark adaptation, illuminance was gradually raised from 20 lx until illuminance level that the patient reached his/her optimal UDVA. This measured illuminance at this point was defined as the minimum required illuminance level (MRIL). MRIL and UDVA for illuminance levels between 20 and 550lx in SG were compared with the corresponding values in CG. MRIL calculation allowed the construction of a predictive mathematical model that estimates the impact of environmental lighting on UDVA.
Results: ICIR for Panoptix eyes ranged from 54.00% to 55.99%. Both groups had significantly higher UDVA at 550lx compared to 20lx (p < 0.05). CG had significantly higher UDVA than SG at 20lx (7.20 letters, p = 0.045), while no significant difference was detected at 550lx (0.40 letters, p = 0.883). SG required significantly more illuminance than CG to maintain their UDVA (MRILSG= 191.05lx, MRILCG= 122lx, p = 0.007). Our predictive model suggests suboptimal UDVA in a series of lighting directives for normophakic and Panoptix eyes.
Conclusion: This is the first study to introduce the VIOL(λ) as a new lighting benefit metric and a mathematical model that quantifies the impact of illuminance on UDVA in normophakic and pseudophakic patients.
Clinicaltrials.gov Identifier: NCT04263636.
Keywords: multifocal intraocular lens, illuminance, light transmission, luminous efficiency function, lighting standards
Introduction
Light is “the natural agent that stimulates sight and enables vision”.1 However, only a small portion of the electromagnetic spectrum of light can be perceived by the human eye, which ranges between 380 and 780 nm.2 Even within the visible spectrum of light, lighting conditions are essential for optimal visual function when performing the activities of daily living. However, our society undervalues light by only focusing on its costs and not properly measuring its benefits. To provide light benefits, lighting manufacturers and lighting designers mainly focus on two formal metrics, the lumen and color rendering index (CRI), which represent only a small part of the benefits actually provided by lighting.3 Since lighting requirements vary according to the different circumstances, such as in the office, during driving, for product highlighting or for the regulation of the sleep-wake cycle, new light benefit metrics have already been accepted. Lately, the International Commission on Illumination (CIE) has defined spectral sensitivity functions and benefit metrics in order to describe the ability of optical radiation to stimulate the photoreceptors that can contribute to retina-mediated non-visual effects of light in humans.4,5 Rea2 has summarized several more lighting metrics such as the Periphery-lx and Brightness-lx. The Periphery-lx metric uses the scotopic luminous efficiency function in order to describe the vision of the eye under low-light levels, which is produced exclusively through rods, while the Brightness-lx metric uses the apparent brightness functions. However, many of the benefits provided by lighting still remain unmeasured increasing the need to introduce new light benefit metrics.2
Optimal lighting is especially essential in middle-aged and senior populations, since it is well known that the ageing process has a negative effect on vision.6 Several theories have been proposed for the reduced visual capacity as the age advances; among them, the alterations in the refractive ocular media of the eye, and the gradually reduced efficacy of the neurosensory optical pathway. In fact, former researchers described that the visual capacity of seniors resembles the corresponding one of young adults when performing in significantly lower lighting conditions.7 Indeed, it is estimated that the average 60-year-old patient needs three to ten times more light energy in order to demonstrate comparable visual capacity to his/her early 20s.6,8 It becomes obvious that light energy needs are increased even more in patients with diseases that either block the light transmittance within the eye, or interfere with the normal function of the photoreceptors.7,9
Evaluation of the necessary environmental lighting is even more challenging in patients that underwent lens extraction surgery, especially, when implanted with a bifocal or trifocal intraocular lens (IOL). It is known that the implantation of an IOL is an integral part of the surgical procedure in cataract operations. Cataract surgery is the most frequently performed operation in Medicine, with patients’ average age ranging from 60 to 77 years.10 Adding the constantly increasing number of refractive lens exchange cases in patients that are usually younger than cataract ones, we assume that a significant percentage of the middle-aged and senior population demonstrates completely different lighting needs due to their implanted IOLs.11
However, the current lighting standards for public and private settings, that were introduced by the corresponding Lighting Societies, are based on spectral sensitivity experimental data that calculated the luminous efficiency function in enucleated eyes.12–15 Even the updated European norms (prEN 12464-1 2019) that are supposed to address the higher lighting needs of the seniors do not take into consideration the possible different characteristics of people that have artificial IOLs implanted.16,17
Implantation of a multifocal IOL completely modifies light transmission to the retina. Since the accommodation of the eye is iatrogenically disrupted, the uniform light distribution within the eye is also disrupted. Preoperatively, the exact same light energy is delivered to the retina, regardless of the focal point. Postoperatively, light distribution depends on the focal point. Multifocal IOLs have complex light distribution mechanisms that constantly split the light to two, three, or even more predefined focal points. It becomes obvious that implantation of a multifocal IOL completely alters the luminous efficiency function V(λ) of the eye, and, prospectively, the lighting needs of the patient.18
The Panoptix IOL (Alcon, FortWorth, TX) is a premium diffractive trifocal IOL for presbyopia correction that has been implanted in thousands of patients in a worldwide scale. Further to the distant focal point, it provides a near (at 40 cm) and an intermediate focal point (at 60 cm), as well.19 Patients that have been implanted with Panoptix IOLs are most likely to present completely different lighting needs than patients with their own crystalline lenses.
Within this context, primary objective of this study is to introduce a contemporary methodology and provide the necessary experimental data that will assist Lighting Societies in their effort to confirm or update lighting standards addressing the lighting needs both of normophakic and pseudophakic patients.
To achieve our study objective: a) we introduce the luminous efficiency function of pseudophakic eyes as a new lighting benefit metric, and b) we develop a mathematical model that quantifies the impact of environmental lighting on visual acuity.
Methods
Setting
This is a prospective, comparative study, which contains a theoretical and an experimental phase. Protocol adhered to the tenets of the Declaration of Helsinki, while written informed consent was provided by all participants. The institutional review board of Democritus University of Thrace approved the study protocol. The study was conducted at the Department of Ophthalmology in the University Hospital of Alexandroupolis, Greece, between January and June 2020. Official registration number of the study is NCT04263636. The authors vouch for the completeness and accuracy of the data and analyses and for the fidelity of the study to the protocol.
Theoretical Phase
To address theoretically our study objective, we introduced the Intraocular-to-Crystalline lens IIluminance Ratio (ICIR), which reflects the light perceived by the pseudophakic eyes in comparison to the normophakic ones, deriving by Equation 1. The construction of the ICIR parameter requires the introduction of the intraocular lens luminous efficiency function VIOL(λ) as a new lighting benefit metric (Table 1).2
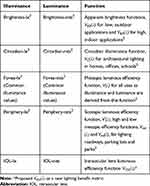 |
Table 1 Lighting Benefit Metrics |
where: ICIR = Intraocular-to-Crystalline lens Illuminance Ratio,
P(λ) = the radiant flux emitted from a light source in the visible spectrum (380 nm – 780 nm),
VIOL(λ) = the photopic intraocular lens luminous efficiency function,
V(λ) = the photopic crystalline lens luminous efficiency function,14
λ = the wavelength for the visible light spectrum (380 nm – 780 nm).
For the VIOL(λ) estimation, it is necessary to estimate the modified total direct transmittance (TDT) of the eye since the transmittance of the crystalline lens [Τ3(λ) in Equation 2] is replaced by the transmittance of the IOL [Τ3IOL(λ) in Equation 3]. Therefore, VIOL(λ) function is calculated by Equation 4.
where: TDT(λ) = Total Direct Transmittance of the normophakic eye,
TDTIOL(λ) = Total Direct Transmittance of the pseudophakic eye,
T1(λ) = the transmittance of the cornea,14
T2(λ) = the transmittance of the aqueous,14
T3(λ) = the transmittance of the crystalline lens,14
T3IOL(λ) = the transmittance of the IOL, and
T4(λ) = the transmittance of the vitreous,14
VIOL(λ) = the photopic intraocular lens luminous efficiency function,
V(λ) = the photopic crystalline lens luminous efficiency function.14
In our study, the ICIR was estimated by substituting the range of transmittance values of the crystalline lens with the corresponding range of values from the Panoptix IOL (ICIRPanoptix) for the wavelength range of 380 nm – 780 nm.14,19 ICIRPanoptix calculations were done for various commercially available luminous sources [light emitting diode (LED), fluorescent (FL), and halogen-incandescent light sources] with different spectral transmittance properties and different combinations of Correlated Color Temperatures (CCT) which varied from 2700 K (warm white) to 6200 K (cool white). The exact radiant flux P(λ) emitted from the selected light sources was measured using a Konica Minolta CL-500A spectrum meter for the wavelength range of 380 nm – 780 nm using a step of 1 nm. The modified intraocular lens luminous efficiency function VIOL(λ) for the pseudophakic eyes with Panoptix IOL [VPanoptix(λ)] was constructed, and compared with the nominal V(λ) function of a 53-year-old normophakic eye for the various light sources.14
Experimental Setting
Participants
Fifty-two participants were enrolled from January to March 2020 in a consecutive-if-eligible basis and populated two distinct groups for the purposes of this study: i) Study group (SG): patients that underwent uneventful pseudophakic presbyopic correction with Panoptix IOL implantation, and ii) Control group (CG): normophakic participants of similar age with no evidence of cataract. Exclusion criteria for both groups included uncorrected distance visual acuity (UDVA) below 0.1 logMAR, astigmatism > 0.75 diopters (D), glaucoma, former incisional eye surgery, corneal or fundus disease, diabetes mellitus, autoimmune diseases, and neurological or psychiatric diseases. Participants with a pseudophakic presbyopic correction having posterior capsule rupture, or lens misalignment were also excluded from the study.
Experimental Layout
An experimental facility at the University Hospital of Alexandroupolis was constructed for the sake of this study (Figure 1). In a hospital room with dimensions 6.87 × 3.5 × 3 m and flat white surface walls, an advanced light diffusion system was installed which secured maximal uniformity at different user-defined lighting settings. In detail: a) LED luminaires were mounted on the ceiling, b) the exact luminaire positioning and the amount of the provided luminous flux were defined using the RELUX light simulation tool (ver. 2019.3.5.0) (Figure 2), c) confirmation of illuminance and on-site adjustments were done with the Extech Lux Meter EA30 (Extech Instruments Corporation, U.S.A.), d) the photometric properties of the luminaires were CCT of 4000 K, Color Rendering Index (CRI) > 90, luminous flux of 2659 lm, installed power of 21 W and luminous efficacy of 126.6 lm/W, e) a digital LED dimmer enabled dimming from 100% to 0% and vice-versa. The maximum light output of the luminaires on the visual acuity chart was 550 ± 15 lx and 860 ± 21 lx at the task area level (height of 0.8 m). The ratio of the vertical illuminance to the horizontal illuminance was 64%.
 |
Figure 1 Layout of experimental facility room (dimmable luminaires, illuminance meter, visual acuity chart, lighting control system, etc.). |
 |
Figure 2 Illumination distribution according to RELUX simulation tool. |
Data Collection
One eye was selected randomly from each study participant. Landolt ring charts at a 3-meter distance were used to estimate UDVA at different vertical illuminance levels in the following consistent way:
First, UDVA was assessed at illuminance of 550 lx, which was considered as the optimal UDVA of each participant. Then, lights were switched off and the patient was allowed to adapt to 0 lx for one minute.20 Following dark adaptation, illuminance was gradually raised from 20 lx until the patient reached his/her optimal UDVA. The measured illuminance at this point was defined as the Minimum Required Illuminance Level (MRIL) that was necessary for optimal UDVA. The illuminance steps were 20 lx, 40 lx, 60 lx, 90 lx, 110 lx, 130 lx, 150 lx, 170 lx, 190 lx, 240 lx, 300 lx, 550 lx, without resting period for the patient among them. Different Landolt ring charts were used in order to avoid memory effect.
Outcome Measures
The primary outcome of this study was the MRIL (in lx) that was necessary for optimal UDVA. Secondary outcomes included the UDVA estimation for each illuminance level (ranging from 20 to 550 lx).
Statistical Analysis
Data distribution was tested with Shapiro–Wilk test. Between-group comparisons of data for which the hypothesis of normality is satisfied were made using independent samples Student’s t-test. Data for which the hypothesis of normality is not satisfied were assessed with Mann–Whitney U-test. Continuous variables will be summarized using the following descriptive statistics: N (non-missing sample size), mean, standard deviation, median, lower and upper values of the interquartile range, minimum and maximum. P-values lower than 0.05 were considered statistically significant. All statistical analyses were performed with the MedCalc version 14.8.1 (MedCalc Software, Mariakerke, Belgium).
An a priori power analysis was performed. The sample size calculation was based upon the difference in MRΙL that patients needed to reach their optimal UDVA between study and control group. The minimal clinically relevant difference in mean score was specified as 0.62 and the standard deviation was expected to be 73 lx. With a significance level of 5%, a power of 80%, and unequal treatment groups with an allocation ratio N2/N1 of 1.32, a sample size of 88 individual’s eyes (38 in SG and 50 in CG) was calculated.
Results
Theoretical Calculations
VPanoptix(λ) plot is presented in Figure 3, while the lower and upper spectral transmittance values for the distant focal point of Panoptix IOL14,19 are presented in Figure 4. The upper values of VPanoptix(λ) plot derived from the upper transmittance values for the distant focal point of Panoptix IOL (T3IOL(λ)upper applying Eq. 3, Figure 4), while the lower values of VPanoptix(λ) plot derived from the lower transmittance values for the distant focal point of Panoptix IOL (T3IOL(λ)lower applying Eq. 3, Figure 4),19 According to Figure 3, the maximum value of VPanoptix(λ) plot is 56% for 550 nm, while the corresponding minimum value is 55.4%. According to Figure 4, the transmittance values with spectrum higher than 580 nm are considered stable, with 43% and 43.6% for the lower and upper value, respectively. The maximum difference in transmittance values is 9.5%, observed at 420 nm. T3IOL estimation was necessary for the construction of the formula that estimates TDTIOL (Equation 3) and V(λ) (Equation 4).
Τhe relative spectral radiant flux P(λ) emitted from the light sources that were used in our study is presented in Figure 5. The theoretical calculations of the ICIRPanoptix, presented in Table 2, were performed by taking into account both the different values of spectral radiant flux P(λ) and the upper and lower values of VPanoptix(λ) (Equation 1). ICIRPanoptix ranged from 54.00% (Halogen lights/2556 Kelvins) to 55.99% (LED lights/6210 Kelvins). Aforementioned outcomes indicate that, theoretically, SG eyes required between 78.60% and 85.19% more illuminance for equal retinal illuminance with the CG ones.21
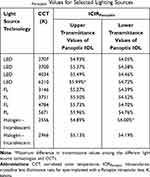 |
Table 2 ICIRPanoptix Values for Selected Lighting Sources |
 |
Figure 5 Actual relative spectral radiant flux emitted from the selected light sources. Abbreviations: K, kelvins; LED, light emitting diode; FL, fluorescent, Halogen – Incandescent. |
According to our theoretical calculations the maximal difference for the different combinations of light source technologies and CCTs in Panoptix eyes was 1.99%. Aforementioned outcome suggests that the ICIR depends primarily on the light transmittance characteristics of the ocular refractive media and not on the technology or the spectrum and CCT of the light source.
Experimental Results
Fifty-two participants [20 men and 32 women (88 eyes), mean age 54.23 ± 7.89 years (range: 40 to 65 years)] were recruited and populated study group (SG: 24 participants – 38 eyes) and control group (SG: 28 participants – 50 eyes) groups. Detailed demographic and clinical parameters are presented in Table 3. Non-significant differences were detected in UDVA (SG: 52.68 ± 6.85 letters, CG: 53.08 ± 10.00, p = 0.88), age (SG: 56.79 ± 7.41, CG: 52.28 ± 7.99, p = 0.06), and refractive error (SG: −0.25 ± 0.31, CG: −0.15 ± 0.52, p = 0.72) between the two groups.
 |
Table 3 Demographic and Clinical Characteristics of Study Participants |
UDVA comparisons between 20 lx and 550 lx on the vertical plane are presented in Table 4. Significant differences were detected between 20 lx and 550 lx for both groups (mean UDVASG difference between 20 lx and 550 lx was 16.68 letters, p < 0.001, while mean UDVACG difference between 20 lx and 550 lx was 9.88 letters, p = 0.005). However, significant difference in UDVA at 20 lx was detected between the two study groups (UDVACG – UDVASG = 7.20 letters, p = 0.04), despite non-significant difference at 550 lx (UDVACG – UDVASG = 0.40 letters, p = 0.88). SG participants required significantly more illuminance than CG ones in order to maintain their UDVA (MRILSG = 191.05 lx, MRILCG = 122 lx, p = 0.007). Everything below aforementioned illuminance levels resulted in a reduction of UDVA.
 |
Table 4 Differences in Uncorrected Distance Visual Acuity and Minimum Required Illuminance Level |
Best fitting curve modeling of our experimental measurements allowed the development of a predictive mathematical model: a) for the impact of illuminance on UDVA, and b) for the additional absolute and percentile illuminance levels for equal UDVA of the multifocal pseudophakic eyes with the normophakic ones. Within this context, the impact of illuminance on the UDVA, expressed in percentile, derives by the formula:
UDVAactual = UDVA for each illuminance level,
UDVAoptimal = UDVA at 550 lx,
x = illuminance (in lx),
aCG = 1.0146, kCG = 5.9612, and aSG = 1.0205 kSG = 11.9105.
The experimental measurements of fCG and fSG, as well as their standard deviations (SDCG and SDSG, respectively) for different illuminance levels are presented in Table 5.
 |
Table 5 Experimental Measurements for Different Illuminance Levels |
Fitting curves for fCG and fSG at different illuminance levels are presented in Figure 6.
According to Equation 5, the necessary illuminance level xSGeq for the SG eyes in order to present equal percentile UDVA with the CG ones derives by the formula:
However, model fitting suggested a tolerance rate of 2.4% when defining the equality fCG = fSG for the xSGeq estimation. Thus, setting fSG = γ fCG and substituting from Equation 5 results in:
where γ = (100–2.4) %.
The necessary additional illuminance of SG eyes for equal percentile UDVA with the CG ones (xSGeq - x) for different illuminance levels is presented in Figure 7 and Table 6. On the other hand, the additional percentile illuminance (xSGeq - x)/x of SG eyes for equal percentile UDVA with CG ones is presented in Figure 8 and Table 6.
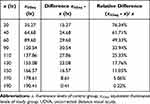 |
Table 6 Necessary Illuminance for Equal Percentile UDVA |
The aforementioned predictive model suggests that for the same percentile UDVA, SG participants demand significantly more illuminance. This additional illuminance ranges from 15.27 lx at illuminance levels of 20 lx (76.34%), to 31 lx for the range between 63 and 94 lx (48–33%), until the 190.41 lx where no difference is detected. At the MRILCG (equal to 122 lx), an additional illuminance of 26 lx (21.3%) is required for the SG. Moreover, SG eyes achieved significantly better percentile UDVA for the same illuminance until about the range of 170 lx, when percentile UDVA difference gradually becomes non-significant (Table 5, Figures 7 and 8).
Discussion
Lighting technology has evolved tremendously over the past 20 years, with the introduction of new light technologies that combine energy efficiency with high illuminance levels. Among the primary objectives of modern architecture, both for urban and rural settings, is the identification of the lighting conditions that will enable full functionality of the human optic system and at the same time utilize the minimal energy. To address this goal, the light transmittance properties of the refractive media of the human optic system were calculated based on phasmatoscopic experiments in enucleated eyes. Phasmatoscopy provided the necessary data for the calculation of the luminous efficiency function V(λ) of the human eye, which reflects the average spectral sensitivity of human visual perception of brightness. The majority of the prevalent directives from the corresponding lighting societies are based on the estimated V(λ) of the human eye.12–16 However, pseudophakic patients were not taken into consideration in the introduction of lighting standards. Moreover, no experiments were conducted that evaluated the actual impact of illuminance on visual acuity and prospectively on the optical performance.
Lens extraction surgery is the most commonly performed surgical intervention in Medicine. In certain western societies, overall prevalence of any type of cataract surgery ranges from 42% in patients above 40 years old to higher than 94% in patients over 70 years.22 However, alongside with the constantly increasing rates of lens-extraction surgery, patient expectations are also increasing.23 Following any operation in the crystalline lens, modern western citizens are not likely to compromise for anything less than a spectacle-free, visual capacity that will enable them to fulfill their social roles.24
In the attempt to meet patient expectations for a spectacle-free vision, multifocal IOL implantations have increased significantly, as well. However, despite impressive postoperative visual acuity at different distances and high satisfaction rates, concerns have been raised about reduced efficacy in certain activities of daily living, especially in low light conditions.25 In fact, several researchers suggested that potential candidates for multifocal IOL implantations should be warned for reduced visual capacity and/or optical phenomena at certain activities like when driving during the night. The reduced efficacy of multifocal IOLs in low light conditions has been primarily attributed to their multifocal design that constantly splits light energy to a series of predefined focal points.26
The Panoptix is a diffractive, non-apodized, multifocal IOL that has an advanced light distribution mechanism, which provides three functional focal points. Panoptix has an impressive 88% overall light transmittance ratio,27 which is almost identical to the light transmittance ratio of the crystalline lens of a young child that reaches 90% at 450 μm.14 However, the human crystalline lens is a monofocal ocular medium that has the ability to modify its shape and geometric properties according to the focal point. Therefore, in pre-presbyopic eyes, it is capable of delivering the exact same light energy to the retina, regardless of the distance of the object. Panoptix’s trifocal design splits the light in three focal points, delivering 44% of the light to the distant focal point, and 22% of the light to the intermediate and near ones, respectively.27 Therefore, Panoptix light transmittance ratio for the distant focal point is almost half than the corresponding one of the crystalline lens.
Although visual acuity is not the sole index of visual capacity, it directly reflects visual performance. Prevalent diseases which reduce visual acuity, like the diabetes mellitus, the age-related macular degeneration, or the keratoconus, impose a tremendous impact on the quality of life and the productivity of the patient.28,29 Therefore, preservation of the optimal visual acuity is top priority for the National Healthcare Systems and should be of major concern to the lighting societies when introducing lighting standards. The recommended lighting standards should reflect the lighting conditions at which visual tasks can be conducted effectively and comfortably in an energy-efficient way which complies with the mandate against light pollution.13,17,30–34
Within this context, our study evaluates whether current lighting standards address the needs both of pseudophakic and normophakic patients by exploring the impact of illuminance on visual acuity both theoretically and experimentally.
Regarding pseudophakic patients, we: a) introduced a new lighting benefit metric, the intraocular lens luminous efficiency function VIOL(λ), which addresses their lighting needs, b) we calculated the VPanoptix(λ), which addresses the specific lighting needs of patients who have been implanted with the prevalent Panoptix IOL. Indeed, the substitution of the spectral transmittance values of the crystalline lens with the corresponding ones for the distant focal point from the Panoptix IOL suggested that, theoretically, pseudophakic eyes would require between 78.60% and 85.19% more illuminance for equal retinal illuminance with the control ones. On the other hand, the light technology and the CCT of the light source had negligible impact on UDVA.
Following the theoretical calculation of VPanoptix(λ), we proceeded to the experimental confirmation in a sample of pseudophakic patients that underwent uneventful Panoptix implantation. As a control group, we recruited normophakic participants with no evidence of cataract in slit-lamp biomicroscopy. Both study and control participants were carefully examined: a) for potential corneal disease that would interfere with cornea’s light transmittance properties, b) for manifest astigmatism above 0.75 D that would interfere with optimal UDVA, and c) for suboptimal macular function that would interfere with their UDVA assessment.
The lighting system that was installed in our experimental facility was carefully designed using the RELUX simulation tool (Figure 2) with all measurements of illumination distribution confirmed by the Extech Lux Meter EA30 to secure maximal uniformity from 20 lx to 550 lx. Unfortunately, below 20 lx our experimental setting failed to provide adequate light uniformity, therefore, experimental data between 0 and 20 lx were excluded from our analysis. Following assessment of UDVA at the illuminance level of 550 lx (optimal UDVA), we examined the MRIL that our patients needed in order to maintain their optimal UDVA. We assumed that any reduction in UDVA under a specific level of light illuminance should be attributed to lower retinal illuminance. Our experimental outcomes were very close to our theoretical calculations, suggesting that Panoptix eyes required 76.34% more light illuminance for equal percentile UDVA with the SG eyes at the illuminance level of 20 lx. The difference in lighting needs between pseudophakic and normophakic eyes was gradually reduced until the level of 191.05 lx, when Panoptix eyes reached their optimal UDVA.
Regarding the fundamental question, whether the current lighting standards address the needs of the modern citizens, we developed a mathematical model based on our experimental data, that directly estimates visual acuity according to the environmental lighting. Our model suggested that for a series of settings the proposed illuminance produces suboptimal visual acuity. For example, at the 6.37.4 setting (Corridors: during night) of the ΕΝ 12464-1 directive, normophakic patients have 90.65% of their optimal UDVA while Panoptix ones have 82.42% of their optimal UDVA. For optimal UDVA, normophakic patients require additional 72 lx while the Panoptix ones require 141.05 lx. At the 5.1.3 setting (Regular vehicle traffic, max speed 40 km) of the ΕΝ 12464-1 directive, normophakic patients have 78.16% of their optimal UDVA while multifocal ones have 63.96% of their optimal UDVA. For optimal UDVA, normophakic patients require additional 102 lx, while the Panoptix ones require 171.05 lx.16
Although we are not aware of any prospective, comparative trials that evaluate the functional capacity of subjects when performing at the aforementioned settings, we cannot justify the proposed illuminance by the EN 12464-1 directive that results in suboptimal visual acuity.
On the other hand, at the 6.26.3 setting (technical drawing) of the ΕΝ 12464-1 directive that proposes illuminance between 750 and 1500 lx, we cannot exclude energy waste since both normophakic and pseudophakic patients would have reached their optimal visual acuity at much lower illuminance levels.
It should be mentioned that our experimental data address the Panoptix IOL. Although Panoptix demonstrates impressive light distribution characteristics, superior to the majority of modern multifocal IOLs, the exact estimation of a and k constants requires the replication of our experimental methods for each prevalent IOL and examination distance. However, the overall mathematical model that addresses the relationship between UDVA and illuminance remains the same, as expressed by the formula 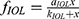 , where
, where 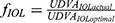 , x = illuminance (in lx) a, k = constants calculated via experimental clinical measurements for each IOL model.
, x = illuminance (in lx) a, k = constants calculated via experimental clinical measurements for each IOL model.
Conclusions
Our study attempts to contribute to the body of knowledge regarding lighting standards with the introduction of the new lighting benefit metric VIOL(λ) and a mathematical model that directly predicts visual acuity according to the illuminance levels for normophakic or pseudophakic patients. Our mathematical is able to address every modern IOL and examination distance, provided that the coefficients a and k are experimentally calculated from the corresponding clinical measurements. We are confident that our study outcomes will assist lighting societies in their effort to confirm or even update lighting standards, which enable full functionality of the human optic system and at the same time comply with the mandates for improved energy efficiency and reduced light pollution.
Data Sharing Statement
The authors intend to share deidentified participant data including study information leaflets and written consent forms for at least one year after the manuscript publication, acceptable in print form. The data are available upon request (email: [email protected]). All relevant data is in Greek.
Acknowledgments
Authors would like to thank Bright Special Lighting S.A. and, specifically, Mr. Vassiliou Georgios (owner) and Mr. Aggelidakis Dimitrios (test engineer) for their support in the luminaire equipment.
Author Contributions
GL conceived and supervised the study, designed the experimental phase, contributed to the data analysis, data interpretation, drafting and critical revision of the manuscript for important intellectual content. EKP contributed to the conception and design, data acquisition, analyzed and interpreted the data, made the statistical analysis, contributed to best fitting curve modeling, wrote and revised the manuscript critically for important intellectual content. ST and AP contributed to the clinical data acquisition and critical revision of the manuscript. KD contributed to the data analysis and data interpretation, best fitting curve modeling, drafting the manuscript, and critical revision of the manuscript. LTD designed the theoretical phase of the study, collected, analyzed and interpreted the data of the theoretical phase, and critically revised the manuscript. All authors read and approved the final report. In general, all authors made substantial contributions to conception and design, acquisition of data, or data analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sliney DH. What is light? The visible spectrum and beyond. Eye (Lond). 2016;30:222–229. doi:10.1038/eye.2015.252
2. Rea MS. The lumen seen in a new light: making distinctions between light, lighting and neuroscience. Light Res Technol. 2015;47:259–280. doi:10.1177/1477153514527599
3. Rea MS. New benefit metrics for more valuable lighting. J Light Vis Environ. 2013;37:41–45. doi:10.2150/jlve.IEIJ130000498
4. CIE. CIE system for metrology of optical radiation for ipRGC-influenced responses to light. Vienna: CIE Central Bureau; 2018. CIE S 026/E: 2018.
5. CIE. What to document and report in studies of ipRGC-influenced responses to light. Vienna: CIE Central Bureau; 2020. CIE TN 011:2020.
6. Nylén P, Favero F, Glimne S, Teär Fahnehjelm K, Eklund J. Vision, light and aging: a literature overview on older-age workers. Work. 2014;47:399–412. doi:10.3233/WOR-141832
7. Werner JS, Peterzell DH, Scheetz AJ. Light, vision, and aging. Optom Vis Sci. 1990;67:214–229. doi:10.1097/00006324-199003000-00013
8. Butler M, McMullan K, Ryan SE. Lighting prescriptions for low vision. J Hous Elderly. 2019;33:189–203. doi:10.1080/02763893.2018.1534175
9. Artigas JM, Felipe A, Navea A, Fandiño A, Artigas C. Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light. Invest Ophthalmol Vis Sci. 2012;53:4076–4084. doi:10.1167/iovs.12-9471
10. Kauh CY, Blachley TS, Lichter PR, Lee PP, Stein JD. Geographic variation in the rate and timing of cataract surgery among US communities. JAMA Ophthalmol. 2016;134:267–276. doi:10.1001/jamaophthalmol.2015.5322
11. Schallhorn SC, Schallhorn JM, Pelouskova M, et al. Refractive lens exchange in younger and older presbyopes: comparison of complication rates, 3 months clinical and patient-reported outcomes. Clin Ophthalmol. 2017;11:1569–1581. doi:10.2147/OPTH.S143201
12. European Norm 12464-1: Light and lighting — lighting of work places. Part 1: indoor work places. BSI Standards Publication; 2011. Available from: https://www.ahjzu.edu.cn/_upload/article/files/50/b5/4e0248ef47278e4dead0447bd16a/89a6cb4c-2303-42e6-97a3-34d8adc7a5eb.pdf. Accessed November 17, 2021
13. European Norm 12464-2: Light and lighting—lighting of work places Part 2: outdoor work places. Brussels, Belgium: CEN; 2014.
14. Boettner EA, Wolter JR. Transmission of ocular media. Invest Ophthalmol. 1962;1:777–783.
15. Illuminating Engineering Society. The IES Lighting Handbook.
16. European Norm 12464-1: Light and lighting - Lighting of work places - Part 1: indoor work places; 2019. Available from: https://www.valosto.com/tiedostot/prEN%2012464-1.pdf. Accessed November 18, 2021.
17. Doulos LT, Tsangrassoulis A, Madias EN, et al. Examining the impact of daylighting and the corresponding lighting controls to the users of office buildings. Energies. 2020;13:4024. doi:10.3390/en13154024
18. Li X, Kelly D, Nolan JM, Dennison JL, Beatty S. The evidence informing the surgeon’s selection of intraocular lens on the basis of light transmittance properties. Eye (Lond). 2017;31:258–272. doi:10.1038/eye.2016.266
19. AcrySof IQ Panoptix, Summary of Safety and Effectiveness data. Fort Worth, TX: Alcon Laboratories, Inc; 2019. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf4/P040020S087B.pdf.
20. Lamb TD, Pugh EN
21. Thibos LN, Lopez-Gil N, Bradley A. What is a troland? J Opt Soc Am a Opt Image Sci Vis. 2018;35:813–816. doi:10.1364/JOSAA.35.000813
22. Ryu SY, Kim J, Hong JH, Chung EJ. Incidence and characteristics of cataract surgery in South Korea from 2011 to 2015: a nationwide population-based study. Clin Exp Ophthalmol. 2020;48:319–327. doi:10.1111/ceo.13705
23. Panagiotopoulou EK, Ntonti P, Vlachou E, Georgantzoglou K, Labiris G. Patients’ expectations in lens extraction surgery: a systematic review. Acta Med (Hradec Kralove). 2018;61:115–124. doi:10.14712/18059694.2018.129
24. Labiris G, Ntonti P, Patsiamanidi M, Sideroudi H, Georgantzoglou K, Kozobolis VP. Evaluation of activities of daily living following pseudophakic presbyopic correction. Eye Vis (Lond). 2017;4:2. doi:10.1186/s40662-016-0067-1
25. Labiris G, Ntonti P, Panagiotopoulou EK, et al. Impact of light conditions on reading ability following multifocal pseudophakic corrections. Clin Ophthalmol. 2018;12:2639–2646. doi:10.2147/OPTH.S180766
26. Lee S, Choi M, Xu Z, Zhao Z, Alexander E, Liu Y. Optical bench performance of a novel trifocal intraocular lens compared with a multifocal intraocular lens. Clin Ophthalmol. 2016;10:1031–1038. doi:10.2147/OPTH.S106646
27. Sudhir RR, Dey A, Bhattacharrya S, Bahulayan A. AcrySof IQ PanOptix intraocular lens versus extended depth of focus intraocular lens and trifocal intraocular lens: a clinical overview. Asia Pac J Ophthalmol (Phila). 2019;8:335–849. doi:10.1097/APO.0000000000000253
28. Brown MM, Brown GC, Sharma S, Landy J, Bakal J. Quality of life with visual acuity loss from diabetic retinopathy and age-related macular degeneration. Arch Ophthalmol. 2002;120:481–484. doi:10.1001/archopht.120.4.481
29. Aydin Kurna S, Altun A, Gencaga T, Akkaya S, Sengor T. Vision related quality of life in patients with keratoconus. J Ophthalmol. 2014;2014:694542. doi:10.1155/2014/694542
30. Pracki P, Skarżyński K. A multi-criteria assessment procedure for outdoor lighting at the design stage. Sustainability. 2020;12:1330. doi:10.3390/su12041330
31. Doulos LT, Sioutis I, Kontaxis PA, Zissis G, Faidas K. A decision support system for assessment of street lighting tenders based on energy performance indicators and environmental criteria: overview, methodology and case study. Sustain Cities Soc. 2019;51:101759. doi:10.1016/j.scs.2019.101759
32. Doulos LT, Kontadakis A, Madias EN, Sinou M, Tsangrassoulis A. Minimizing energy consumption for artificial lighting in a typical classroom of a Hellenic public school aiming for near zero energy building using LED DC luminaires and daylight harvesting systems. Energy Build. 2019;194:201–217. doi:10.1016/j.enbuild.2019.04.033
33. Papalambrou A, Doulos LT. Identifying, examining, and planning areas protected from light pollution. The case study of planning the First National Dark Sky Park in Greece. Sustainability. 2019;11:5963. doi:10.3390/su11215963
34. Technical report of commission Internationale de l’Eclairage CIE 150: guide on the limitation of the effects of obtrusive light from outdoor lighting installation; Vienna, Austria: CIE; 2017.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.