Back to Journals » OncoTargets and Therapy » Volume 15
Leukoencephalopathy During Daratumumab-Based Therapy: A Case Series of Two Patients with Multiple Myeloma
Authors Kareem SS , Viswanathan N, Sahebjam S, Tran ND , Gatewood T, Tobon K, Baz R, Piña Y, Shain KH, Mokhtari S
Received 10 March 2022
Accepted for publication 23 August 2022
Published 6 September 2022 Volume 2022:15 Pages 953—962
DOI https://doi.org/10.2147/OTT.S365657
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Leo Jen-Liang Su
Syeda Saba Kareem,1 Neena Viswanathan,2 Solmaz Sahebjam,2 Nam D Tran,2 Tyra Gatewood,2 Katherine Tobon,1 Rachid Baz,1 Yolanda Piña,2 Kenneth H Shain,1,3 Sepideh Mokhtari2
1Malignant Hematology Department, Moffitt Cancer Center, Tampa, FL, USA; 2Neuro-Oncology Department, Moffitt Cancer Center, Tampa, FL, USA; 3Tumor Biology Department, Moffitt Cancer Center, Tampa, FL, USA
Correspondence: Syeda Saba Kareem, Malignant Hematology Department, Moffitt Cancer Center, 12902 USF Magnolia Drive, Tampa, FL, 33612, Tel +850-321-4383, Fax +813-449-8246, Email [email protected]
Abstract: Leukoencephalopathy in the setting of multiple myeloma (MM) is a rare demyelinating condition, with few reported cases in literature. Daratumumab is a CD38 targeted monoclonal antibody that has been widely used for the management of MM. In the absence of central nervous system (CNS) disease, many medication-induced leukoencephalopathy cases reported with MM, including daratumumab-induced, are associated with progressive multifocal leukoencephalopathy (PML) and John Cunningham (JC) virus. Currently, there are no reported cases of daratumumab-induced leukoencephalopathy among patients without CNS involvement or PML. We discuss 2 patients who developed leukoencephalopathy while receiving daratumumab-based therapy without evidence of PML or CNS disease. Both patients had baseline MRIs without significant white matter changes before daratumumab-based therapy. Patients began experiencing neurological deficits about 6 to 8 months after daratumumab-based therapy initiation. One patient passed away before being assessed for improvement of symptoms with daratumumab cessation. The second patient had some stabilization of symptoms after cessation; however, the leukoencephalopathy remained irreversible. As the class of anti-CD38 monoclonal antibodies expands in MM therapy, we highlight a potential treatment complication and the importance of detecting leukoencephalopathy early among patients receiving anti-CD38 therapy. We recommend vigilant monitoring of any new or worsening neurological symptoms to avoid serious complications of irreversible leukoencephalopathy.
Keywords: anti-CD38, monoclonal antibody, neurotoxicity, plasma cell disorder, white matter changes, daratumumab, leukoencephalopathy, case report
Background
Leukoencephalopathy is a clinical condition associated with functional decline in neuronal capabilities secondary to structural alterations in cerebral white matter, which are induced by changes to the myelin. There are many different types of leukoencephalopathy, which can result from genetic mutations; can be acquired via vascular injury, infections, inflammatory processes, nutritional deficiencies, traumatic events, or neoplastic disease; or can be caused by toxicity/adverse effects from medications.1,2 In the setting of multiple myeloma (MM), leukoencephalopathy is rare and has been predominantly documented secondary to central nervous system (CNS) disease, paraneoplastic syndrome, infectious processes, genetic conditions, and medication use.3–6
Leukoencephalopathy—primarily John Cunningham (JC) virus–associated progressive multifocal leukoencephalopathy (PML)—has been reported in association with monoclonal antibody treatment.7 Daratumumab is a human monoclonal antibody that targets CD38, a cell surface glycoprotein that is highly expressed on myeloma cells. Daratumumab induces cell death through various mechanisms, including direct cytotoxicity, indirect death via complement-dependent cytotoxicity, antibody-dependent cellular phagocytosis, and antibody-dependent cytotoxicity.8 To our knowledge, PML cases compose the only prior reports of daratumumab-associated leukoencephalopathy.9,10 Here, we describe 2 patients who developed leukoencephalopathy, without evidence of PML or CNS involvement, while receiving daratumumab-based therapy for relapsed and refractory MM.
Case Series
Case Report 1
A 65-year-old right-handed woman was diagnosed with IgG κ MM in 2002. Her treatment began in 2012, and she received multiple lines of therapy, including lenalidomide, bortezomib, and dexamethasone (RVd) followed by high-dose melphalan and peripheral blood autologous stem cell transplant (HDM-ASCT). She had mild peripheral neuropathy from chemotherapy, and because of progressive disease, she began receiving lenalidomide and dexamethasone (Rd) by late 2014. She developed a pulmonary embolism in 2016 and was prescribed warfarin therapy, which was eventually switched to aspirin. She continued to receive lenalidomide and dexamethasone until 2019.
In March 2019, after biochemical disease progression, ixazomib was added to her chemotherapy regimen. She remained neurologically intact but developed intractable headaches, which prompted a brain magnetic resonance imaging (MRI) scan. The MRI showed microvascular ischemic changes and a small subdural hematoma, without any other abnormalities (Figure 1A). Because of intolerable side effects of vomiting and disease progression, she was prescribed daratumumab, pomalidomide, and dexamethasone (DPd) in April 2019. In October 2019, the pomalidomide dose was decreased because of cytopenia. The RBC morphology was normal and lactate dehydrogenase (LDH) level was 142 U/L. By December 2019, within 8 months of treatment initiation, her family noted mild cognitive changes, including short-term memory loss and difficulty with executive functioning and planning. During this time, the patient was prescribed 0.4 gm/kg monthly intravenous immunoglobulin (IVIG) for recurrent infections. By February 2020, her symptoms progressed into a more severe encephalopathy, with right upper extremity weakness and right hemibody neglect. In April 2020, a brain MRI showed patchy FLAIR changes in posterior periventricular and subcortical white matter bilaterally, with mild restricted diffusion.
A lumbar puncture (LP) on May 5, 2020, showed an elevated myelin basic protein (MBP) level of 16.2 ng/mL (normal value <4 ng/mL), which was concerning for active demyelination; the LP results were otherwise unremarkable. Cerebrospinal fluid (CSF) was negative for plasma cells; lactate dehydrogenase (LDH) levels were within normal limits; and JC virus, tested via polymerase chain reaction, was not detected. The patient’s encephalopathy continued to rapidly worsen, with right facial droop, word-finding difficulties, pseudobulbar affect, and dysphagia. A repeat MRI brain performed on May 18, 2020, showed worsening T2-FLAIR changes in bilateral frontal regions (left greater than right) and the right temporal lobe, with low–level enhancement in some of these areas and microvascular ischemic changes; the hematoma that was seen at baseline had resolved (Figure 1B). Upon examination, the patient was disoriented, intermittently followed simple commands, and was able to read. She could name and repeat but was not able to write, and she had significant difficulty with delayed word recall (ie, 0/4 words in 2 minutes). She also had acalculia, agraphia, and apraxia. She had right hemibody neglect and left upper extremity dysmetria, with symmetric hyperreflexia in all upper and lower extremities.
A repeat LP on May 28, 2020 was still negative for malignancy and showed a continued elevated MBP level of 13.7 ng/mL. She had an elevated serum factor VIII level of 277% (normal value, 50%–150%) and was evaluated for a cerebral vascular accident; however, MRI findings did not indicate a stroke. During this time, she was still receiving DPd therapy and IVIG (0.4 gm/kg) monthly, with an overall partial response. However, because of worsening clinical status and progressive white matter changes seen via imaging, the decision was made on June 9, 2020, to halt the DPd regimen and increase the IVIG to 1 gm/kg monthly. No further responses to the treatment change were able to be assessed, as the patient passed away on June 18, 2020, and a postmortem evaluation was not performed. The patient was not tested for COVID-19.
Case Report 2
A 56-year-old right-handed man was diagnosed with IgG κ MM in 2011 and received multiple lines of therapy, including RVd, followed by HDM-ASCT in October 2012. The patient continued to receive maintenance lenalidomide and dexamethasone from 2012 to 2016. Because of vertigo, a brain MRI was performed in April 2016 (Figure 1C); mild microvascular ischemic changes were seen, but the results were otherwise unremarkable. After disease progression in late 2016, he was prescribed ixazomib, pomalidomide, and dexamethasone (IPd). He discontinued treatment in November 2018 because of intolerability related to fatigue, dizziness, and peripheral neuropathy that persisted even after multiple dose reductions. In February 2019, the patient’s disease progressed, and he was prescribed daratumumab, lenalidomide, and dexamethasone (DRd).
In August 2019, within 6 months of starting DRd, he presented with a significant decline in functional status: he required a wheelchair to ambulate and had profound dysphagia. The lenalidomide dose had been reduced because of cytopenia, dizziness, and fatigue, and it was discontinued by August 2019. The patient continued to receive monthly daratumumab as a monotherapy while he experienced significant cognitive decline (ie, Montreal Cognitive Assessment [MoCA] score of 24/30) and diffuse hyperreflexia. A brain MRI was performed in November 2019 (Figure 1D) and showed mild interval increases in ventriculomegaly and extensive bilateral confluent foci of increased T2-FLAIR changes within the cerebral white matter, with some microvascular ischemic changes.
An LP revealed an elevated opening pressure of 21 cm H2O, with lymphocytic pleocytosis (7 nucleated cells, with 91% lymphocytes), elevated protein level (101 mg/dL), elevated number of oligoclonal bands (5), and elevated MBP level (6.79 ng/mL). CSF was negative for evidence of plasma cells; paraneoplastic laboratory results were negative; LDH was slightly elevated at 289 U/L (upper normal limit 225 U/L); and JC virus, tested via polymerase chain reaction, was not detected. The patient had some improvement in his gait and cognition that lasted for 24 hours after the LP, and a diagnosis of hydrocephalus was considered at that time. He was prescribed 1 gm/kg of IVIG for 2 days in January 2020, and daratumumab was discontinued in February 2020. On February 25, 2020, he had a slight improvement in MoCA score from 24/30 to 26/30, and a brain MRI showed stable T2-FLAIR changes. Because of the patient’s ventriculomegaly, elevated opening pressure, and improvement after high-volume lumbar puncture, a ventriculoperitoneal shunt was placed in March 2020. The patient was also given a trial of rituximab therapy, but it did not improve his symptoms further. For his MM treatment, he was prescribed venetoclax and dexamethasone in April 2020. By June 2020, he had worsening gait, balance, and cognition, and he was began receiving monthly IVIG 1 gm/kg. A COVID-19 test performed in July 2020 was negative.
A repeat LP performed in September 2020 was negative for malignancy and showed resolution of oligoclonal bands; however, the MBP level remained elevated at 6.59 ng/mL. After MM progression in December 2020, he began receiving low-dose cyclophosphamide and prednisone. This treatment was later discontinued because of worsening neurological symptoms and gastrointestinal intolerability to chemotherapy. IVIG was also discontinued at this time, as the patient considered entering hospice care. In May 2021, the patient decided to resume treatment and began receiving elotuzumab, pomalidomide, and solumedrol. Of note, a coagulopathy panel was performed on May 24, 2021, and he was found to have an elevated factor VIII level of 269%. Currently, the patient’s cognition has stabilized; however, he continues to experience progressive lower extremity weakness and fatigue, and he still requires use of a wheelchair. Unfortunately, his multiple myeloma has progressed, and he is not tolerating any further treatment. He was placed in hospice care for palliative management.
Discussion
The presentation of leukoencephalopathy with demyelinating processes among patients with MM who are receiving active treatment generally prompts a differential diagnosis that includes meningoencephalitis, malignant CNS involvement, PML, posterior reversible encephalopathy syndrome (PRES), genetic leukodystrophy, and chemotherapy-induced leukoencephalopathy.4–6,11–13 For the patients in our series, we ruled out infection and malignant cerebral involvement because of negative CSF results throughout their courses of therapy. A summary of the cases is presented in Table 1 with patient timelines in Figure 2.
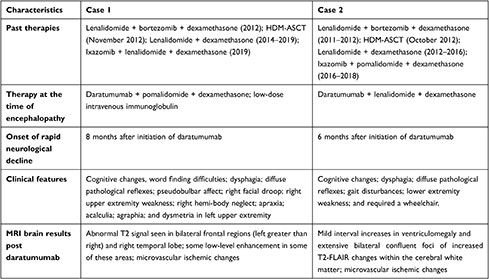 |  |
Table 1 Clinical Summary |
 |
Figure 2 Continued. |
 |
Figure 2 Patient case timelines. Patient Case 1 and 2 timelines. |
In Case 1, 8 months after initiation of daratumumab, the patient was noted to have mild cognitive changes, including short-term memory loss and difficulty with executive functioning and planning. Two months after initial neurologic presentation, she developed severe cognitive impairment, with right upper extremity weakness and right hemi-body neglect. Two months after that, her brain MRI showed patchy FLAIR changes in posterior periventricular and subcortical white matter bilaterally, with mild restricted diffusion. Her CSF studies showed an elevated myelin basic protein (MBP) level of 16.2 ng/mL (normal value <4 ng/mL). In Case 2, within 6 months of starting daratumumab, the patient became wheelchair-bound and had profound dysphagia with progressive cognitive decline. Nine months after initiation of daratumumab (Figure 1D), a brain MRI showed mild interval increases in ventriculomegaly and extensive bilateral confluent foci of increased T2-FLAIR changes within the cerebral white matter. For both patients, elevated CSF MBP levels and MRI findings of progressive leukoencephalopathy seen after daratumumab therapy were indicative of active demyelination.
We sought to determine whether the etiology for leukoencephalopathy was related to treatment. Both patients were receiving combination therapy with an anti-CD38 monoclonal antibody, an immunomodulatory drug (IMiD), and a steroid when symptoms began. There have been IMiD-associated PML and PRES cases reported in the literature; however, both patients’ negative JC virus PCR assays and lack of evidence of PRES on MRIs suggested that these diseases were not present. Case reports of IMiD-related neurotoxicity have been published for both lenalidomide and pomalidomide.23–27 Peripheral neuropathy is a commonly reported adverse effect for IMiDs, but central neurotoxicity, including ataxia, amnesia, aphasia, and reversible comas, are rare, with only a few cases reported.28 Notably, both of our patients were receiving IMiDs for many years during their previous therapies without significant central neurotoxicity and specifically without any white matter degenerative changes, as was seen in both baseline MRIs.
Having mainly ruled out IMiDs, we next evaluated whether anti-CD38 monoclonal antibodies may have been associated with leukoencephalopathy for these cases. In addition to being expressed on plasma cells, CD38 is expressed in the CNS, including, but not limited to, neurons, astrocytes, and microglial cells.14–20 Despite being a monoclonal antibody, daratumumab has the potential to cross the blood-brain barrier;21 once in the CNS, daratumumab can potentially induce demyelination by suppressing CD38.22 For both of our patients, elevated MBP levels and MRI findings of leukoencephalopathy seen after daratumumab therapy were indicative of active demyelination. Currently, PML is the only neurotoxicity associated with daratumumab in published case reports.9,10 PML is a demyelinating condition caused by the JC virus; radiographically, it is asymmetric and well-demarcated.29 For our report, leukoencephalopathy was asymmetric in Case 1 and symmetric in Case 2. However, neither of the patients were positive for JC virus, indicating that these cases were unlikely to be related to PML. The symmetry seen in Case 2 was similar to whole-brain radiation–related leukoencephalopathy.30
To assess the likelihood of daratumumab’s association with leukoencephalopathy in these cases, the Naranjo Adverse Drug Reaction (NADR) assessment, which evaluates a medication as possibly, probably, or definitively related to a clinical event, was used.31 The NADR score was 7 out of 13 for Case 1 and 6 out of 13 for Case 2, and both of these scores correspond to a probable adverse drug reaction. A NADR score of 9 or above would have been considered definitive; however, there were some NADR categories we were unable to assess. Neither patient had their dose increased or re-administered, so it is unknown whether they may have had a more severe reaction in this context. Daratumumab was the only anti-CD38 medication that these patients were exposed to, so we could not assess whether a similar reaction was seen with other agents in the same class. The NADR assessment was also performed for the IMiDs, which had a score of 1 for both patients; this denotes a possible adverse drug reaction. It is important to note that the patient in Case 1 had their pomalidomide dose reduced because of cytopenia and the patient in Case 2 had lenalidomide discontinued because of intolerability; however, neurological symptoms continued to progress for both patients. The patients in Cases 1 and 2 also received lenalidomide during previous therapies for 5 and 4 years, respectively, without developing leukoencephalopathy.
Since the initial approval of daratumumab by the Federal Drug Administration (FDA) for patients with previously treated MM in 2015, its use has rapidly expanded to many other indications, including first-line therapy.32–34 The anti-CD38 monoclonal antibody class has also expanded, with the addition of the subcutaneous product daratumumab-hyaluronidase and the recent approval of isatuximab in 2020.35,36 With these approvals, we are likely to see more patients receiving anti-CD38 therapy for MM.
There is a need to further identify risks of cognitive impairment or neurodegenerative changes among patients with MM without CNS involvement who are being treated with anti-CD38 therapies. Both patients in our series had mild small vessel disease prior to daratumumab initiation, which could have increased their risk of post-therapeutic complications. Though most metabolic treatment–induced leukoencephalopathy cases reported in literature are reversible, both of our patients had irreversible encephalopathy.11
Based on our observations, it would be prudent to recognize any central neurotoxicity, leukoencephalopathy, or hydrocephalus that may occur while patients are receiving anti-CD38–based therapy. If a patient develops any new neurological deficits such as cognitive impairment, ataxia, aphasia, dysphasia, or profound peripheral neuropathy, we recommend withholding anti-CD38 therapy until the patient is evaluated with at least an LP and MRI by neurologists/neuro-oncologists. If the brain MRI scan shows significant leukoencephalopathy and CSF shows elevated MBP and protein, concerns should be raised for active demyelination. Pharmaceutical intervention with 1 gm/kg of IVIG monthly can also be considered to improve or stabilize neurologic deficits.
As with any observational study, there are confounding factors to consider. Concurrent medications, vascular risk factors, or autoimmune conditions could have contributed to our patients’ leukoencephalopathy. Both patients had elevated factor VIII levels and microvascular ischemic changes, and the patient in Case 1 had a history of thromboembolic events and a baseline hematoma that could have contributed to her decline being more rapid than that of the patient from Case 2. The patient from Case 2 had mild gait disturbances prior to daratumumab-based therapy; however, his baseline MRI was negative for active demyelination. His symptoms initially stabilized after the cessation of daratumumab, placement of the ventriculoperitoneal shunt, and initiation of IVIG therapy; however, the demyelination was irreversible, and the patient continued to have progressive symptoms. The positive oligoclonal bands and lymphocytic pleocytosis could be indicative of an underlining autoimmune disorder or encephalitis. Rituximab is a CD20 monoclonal antibody that causes depletion of B cells and is used in managing autoimmune conditions; when the patient from Case 2 was given a trial of rituximab, there was no further improvement or resolution of symptoms.37
Conclusion
In conclusion, to the best of our knowledge, these are the first cases of leukoencephalopathy reported among patients without a PML diagnosis who are receiving daratumumab-based therapy. Patients with MM, who may have preexisting peripheral neuropathy, should be further tested if they exhibit worsening neurological symptoms. Diagnostic MRIs and LPs with a neurology consult are recommended for patients exhibiting central neurotoxicity while receiving CD38-based chemotherapy. There is a need to further study possible risks associated with this drug class as its use continues to expand in MM treatment.
Abbreviations
CNS, central nervous system; CSF, cerebrospinal fluid; DRd, daratumumab, lenalidomide, and dexamethasone; DPd, daratumumab, pomalidomide, and dexamethasone; HDM-ASCT, high-dose melphalan and peripheral blood autologous stem cell transplant; IVIG, intravenous immunoglobulin; IPd, ixazomib, pomalidomide, and dexamethasone; JC, John Cunningham (virus); LDH, lactate dehydrogenase; Rd, lenalidomide and dexamethasone; RVd, lenalidomide, bortezomib, dexamethasone; LP, lumbar puncture; MM, multiple myeloma; MBP, myelin basic protein; PML, progressive multifocal leukoencephalopathy; T2-FLAIR, T2 fluid-attenuated inversion recovery.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Editorial assistance was provided by the Moffitt Cancer Center’s Office of Scientific Publishing by Daley Drucker. No compensation was given beyond her regular salary.
Consent
Written informed consent was obtained from each patient/patient representative for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. Institutional approval was not required to publish the case details.
Disclosure
R.B has received research funding from Janssen, Celgene, Karyopharm, Abbvie, BMS, Merck, Sanofi and is on the advisory board for Janssen, Karyopharm, GSK, Sanofi; K.S has received research/grant funding from Abbvie, Karyopharm and is on the advisory board for celgene, BMS, Amgen, Takeda, Janssen, Karyopharm, GSK, Sanofi, Genzyme and is a consultant for Adaptive. S.S reports personal fees from Merck, personal fees from Boehringer-Ingelheim, grants from Bristol Myers-Squibb, grants from Merck, grants from Brooklyn ImmunoTheraputics, other from Eli Lilly, outside the submitted work. The other authors have no disclosures.
References
1. Patterson MC. Leukoencephalopathy. Encyclopedia Neurol Sci. 2014;1:878–879.
2. Sobol U, Stiff P. Neurologic aspects of plasma cell disorders. Handb Clin Neurol. 2014;120:1083–1099.
3. Egan P, Elder P, Deighan I, O’Connor SJM, Alexander HD. Multiple myeloma with central nervous system relapse. Haematologica. 2020;105(7):1780–1790. doi:10.3324/haematol.2020.248518
4. Aquino C, Connolly B, Lang A. Smoldering multiple myeloma associated with leukoencephalopathy presenting with holmes tremor, ataxia, and pyramidal syndrome. Movement Disorders Clin Practice. 2018;5(4):433–435. doi:10.1002/mdc3.12623
5. Lebrun C, Chanalet S, Frenay M, Chatel M. Leukoencephalopathy in multiple myeloma: two case reports. Ann Oncol. 1999;10(12):1515–1517. doi:10.1023/A:1008353312410
6. Knight K, Chien S, Koutsavlis I, Campbell V. Progressive multifocal leukoencephalopathy following five lines of therapy and three autologous bone marrow transplants for multiple myeloma. BMJ Case Rep. 2020;13(3):e233552. doi:10.1136/bcr-2019-233552
7. Bohra C, Sokol L, Dalia S. Progressive multifocal leukoencephalopathy and monoclonal antibodies: a review. Cancer Control. 2017;24:4. doi:10.1177/1073274817729901
8. Sanchez L, Wang Y, Siegel DS, Wang ML. Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol. 2016;9(1):51. doi:10.1186/s13045-016-0283-0
9. Monge J, Contreras J, Elsoukkary S, et al. Progressive multifocal leukoencephalopathy in a patient with multiple myeloma receiving daratumumab and pomalidomide. Blood. 2019;134:4876. doi:10.1182/blood-2019-122134
10. Steinhardt MJ, Wiercinska E, Pham M, et al. Progressive multifocal leukoencephalopathy in a patient post allo-HCT successfully treated with JC virus specific donor lymphocytes. J Transl Med. 2020;18(1):177. doi:10.1186/s12967-020-02337-5
11. Sarbu N, Shih R, Jones R. White matter diseases with radio-logic-pathologic correlation. Radio Graphics. 2016;36:1426–1447.
12. Isaacs D, Olmez I, Claassen D. Adult-onset adrenoleukodystrophy presenting after chemotherapy. Neurol Clin Pract. 2014;4(2):168–170. doi:10.1212/01.CPJ.0000435751.17621.93
13. Cai X, Bhattacharyya S, Plitt A, et al. Management of posterior reversible encephalopathy syndrome induced by carfilzomib in a patient with multiple myeloma. JCO. 2016;34(2):e1–e5. doi:10.1200/JCO.2013.49.6166
14. Yamada M, Mizuguchi M, Otsuka N, Ikeda K, Takahashi H. Ultrastructural localization of CD38 immunoreactivity in rat brain. Brain Res. 1997;756(1–2):52–60. doi:10.1016/S0006-8993(97)00117-0
15. Mizuguchi M, Otsuka N, Sato M, et al. Neuronal localization of CD38 antigen in the human brain. Brain Res. 1995;697(1–2):235–240. doi:10.1016/0006-8993(95)00885-T
16. Ma Y, Wu D, Ding X, Ying W. CD38 plays key roles in both antioxidation and cell survival of H2O2-treated primary rodent astrocytes. Int J Physiol Pathophysiol Pharmacol. 2014;6(2):102–108.
17. Kou W, Banerjee S, Eudy J, et al. CD38 regulation in activated astrocytes: implications for neuroinflammation and HIV-1 brain infection. J Neurosci Res. 2009;87(10):2326–2339. doi:10.1002/jnr.22060
18. Ma Y, Jiang J, Wang L, et al. CD38 is a key enzyme for the survival of mouse microglial BV2 cells. Biochem Biophys Res Commun. 2012;418(4):714–719. doi:10.1016/j.bbrc.2012.01.084
19. Ma Y, Cao W, Wang L, et al. Basal CD38/cyclic ADP-ribose-dependent signaling mediates ATP release and survival of microglia by modulating connexin 43 hemichannels. Glia. 2014;62(6):943–955. doi:10.1002/glia.22651
20. Mayo L, Jacob-Hirsch J, Amariglio N, et al. Dual role of CD38 in microglial activation and activation-induced cell death. J Immunol. 2008;181(1):92–103. doi:10.4049/jimmunol.181.1.92
21. Vercruyssen M, Hachem GE, Maerevoet M. The Daratumumab crosses the blood brain barrier. Clin Lymphoma Myeloma Leuk. 2018;18:S289. doi:10.1016/j.clml.2018.07.229
22. Roboon J, Hattori T, Ishii H, et al. Deletion of CD38 suppresses glial activation and neuroinflammation in a mouse model of demyelination. Front Cell Neurosci. 2019;13:258. doi:10.3389/fncel.2019.00258
23. Ueno H, Kikumto M, Takebayashi Y, et al. Pomalidomide-associated progressive multifocal leukoencephalopathy in multiple myeloma: cortical susceptibility-weighted imaging hypointense findings prior to clinical deterioration. J Neurovirol. 2020;26(3):452–455. doi:10.1007/s13365-020-00845-0
24. Anderson S, Kiernan M, Ho PJ. Lenalidomide-related progressive multifocal leukoencephalopathy: a case report and review of drug-related cases in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019;19(4):e169–e171. doi:10.1016/j.clml.2018.12.021
25. Sawicki CP, Climans SA, Hsia CC, Fraser JA. Progressive multifocal leukoencephalopathy during ixazomib-based chemotherapy. Curr Oncol. 2018;25(1):e99–e102. doi:10.3747/co.25.3674
26. Usui Y, Nakano H, Komatsu J, et al. Progressive multifocal leukoencephalopathy during treatment with lenalidomide and elotuzumab for multiple myeloma. Leuk Lymphoma. 2020;61(9):2234–2237. doi:10.1080/10428194.2020.1765237
27. Rollin-Sillaire A, Delbeuck X, Pollet M, et al. Memory loss during lenalidomide treatment: a report on two cases. BMC Pharmacol Toxicol. 2013;14:41. doi:10.1186/2050-6511-14-41
28. Patel UH, Mir MA, Sivik JK, Raheja D, Pandey MK, Talamo G. Central neurotoxicity of immunomodulatory drugs in multiple myeloma. Hematol Rep. 2015;7(1):5704. doi:10.4081/hr.2015.5704
29. Küker W, Mader I, Nägele T. Progressive multifocal leukoencephalopathy value of diffusion-weighted and contrast-enhanced magnetic resonance imaging for diagnosis and treatment control. Eur J Neurol. 2006;13:819. doi:10.1111/j.1468-1331.2006.01362.x
30. Monaco EA, Faraji AH, Berkowitz O, et al. Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer. 2013;119(1):226–232. doi:10.1002/cncr.27504
31. Busto U, Naranjo CA, Sellers EM. Comparison of two recently published algorithms for assessing the probability of adverse drug reactions. Br J Clin Pharmacol. 1982;13:223–227. doi:10.1111/j.1365-2125.1982.tb01361.x
32. Shin YW, Lee ST, Park KI, et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord. 2017;11:1756285617722347. doi:10.1177/1756285617722347
33. Janssen Pharmaceutical Companies. Darzalex (Daratumumab) [Package Insert]. Janssen Pharmaceutical Companies; 2020.
34. Multiple Myeloma. NCCN Clinical Practice Guidelines in Oncology Version 2; 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf.
35. Sanofi-Aventis. Sarclisa (Isatuximab-Irfc) [Package Insert]; 2020.
36. Janssen pharmaceudical Companies. Darzalex Faspro (Daratumumab Hyaluronidase-Fihj) [Package Insert]. Janssen pharmaceudical Comapnies; 2020.
37. Kosmidis ML, Dalakas MC. Practical considerations on the use of rituximab in autoimmune neurological disorders. Adv Neurol Disord. 2010;Mar(2):93–105. doi:10.1177/1756285609356135
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

