Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Lifestyle Influence on Mild Cognitive Impairment Progression: A Decision Tree Prediction Model Study
Authors Hou J, Jiang H , Han Y, Huang R, Gao X, Feng W, Guo Z
Received 15 August 2023
Accepted for publication 29 January 2024
Published 12 February 2024 Volume 2024:20 Pages 271—280
DOI https://doi.org/10.2147/NDT.S435464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Yuping Ning
Jiwen Hou,1,2,* Hua Jiang,1,* Yan Han,1 Rong Huang,1 Xiang Gao,1 Wei Feng,1 Zongjun Guo2
1Department of Geriatrics, Affiliated Hospital of Chengdu University, Chengdu, People’s Republic of China; 2Department of Geriatrics, Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zongjun Guo, Department of Geriatrics, Affiliated Hospital of Qingdao University, No. 16, Jiangsu Road, Qingdao, Shandong Province, People’s Republic of China, Tel +8618661808736, Email [email protected]
Purpose: This study assessed the influences of different lifestyle on mild cognitive impairment (MCI) progression and established a decision tree prediction model to analyse their predictive significance on MCI progression incidence.
Patients and Methods: From October 2015 to February 2020,330 patients with MCI were recruited, and demographic and lifestyle information collected. They were followed up for 19.04 ± 10.227 months. Cognitive function was assessed using the Mini-Mental State Examination Scale every 6 months, and they were divided into MCI stable group and MCI progression group.
Results: The Kaplan Meier survival analysis showed an overall cohort survival rate of 33.2%; the annual conversion rate of MCI progression was 20%. Physical exercise, social engagement, high-fat diet, age, napping, and tea drinking were decision tree prediction model nodes. Hobbies were the most important factor for predicting MCI progression. The MCI progression probability rates were: with hobbies 26.829% (44 cases), without hobbies 57.831% (96 cases); for those withot hobbies, with physical exercise 43.077% (28 cases) without physical exercise 72.340% (68 cases); for those without hobbies with physical exercise and social engagement 20.000% (4 cases), without social engagement 53.333% (24 cases); for those without hobbies, physical exercises and social engagement and with nap habits 48.485% (16 cases), without nap habits 66.667% (8 cases). The decision tree prediction model AUC for predicting the MCI progression receiver operating characteristic curve was 0.737 (95% confidence interval: 0.685– 0.785) (75.71% sensitivity, 71.75% specificity, P < 0.001.
Conclusion: Hobbies, physical exercise, social engagement, napping, and drinking tea can help prevent MCI progression, while a high-fat diet may exacerbate MCI progression. In this study the rule with the lowest MCI progress probability for those who had hobbies, high-fat diet, and social engagement. And the decision tree model had good prediction efficiency.
Keywords: lifestyle, mild cognitive impairment progression, survival analysis, decision tree prediction model
Introduction
Along with the aging of the population, the prevalence of Alzheimer’s disease has also increased significantly. At present, there are 50 million patients with dementia in the world, which is expected to triple to more than 152 million by 2050. Dementia will be the biggest global health and social care challenge of the 21st century.1 At present, the efficacy of medication for cognitive dysfunction is not clear,2 That is the reason the World Health Organization suggested in 2019 the key to reducing dementia prevalence is early identification and prevention.3 Mild cognitive impairment (MCI) is an unstable state between normal cognitive function and dementia,4 such as somebody have a memory impairment beyond that expected for age and education yet are not demented. According to research, one in five patients with MCI over 65 will eventually develop dementia.1 Fortunately, there are interventions available for MCI that may restore cognitive impairment and prevent or delay the progression of cognitive dysfunction to dementia.5 Therefore, research of MCI related influencing factors is of great significance for the clinical control of cognitive dysfunction.
MCI is influenced by many factors, among them is lifestyle, which can be changed and clearly recognized.6 Numerous studies have shown that lifestyle factors such as eating habits, physical exercise, social engagement, hobbies, smoking, alcohol consumption, and others can have good or bad effects on cognitive function.7–11 In a previous study of 625 older people, the results concluded social engagement, high-fat diet, hobbies, living style, tea drinking and smoking were closely related to MCI occurrence, and social engagement was the most important predictor of MCI.9 MCI progression to dementia is an insidious and relatively slow process, moreover, the stage between MCI and dementia has been poorly studied. In this study, we wanted to explore how lifestyle influence on patients who had already been diagnosed with MCI. Therefore, patients with MCI based on original lifestyle were followed up in this study. Then, our team established a decision tree prediction model to analyse the influencing factors of MCI progression, and hope to provide some optional beneficial lifestyle for delaying or avoiding MCI progression to dementia.
The decision tree method is a machine learning method, which includes C5.0, CART, CHAID, ID3, SLIQ and other algorithms. The C5.0 algorithm adopted in this study was for discovering the best classification indexes of the data samples by using information gain, and these classification indexes became each node of the decision tree, and finally formed a tree structure diagram.12–14 It not only screened out the influencing factors for the disease, it reflected the interaction between the factors,15–17 and could be used to predict probability of disease occurrence.18,19
Patients and Methods
Research Patients
Participants: In our earlier cohort of studies on cognitive function,9 those who met the inclusion and exclusion criteria in the geriatrics department and physical examination center from October 2015 to February 2020 were included, which according to the MCI diagnostic criteria,20 (cognitive problems, at least one cognitive impairment, independence of daily function, and absence of dementia), were 330 patients with MCI. All participants and their family members participated in this study voluntarily, and informed consent was obtained.
Inclusion criteria: (1) Patients aged ≥ 60 years with a definite diagnosis of MCI (The elderly age in developing countries is 60 years or older and ≥ 65 years in developed countries); and (2) ability to complete all the questionnaire data and agree to participate in the follow-up.
Exclusion criteria: (1) Any important neurological diseases other than MCI (such as Parkinson’s disease, epilepsy, craniocerebral trauma);21 (2) diagnosis of dementia by the Clinical Dementia Rating scale;22 (3) clinically advanced illnesses (such as advanced tumours, severe infections) or unstable diseases such as schizophrenia, mania or severe depression history.23
Criteria for MCI progression:24 (1) MCI had been diagnosed in advance; (2) patients or insiders stated their cognitive function had decreased significantly; (3) Mini-Mental State Examination (MMSE) score decreased by more than 2 points compared with the initial diagnosis; (4) factors such as new-onset craniocerebral mass, and acute cerebral infarction that cause cognitive function progression were excluded.
Quality control: The investigators of this study were geriatric doctors and neurologists. Before starting the survey, they received standardized training, and had to be familiar with the selection criteria table of the survey quantity questions. After the questionnaires were completed, data were entered by two investigators, checked, and numbered for archiving.
Research Methods
Follow-up methods: Demographic information, lifestyle, behavioural habits of participants who met the inclusion and exclusion criteria were collected, and the study variables were assigned (Table 1). The cognitive function of the patients was followed up once every 6 months in the outpatient department, and they were divided into MCI stable group and MCI progression group according to the MCI progression criteria. The follow-up time was February 2020. During this period, we only observed the study and did not conduct lifestyle interventions.
 |
Table 1 Variable Assignment Table of Influencing Factors of MCI Progression |
Statistical methods: MedCalc15.2.2 was used for statistical analyses. Normal distribution measurement data were expressed as (x ± s), categorical variables were expressed as percentages. A Kaplan-Meier survival analysis was used for the univariate survival analysis, and survival curves were drawn. IBM SPSS Modeler 18.0 (IBM Corp., Armonk, NY, USA) was used to establish the decision tree prediction model. The receiver operating characteristic (ROC) curve was drawn to evaluate the predictive ability of decision tree prediction model. All results were considered statistically significant with P < 0.05.
Results
General Information of Patients with MCI
There were 164 men and 166 women in the MCI group. According to age, 79 patients were 60–64 years old, 83 were 65–69 years old, 76 were 70–74 years old, 60 were 75–79 years old, and 32 were ≥80 years old. The average age was 70.280 ± 6. 958 years (Table 2).
 |
Table 2 Analysis of the Influencing Factors of Lifestyle on the Progress of MCI |
Follow Up results of Patients with MCI
The mean follow-up time of participants was 19.040 ± 10.227 months. A total of 330 patients with MCI were followed up. The follow-up observations revealed that 140 patients with MCI progressed and 177 with MCI were stable Thirteen participants were lost to follow-up, of which 6 died of other diseases, 1 with craniocerebral trauma stopped follow-up, and 6 withdrew due to other reasons. The lost follow-up rate was 3.9%.
Survival Analysis of Patients with MCI
A Kaplan-Meier survival analysis was used. Survival status was whether MCI progresses or not, and follow-up time was survival time. The median survival time of progression in 330 patients with MCI was 26 ± 2.5 months, of which the shortest was 4 months; the overall survival rate was 33.2%; because only two cases were followed up for 52 months, and the adjusted annual conversion rate of MCI progression was 20% (Figure 1). The Log rank test was selected for the univariate analysis, and when compared with the stable survival curve, MCI progression was statistically significant in terms of nap habits, physical exercise, social engagement, and hobbies (P < 0.05) (Table 2).
 |
Figure 1 This curve represents a gradual decrease in the probability of cognitive stability over time in MCI patients. By the end of follow-up, the probability of stable MCI was 33. 2%. |
Decision Tree Prediction Model for Lifestyle Influence on MCI Progression
Taking the MCI progression as the target variable, 12 predictors including demographic and lifestyle factors were included to establish a decision tree prediction model. Using 10 cross-validation methods, the average accuracy of the decision tree prediction model was 65.7%, and the standard error 2.8%. Hobbies, physical exercise, social engagement, high-fat diet, age, napping, and drinking tea entered each node of the decision tree prediction model, and hobbies were the root node variables. The probability of MCI progression with hobbies was 26.829% (44 cases), which was lower than 57.831% (96 cases) without hobbies. On the right side of the decision tree prediction model, among participants without hobbies, the probability of MCI progression of those with physical exercise was 43.077% (28 cases), lower than without physical exercise, 72.340% (68 cases). Among the participants with no hobbies or physical exercise, the probability of MCI progression of those with social engagement was 20.000% (4 cases), which was lower than that of those without social engagement (53.333%, 24 cases). Among the participants with no hobbies, physical exercise, but no social engagement, the probability of MCI progress of those with nap habit was 48.485% (16 cases), lower than 66.667% (8 cases) for those without a nap habit. Among the participants with no hobbies, physical exercise, social engagement, and nap habit, the probability of MCI progression of those aged 65 years and below was 20.000% (2 cases), lower than 60.870% (14 cases) for those older than 65 years. The resulting interpretation rules of the decision tree prediction model on the other side are the same as before (Figure 2). In addition, we easily analysed among lifestyle factors the rule with the lowest MCI progress probability was 12.000% for those who had hobbies, high-fat diet, and social engagement, and 75% for those who did not have hobbies and did not exercise.
Importance of Factors Influencing MCI Progression in Decision Tree Prediction Model
In the decision tree prediction model, the importance of each node predictor variable to the construction of the model was different. The importance of hobbies as the root node variable was 34.63%; the importance of physical exercise, social engagement, high-fat diet, age, napping, and tea drinking in the leaf nodes were 32.16%, 12.87%, 6.98%, 6.18%, 4.44%, and 2.73%, respectively (Figure 3).
ROC Curve of Decision Tree Prediction Model Predicting MCI Progress
The AUC of the decision tree prediction model for predicting MCI progression was 0.737 (95% confidence interval [CI]: 0.685–0.785), 75.71% sensitivity, and 71.75% specificity, P < 0.001 (Figure 4).
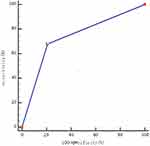 |
Figure 4 The broken line in the figure represented the ability of the decision tree prediction model to predict the occurrence of MCI progression. The AUC was 0.737, P < 0.001. |
Discussion
In this study, the incidence of cognitive deterioration in MCI patients was found through follow-up observation, and the decision tree prediction model was used to predict the influence of lifestyle on the progression of MCI.
During the follow-up of patients with MCI, we found that the survival probability of cognitive stability decreased with time, with an overall survival probability of 33.2% and an annual conversion rate of 20%. The disease process of dementia is continuous and hidden, and there are few studies on the progress of MCI. Feng et al followed up patients with MCI for an average of 1.7 years, and found that the annual conversion rate for MCI progression was 11.8%.24 At present, it is generally accepted that the annual conversion rate from MCI to Alzheimer’s disease [AD] is between 10% and 15%.25 It is reasonable that the annual conversion rate for MCI progression is higher than that for MCI to AD.
In the decision tree prediction model, hobbies, physical exercise, social engagement, high-fat diet, age, napping, tea drinking entered each node of the decision tree prediction model. Hobbies were the root node variable and the most important predictive variable (the importance was 47.48%). Patients with MCI with hobbies had significantly lower cognitive function progression than those without hobbies. Studies have shown that hobbies (such as reading, music, playing chess) can delay the onset of dementia and the progression of cognitive impairment by making relevant neural networks more efficient or plastic.26 This study results are supported by a 4-year cohort study showing that hobbies improved cognitive function in patients with MCI (OR 1.09, 95% CI 1.03–1.16).10 The probability of MCI progression in patients with MCI who persisted in physical exercise was significantly lower than that for those without physical exercise. At present, a large number of studies have proven the protective effect of physical exercise on cognitive function.27–29 A recent basic study suggests that physical exercise may increase the content of myelin in white matter of the whole brain, which is one of the reasons for its improvement in cognitive function.30 Research suggest that social engagement can better compensate for brain changes (aging, cognitive decline, AD) by activating and strengthening neurobiological pathways to Increase cognitive reserve.31 In this study, patients with MCI with frequent social engagement had a lower probability of cognitive decline than those without social engagement, which was consistent with previous studies.32,33 The probability of MCI progression in patients with MCI with a high-fat diet was significantly higher than that in patients with a non-high-fat diet, which may be due to cholesterol increasing oxidative stress in the body and increasing the concentration of amyloid protein in the hippocampus, thus aggravating cognitive impairment.34 Tea polyphenols in tea have the effect of anti-oxidative stress to reduce the damage of nerve cells, thus it has a protective effect on cognitive function.35 The relationship between napping and cognitive function is not completely clear. Currently, the mainstream view is that there is a biphasic relationship between nap time and cognitive function, that is, moderate nap time can delay the decline of cognitive function, while prolonging nap time may aggravate cognitive impairment.36–39 In this study, participants with nap habits were less likely to develop MCI than those without nap habits, therefore it is very important to further clarify the times for naps. It can be seen that, Hobbies, physical exercise, social engagement, napping, tea drinking were beneficial in delaying MCI progression; However, high-fat diet was detrimental.
The importance of lifestyle in relation to MCI progression in the decision tree prediction model was different. Hobbies were the root node variable with the highest importance value, followed by physical exercise, social engagement, high-fat diet, age, napping, and tea drinking. However, previous research of this research group showed that in the lifestyle decision tree prediction model on MCI occurrence, social engagement was the root node variable with the highest importance value, followed by high-fat diet, tea drinking, hobbies, living style, smoking.9 Therefore, we can make the most appropriate clinical intervention strategy according to the importance of different influencing factors and the changes of the importance for influencing factors in different stages of cognitive function. In addition, we can also observe that the decision tree prediction model can directly and vividly show researchers the rules of minimum probability and maximum probability for MCI progression. In this study the rule with the lowest MCI progress probability was 12.000% for those who had hobbies, high-fat diet, and social engagement, Therefore, the decision tree prediction model has more advantages than COX regression in these aspects. The AUC of ROC evaluated by this decision tree prediction model was 0.733 (95% CI 0.681–0.780), which fully shows the advantage and feasibility of decision tree prediction model in a medical statistical analysis.
Although this study conducted a relatively comprehensive investigation on the influencing factors of lifestyle on the progression of MCI, there are several limitations. For example, lifestyle content can be further refined, such as the time, intensity, and type of physical exercise, type of drinking, amount of alcohol consumed, and specific nap times, which can make the research results more instructive. MCI is an unstable state between a normal cognitive state and dementia, which can not only develop into dementia, but also reverse to normal cognitive function. A meta-analysis of 25 studies has shown that the overall reversal rate of MCI is approximately 24%.40 Therefore, it is necessary to further improve the research regarding MCI reversal to normal cognitive function in the future.
Statement of Ethics
This study was approved by the Ethics Committee of Qingdao University (approval number: QYFYWZLL25697), and complied with the Declaration of Helsinki.
Acknowledgments
The study was supported by the following organizations: Jinniu District Medical Association Scientific Research Project, Key Project (jnky2021-14); Jinniu District Medical Association Scientific Research Project, Nursery Project (jnky2021-15); the Affiliated Hospital of Chengdu University Project (Y2021044); Qingdao Social Science Planning Project (qdskl2201376); and Shandong Geriatric Science and Technology Plan (lkjg2021z013).
Disclosure
The authors have no conflicts of interest regarding the content of this article. An unauthorized version of the Chinese MMSE was used by the study team without permission. The MMSE is a copyrighted instrument and may not be used or reproduced in whole or in part, in any form or language, or by any means without written permission of PAR (www.parinc.com).
References
1. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi:10.1016/S0140-6736(17)31363-6
2. Fink HA, Jutkowitz E, Mccarten JR, Hemmy LS, Kane RL. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Internal Med. 2017;168(1):1
3. WHO(WHO). Risk reduction of cognitive decline and dementia; 2019.
4. Rc P, Ge S, Sc W, Rj I, Eg T, K E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56(3):303–308. doi:10.1001/archneur.56.3.303
5. M M-A. Reversion from mild cognitive impairment to normal cognition: a meta-analysis. Alzheimer Dis Associated Disord. 2016;30(4):324–330. doi:10.1097/WAD.0000000000000145
6. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413–446. doi:10.1016/S0140-6736(20)30367-6
7. Dominguez L, Veronese N, Vernuccio L, et al. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients. 2021;13(11):4080. doi:10.3390/nu13114080
8. Wang Z, Pang Y, Liu J, Wang J, Xie Z, Huang T. Association of healthy lifestyle with cognitive function among Chinese older adults. Eur J Clin Nutr. 2021;75(2):325–334. doi:10.1038/s41430-020-00785-2
9. Wang Z, Hou J, Shi Y, et al. Influence of lifestyles on mild cognitive impairment: a decision tree model study. Clin Interventions Aging. 2020;15:2009–2017. doi:10.2147/CIA.S265839
10. Shimada H, Doi T, Lee S, Makizako H. Reversible predictors of reversion from mild cognitive impairment to normal cognition: a 4-year longitudinal study. Alzheimer’s Res Ther. 2019;11(1):24. doi:10.1186/s13195-019-0480-5
11. Bielak A, Gow A. A decade later on how to ”use it” so we don’t ”lose it”: an update on the unanswered questions about the influence of activity participation on cognitive performance in older age. Gerontology. 2022;22:1–20.
12. Kuhn M, Johnson K. Applied predictive modeling || nonlinear classification models. Springer. 2013;13:329–367.
13. Morris K, Perna F. Decision tree model vs traditional measures to identify patterns of sun-protective behaviors and sun sensitivity associated with sunburn. JAMA dermatol. 2018;154(8):897–902. doi:10.1001/jamadermatol.2018.1646
14. Wang Y, Wu S, Yan D, et al. Determining and mapping the spatial mismatch between soil and rice cadmium (Cd) pollution based on a decision tree model. Environ Pollution. 2020;265:115029. doi:10.1016/j.envpol.2020.115029
15. Tufféry S Data mining and statistics for decision making; 2011.
16. Hh Y, K C, B R, K S, M F-T, Y J. Identifying smoker subgroups with high versus low smoking cessation attempt probability: a decision tree analysis approach. Addict Behav. 2020;103:106258. doi:10.1016/j.addbeh.2019.106258
17. Tan E, Lin F, Sheck L, Salmon P, Ng S. A practical decision-tree model to predict complexity of reconstructive surgery after periocular basal cell carcinoma excision. J Eur Acad Dermatol Venereol. 2017;31(4):717–723. doi:10.1111/jdv.14012
18. L H, W TT, Y DL, et al. Decision tree model for predicting in-hospital cardiac arrest among patients admitted with acute coronary syndrome. Clinical Cardiol. 2019;42(11):1087–1093. doi:10.1002/clc.23255
19. Fj B, Tm M, D I, et al. A novel clinical prediction model for prognosis in malignant pleural mesothelioma using decision tree analysis. J Thoracic Oncol. 2016;11(4):573–582. doi:10.1016/j.jtho.2015.12.108
20. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the international working group on mild cognitive Impairment. J Internal Med. 2004;256(3):240–246. doi:10.1111/j.1365-2796.2004.01380.x
21. Sw H, D A, F C, Mo M. A framework for secondary cognitive and motor tasks in dual-task gait testing in people with mild cognitive impairment. BMC Geriatr. 2018;18(1):202. doi:10.1186/s12877-018-0894-0
22. Jc M. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi:10.1212/WNL.43.11.2412-A
23. Be S, W T, Yk C, et al. Risk of progression from subjective cognitive decline to mild cognitive impairment: the role of study setting. Alzheimer’s & Dementia. 2018;14(6):734–742. doi:10.1016/j.jalz.2017.12.003
24. Chunhua F, Xiaoyun X, Yue W, et al. Factors related to the progression of mild cognitive impairment toward Alzheimer’s disease. Chin J Phys Med Rehabil. 2016;38(2):108–112.
25. Farias S, Mungas D, Reed B, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch. Neurol. 2009;66(9):1151–1157. doi:10.1001/archneurol.2009.106
26. S Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi:10.1016/S1474-4422(12)70191-6
27. C K, S CY, B T, C L, R S, G T. Practical applications of physical activity for successful cognitive aging. JAAPA. 2017;30(8):30–35. doi:10.1097/01.JAA.0000520537.00581.f1
28. Erickson KI, Hillman C, Stillman CM, et al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exercise. 2019;51(6):1242–1251. doi:10.1249/MSS.0000000000001936
29. Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Internal Med. 2011;269(1):107–117. doi:10.1111/j.1365-2796.2010.02281.x
30. Sorte Silva N B, Dao E, Hsu C, et al. Myelin and physical activity in older adults with cerebral small vessel disease and mild cognitive impairment. . J Gerontol Series a Bio Sci Med Sci. 2022;2022:1.
31. Nikolaos S, Yaakov S. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25(5):625–633. doi:10.1076/jcen.25.5.625.14576
32. Oh SS, Cho E, Kang B. Social engagement and cognitive function among middle-aged and older adults: gender-specific findings from the Korean longitudinal study of aging (2008–2018). Nat Publishing Group. 2021;2021:1.
33. Ahmed MM, Lindauer A, Pruitt A, Mattek N, Kaye JA. Social engagement and cognitive function are related in older African Americans. Alzheimer’s Dementia. 2018;78:(7):14.
34. Gamba P, Testa G, Gargiulo S, Staurenghi E, Poli G, Leonarduzzi G. Oxidized cholesterol as the driving force behind the development of Alzheimer’s disease. Front Aging Neurosci. 2015;7:119. doi:10.3389/fnagi.2015.00119
35. Qi G, Mi Y, Wang Y, et al. Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct. 2017;8(12):4421–4432. doi:10.1039/C7FO00991G
36. Li P, Gao L, Yu L, et al. Daytime napping and Alzheimer’s dementia: a potential bidirectional relationship. Alzheimer’s & Dementia. 2022;19(1):158–68.
37. Li C, Yan Y. Afternoon napping durations in Chinese population over 60 years old: longitudinal associations with cognitive performance. Front Public Health. 2022;10:911498. doi:10.3389/fpubh.2022.911498
38. Alqurashi Y, AlHarkan K, Aldhawyan A, et al. Association between nap duration and cognitive functions among Saudi older adults. Front Neurosci. 2022;16:917987. doi:10.3389/fnins.2022.917987
39. Chang H, Yang K, Chu M, Yun C, Kim D. Association between nap and reported cognitive function and role of sleep debt: a population-based study. J Clinical Neurol. 2022;18(4):470–477. doi:10.3988/jcn.2022.18.4.470
40. Reversion From M-AM. Mild cognitive impairment to normal cognition: a meta-analysis. Alzheimer Dis Assoc Disord. 2016;30(4):324–330.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


