Back to Journals » Infection and Drug Resistance » Volume 17
Lemierre Syndrome: Report of a Case with an Innovative Diagnostic Method and Literature Review
Authors Xie M, Liu J, Zheng J, Wang J, Han D
Received 7 September 2023
Accepted for publication 14 December 2023
Published 3 January 2024 Volume 2024:17 Pages 1—10
DOI https://doi.org/10.2147/IDR.S439069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Mengxiao Xie,1,* Jian Liu,2,* Jieyuan Zheng,1 Jingchao Wang,1 Dongsheng Han1,3,4
1Department of Laboratory Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of Intensive Care Unit, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 3Key Laboratory of Clinical in vitro Diagnostic Techniques of Zhejiang Province, Hangzhou, People’s Republic of China; 4Institute of Laboratory Medicine, Zhejiang University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongsheng Han, Department of Laboratory Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou, 310003, People’s Republic of China, Email [email protected]; [email protected]
Objective: To understand the clinical features, diagnosis and treatment of Lemierre syndrome (LS), a high-risk and low-prevalence infectious disease.
Methods: We present the severe LS case that was diagnosed using metagenomic next-generation sequencing (mNGS) in our hospital, and systematically summarized the diagnosis and treatment strategies of patients that reported LS from 2006 to 2022.
Results: The 24-year-old patient in our hospital suffered from cranial nerve paralysis, a neurological complication rarely seen in LS cases. The causative agent (Fusobacterium necrophorum, Fn) of this patient was only detected by mNGS tests, and the reads number of Fn detected by plasma mNGS tests was decrease as the patients gradually improved, indicating plasma mNGS is valuable in monitoring treatment efficacy. Although most of the cases retrieved from the literature showed typical symptoms, such as a history of sore throat, septic emboli, and internal jugular vein thrombosis, clinical manifestations were still relatively heterogeneous (eg, diversity of predisposing factors and pathogens, differences in pulmonary imaging features).
Conclusion: We summarized the clinical presentation, diagnosis, treatment, and regression of 17 symptomatic cases reported LS to provide clinicians with knowledge about this rare but fatal disease. mNGS assays should be considered as early as possible to identify the responsible pathogens for acute and critically ill patients with suspected infections in order to implement accurate and effective treatment.
Keywords: lemierre syndrome, Fusobacterium necrophorum, metagenomic next-generation sequencing, thrombophlebitis, sepsis
Introduction
Lemierre syndrome (LS) is a high-risk, rare infectious disease characterized by anaerobic sepsis, septic thrombophlebitis of the internal jugular vein (IJV), and metastatic abscesses.1 Young people aged 16–24 years old are a susceptible group for this disease, with data showing an incidence of about 0.8–3.6/million per year.2 LS usually begins with pharyngitis, but is prone to be missed because of the variable atypical clinical manifestations and challenging diagnosis. Fusobacterium necrophorum (Fn), an obligate Gram-negative anaerobic bacterium from the oral normal flora isolated in 48–82% of LS cases, is thought to be the most common causative agent of LS.2 Other pathogens that may be associated with LS include Streptococci, Klebsiella pneumonia, Bacteroides species, and Staphylococcus aureus.3 It has been hypothesized that in patients with LS, Fn in the oral cavity may invade the pharyngeal mucosa and the lateral pharyngeal space through the pharyngeal mucosa damaged by bacterial/viral pharyngitis or other causes, which then leads to IJV thrombophlebitis and metastatic septic emboli secondary to acute pharyngeal infections.4,5 Various pathogenic factors of Fn including endotoxin, leukotoxin, platelet agglutination, hemagglutinin and hemolysin may play a role in the development of the disease.6 However, as a strictly anaerobic bacterium, Fn has a high rate of missed detection by conventional microbiological culture, which poses a challenge for timely and accurate diagnosis of the disease.7 As an emerging nucleic acid molecular diagnostic method, metagenomic high-throughput sequencing (mNGS) has the characteristics of being independent of microbial culture and being less affected by empirical antimicrobial therapy, and can directly detect pathogens in clinical specimens that are difficult to identify by conventional clinical laboratory methods, providing rapid and accurate pathogenic evidence for clinical diagnosis.8,9
In this study, we present a severe LS case caused by Fn that was diagnosed with the help of metagenomic next-generation sequencing (mNGS) in a timely manner. We also discuss the timely diagnosis and effective treatment strategies for these patients by reviewing some typical reported literature to provide a scientific and useful data for patient management.
Case Presentation
Clinical Presentation
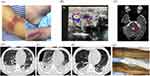
The patient was a 24-year-old male who was healthy in the past, with no history of infectious diseases, genetic diseases, drug use or animal contact. 10 days before admission, the patient had a sore throat and loss of appetite after drinking alcohol. He took some over-The-counter drugs, but the symptoms did not improve. 5 days before admission, he suffered from neck pain, lumbago, loss of appetite, occasional gibberish and sleep disorders, but no diagnosis was made in a local hospital and the patient was not admitted. Five days later, the patient’s condition continued to worsen, with high fever (38.1–39.3°C), chest pain, shortness of breath, heavy sweating, yellow staining of skin and sclera, and large purple patches on both lower limbs (Figure 1). He was initially diagnosed with septic shock with multiple organ failure and admitted to the intensive care unit (ICU). The laboratory examination results showed that the neutrophil percentage (85.9%), D-dimer (2422μg/L), lactic acid (5.3 mmol/L), procalcitonin (>100ng/mL), and total bilirubin/direct bilirubin (240.5/211.8 μmol/L) increased significantly. However, the platelet count was severely reduced (only 2×109/L). Chest CT showed multiple nodules and mass shadows in both lungs and pleural effusion on both sides (Figure 1). Considering his pulmonary condition, bronchoscopy and closed thoracic drainage were performed, and both bronchoalveolar lavage fluid(BALF) and pleural drainage fluid were obtained. Endotracheal intubation was performed and mechanical ventilation started.
Meropenem (1g, Q8H) combined with Vancomycin (0.5g, Q12H) and Cefazolin (2g, Q8H) were used for empirical antibiotic treatment. Intravenous Ademetionine 1.4 - Butanedisulfonate (Ade-SD4) was used to reduce bilirubin, Recombinant human thrombopoietin (rhTPO) was used to increase platelets. Heparin sodium was used for thromboprophylaxis.
Diagnosis and Treatment
In order to identify the potential pathogens as soon as possible, the patient’s samples were collected immediately after admission for pathogenic screening, including the microbial culture of blood/sputum/BALF/urine/pleural drainage fluid, polymerase chain reaction (PCR) assays for Cytomegalovirus (CMV) and Epstein-Barr virus (EBV), serological assays for HIV and Legionella, and plasma/BALF metagenomic next-generation sequencing (mNGS) testing. On the third day of admission, Fusobacterium necrophorum(Fn) was detected by both the plasma and BALF mNGS testing, with the normalized reads per million reads (RPM) of 32,115 and 670,377, respectively (Figure 2). Based on clinical evaluation and the fact that Fusobacterium is an anaerobe, the antibiotic strategy was adjusted to metronidazole (500mg, Q6H), meropenem (1g, Q8H) and cefazolin (2g, Q8H). Subsequently, the patient’s inflammatory indicators such as CRP and PCT decreased slowly. Day 7, repeat CT scan of the chest for follow-up revealed multiple new cavitary lesions bilaterally, right hydropneumothorax, and left pleural effusion (Figure 1). However, except for plasma/BALF mNGS tests, the results of other microbiological methods were negative. In view of the clinical characteristics of Fn and neck pain as an initial symptoms, regular cervical vascular ultrasound is required. On day 8, direct bedside ultrasound suggested left internal jugular vein thrombosis (Figure 1). Based on the pathogen detected (Fn) and several typical clinical symptoms that the patient had presented since the onset of the disease (history of onset of sore throat, neck pain, internal jugular vein thrombosis and sepsis), the patient was finally diagnosed with LS. Fn was again detected in the plasma mNGS testing performed on the same day, with a decreased RPM value (RPM=312) compared to that of first test (RPM=32,115) (Figure 2). Then, the anticoagulant therapy was adjusted to subcutaneous enoxaparin (4000U, QD), and cefazolin was discontinued.
From day 9, the patient had only intermittent fever, the level of inflammatory indicators (such as PCT and CRP) and bilirubin continued to decrease, the platelet counts increased and liver and kidney function gradually recovered. Plasma mNGS performed on that day showed that the RPM value (RPM=71) of Fn was significantly lower than before (Figure 2). Day 17, the ventilator was removed due to the obvious improvement of oxygenation. Repeat bedside ultrasound showed the left internal jugular vein thrombosis disappeared. However, on day 19, the patient presented with masticatory weakness, speech impairment and dysphagia. Magnetic resonance imaging (MRI) of the skull base suggested patchy abnormal signal foci in the pons, suggesting central pons myelinolysis (Figure 1), which was suspected to be a rare central nervous system damage (long-lasting cranial nerve palsy) caused by LS. Day 26, plasma mNGS testing was negative (Figure 2), the patient’s circulatory condition stabilized, the pulmonary infection improved, and the ecchymosis of lower limbs receded (Figure 1). The patient was then transferred to a rehabilitation hospital for treatment of symptoms such as vocal dysfunction and vocal incomplete closure caused by neurological dysfunction. At the follow-up visit after 6 weeks, the neurological dysfunction was significantly relieved and the patient was discharged from the rehabilitation hospital. Unfortunately, long-term follow-up was lost. The patient’s medical history is shown in Figure 3.
 |
Figure 3 Brief review of the clinical course of the patient. |
Discussion
Currently, the diagnosis of LS is based mainly on medical history, microbiological and imaging findings, usually with the following three manifestations: (i) recent pharyngeal disease, (ii) septic emboli, and (iii) internal jugular vein thrombosis or detection of Fusobacterium necrophorum.10
The patient in this study was 24 years old. The earliest symptoms were sore throat and neck pain, followed by sepsis, multiple nodules and cavities in both lungs and left internal jugular vein thrombosis. Fusobacterium necrophorum was identified by multiple mNGS tests. These characteristics are consistent with the typical characteristics of LS cases. However, the clinical presentation can show a large heterogeneity from case to case, posing great difficulties for timely clinical diagnosis. We reviewed cases published with a definitive clinical diagnosis of Lemierre syndrome between 2006 and 2022 and selected cases with different clinical manifestations. In the Table 1, we found that there is a large age span ranging from 15 to 79 years, with an average age of 40 years. Most of patients started from sore throat or fever. The causative organisms were diverse in these cases, with Fusobacterium necrophorum being the most common, accounting for 64.7% (11/17). Other microorganisms included Fusobacterium nucleatum, Streptococcus constellatus, Prevotella, and Streptococcus pharyngitis (Table 1). The pathogen first invades the lateral pharyngeal tissues and internal cervical vessels, followed by hematogenous dissemination to other organs (eg, lungs, liver and large joints) causing proliferative metastatic abscesses. Thrombosis can present as internal jugular thrombophlebitis in Lemierre syndrome, while as portal and superior mesenteric vein thromboses in the GI variant of Lemierre syndrome.11 The lung is the most frequently involved organ, manifesting as septic pulmonary embolism, pleural effusion, abscess chest, and pneumothorax.12 In this case of the present study, we not only detected pathogenic bacteria (Fusobacterium necrophorum) in the BALF sample using mNGS testing, but also found significant pathological changes in the lungs (eg, multiple cavitary lesions bilaterally, left hydropneumothorax, and left pleural effusion) (Figure 1). Similar to the two cases (cases 5 and 15) in Table 1, the patient in this study also had pharyngeal pain that occurred after drinking alcohol prior to the onset of the disease, suggesting that alcohol consumption may be a risk factor in causing LS.
 |
Table 1 A Review of Clinical Information on Lemierre Syndrome Cases Reported in Recent Years |
Clinical sequelae of LS are worthy of attention. A previously study showed that about 10% of LS patients have long-term sequelae such as cranial nerve palsy, blindness or vision loss and paralysis, which seriously affect the quality of life after rehabilitation.30 The patient in this study suffered from chewing weakness, language disorder and dysphagia caused by cranial nerve paralysis. Fortunately, after rehabilitation treatment, the patient recovered.
For patients with sepsis and septic shock, early diagnosis and treatment are key to reducing patient mortality. Studies have shown that delays in effective antimicrobial therapy have resulted in decreased survival rates among patients with LS.31 However, current culture-based diagnostic procedures (eg, blood and BALF cultures) often yield negative results even when an infection is strongly suspected.7 This may be partially due to concurrent antibiotic exposure and fastidious and atypical causative organisms. Recently, with the introduction of culture-independent metagenomic next-generation sequencing (mNGS), which can theoretically detect any organism directly from clinical samples in a single assay without a priori selection of target pathogens, many unexpected, atypical and slow-growing pathogens have been identified within a clinically actionable time frame.32 In this study, we detected the causative agent (ie, Fusobacterium necrophorum) by both plasma and BALF mNGS, while all other microbial tests were negative, indicating that mNGS played a very important role in the rapid and accurate pathogenic diagnosis of this case. In Table 1, three LS cases (cases 2, 4 and 5) were also identified Fn using mNGS and all subsequently improved with targeted treatment. In addition, plasma mNGS was consecutively used five times in this study to monitor pathogens in the blood at different stages of the patient’s infection and treatment (Figure 2). We found that the RPM value of Fn detected in the plasma was decreasing as the patient gradually improved, which seems to indicate that the longitudinal detection of blood samples from the same patient with plasma mNGS assay may have value in monitoring the effectiveness of treatment.
Table 1 shows that antibiotic regimens for LS always included metronidazole and carbapenems. Up to half of the patients received antibiotic combination therapy. Data shows that Fn is usually sensitive to penicillin, cephalosporin, metronidazole, clindamycin, tetracyclines, and chloromycetin.33 It is naturally resistant to quinolones and aminoglycosides and lack sensitivity to macrolides and tetracyclines. In recent years, it has been found that some Fn isolates will produce β-lactamase, which is resistant to penicillin. For pathogens in pus cavities and/or bacterial thrombi, although sensitive antibiotics are selected, the drug regimen usually needs to be extended to 4–8 weeks because it is not easy to achieve an effective bactericidal concentration locally.10 Except for the administration of antibiotics, it must be noted that surgical drainage of empyema and abscess and debridement of necrotic tissue are also necessary at most times.33 Life-saving measures such as tracheal intubation or tracheotomy were performed in more than half of the patients (Table 1).
The use of anticoagulants is controversial in the treatment of LS. As septic thrombophlebitis in LS is formed by infected fragments, it has been suggested that anticoagulation promotes the exposure of bacteria within the thrombus to high concentration of antibiotics during thrombolysis, shortening the disease process and preventing the spread of the thrombus.30 Although patients are usually associated with thrombocytopenia and renal insufficiency, the use of anticoagulants does not increase the risk of bleeding.34 However, there was no statistical difference found in the rate of recanalisation and mortality between patients who did not receive anticoagulation and those who did.35 In Table 1, over half of the patients (11/17) were receiving anticoagulants, and all of them survived. Nevertheless, due to the small sample size, this outcome lacks sufficient statistical power to be conclusive. Because of the low prevalence, there are currently no prospective trials investigating the concrete mechanism of anticoagulation in LS.
In summary, LS is a disease characterized by septic thrombophlebitis of the internal jugular vein, which is rare and difficult to diagnose without delay. For the diagnosis of acute and critically ill patients with suspected infections, the use of mNGS assays should be considered as early as possible to identify the responsible pathogens in order to implement accurate and effective treatment.
Ethical Approval Statement
This study was approved by the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) institutional review board (IIT20220714A). The informed consent was obtained from the patient for the publication of all images, clinical data and other data.
Acknowledgments
We thank all clinicians who provided detailed diagnostic and treatment data of the patient for our study, as well as all infectious disease (ID) physicians and clinical microbiologists who received our infectious disease consultations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Zhejiang Provincial Natural Science Foundation (grant number LY23H200001 by D.H.) and China International Medical Foundation (grant numbers Z-2017-24-2202 by D.H.).
Disclosure
The authors declare no competing interests.
References
1. Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(5874):701–703. doi:10.1016/S0140-6736(00)57035-4
2. Hagelskjær Kristensen L, Prag J. Lemierre’s syndrome and other disseminated Fusobacterium necrophorum infections in Denmark: a prospective epidemiological and clinical survey. Eur J Clin Microbiol Infect Dis. 2008;27(9):779–789. doi:10.1007/s10096-008-0496-4
3. Sacco C, Zane F, Granziera S, et al. Lemierre syndrome: clinical update and protocol for a systematic review and individual patient data meta-analysis. Hämostaseologie. 2019;39(01):76–86. doi:10.1055/s-0038-1654720
4. Kjaerulff AM, Thomsen MK, Ovesen T, Klug TE. Clinical and biochemical characteristics of patients with Fusobacterium necrophorum-positive acute tonsillitis. Eur Arch Otorhinolaryngol. 2015;272(6):1457–1463. doi:10.1007/s00405-015-3535-7
5. Kuppalli K, Livorsi D, Talati NJ, Osborn M. Lemierre’s syndrome due to Fusobacterium necrophorum. Lancet Infect Dis. 2012;12(10):808–815. doi:10.1016/S1473-3099(12)70089-0
6. Nagaraja TG, Narayanan SK, Stewart GC, Chengappa MM. Fusobacterium necrophorum infections in animals: pathogenesis and pathogenic mechanisms. Anaerobe. 2005;11(4):239–246. doi:10.1016/j.anaerobe.2005.01.007
7. Riordan T. Human Infection with Fusobacterium necrophorum (Necrobacillosis), with a Focus on Lemierre’s Syndrome. Clin Microbiol Rev. 2007;20(4):622–659. doi:10.1128/CMR.00011-07
8. Han D, Li R, Shi J, Tan P, Zhang R, Li J. Liquid biopsy for infectious diseases: a focus on microbial cell-free DNA sequencing. Theranostics. 2020;10(12):5501–5513. doi:10.7150/thno.45554
9. Han D, Li Z, Li R, Tan P, Zhang R, Li J. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019;45(1):1–18. doi:10.1080/1040841X.2018.1514366
10. Johannesen KM, Bodtger U. Lemierre’s syndrome: current perspectives on diagnosis and management. Infect Drug Resist. 2016;9:221–227. doi:10.2147/IDR.S95050
11. Jaber F, Alsakarneh S, Campbell J, et al. Pylephlebitis Complicated by Hepatic Abscesses due to Fusobacterium Nucleatum: a Case of Lemierre’s Syndrome Variant and Literature Review. ACG Case Rep J. 2023;10(5):e01046. doi:10.14309/crj.0000000000001046
12. Kherabi Y, Chevrel G, Roux D, Federici L. Gynecological Lemierre’s syndrome: a case report and literature review. Rev Med Interne. 2020;41(7):493–495. doi:10.1016/j.revmed.2020.02.012
13. Sattar Y, Susheela AT, Karki B, Liaqat A, Ullah W, Zafrullah F. Diagnosis and Management of Lemierre’s Syndrome Presented with Multifocal Pneumonia and Cerebral Venous Sinus Thrombosis. Case Rep Infect Dis. 2020;2020:6396274. doi:10.1155/2020/6396274
14. Wang S, Huang Y, Du S, Song Z, Tong C. Lemierre’s syndrome: a case report[J/CD]. Chin Med Case Rep. 2022;04:E3199.
15. Li Z, Wang Y, Zhao H, et al. Two cases of Lemierre syndrome causes death[J/CD]. Chin Med Case Rep. 2022;04:E2277.
16. Ling J, Ran X, Wang Z, Li S, Yan L. Severe thrombocytopenia and jaundice associated with Lemierre’s syndrome: a case report [J/CD]. Chin Med Case Rep. 2022;04:E937.
17. Zhang Y, Wang T, Xing X, Zhang K. Lemierre syndrome caused by Fusobacterium necrophorum: a case report[J]. Chin J Tuberc Respir Dis. 2021;44:4.
18. Chen F, Jean S, Ou T, Yu F, Lee W. Pulmonary empyema caused by co-infections of Mycoplasma pneumoniae and Fusobacterium necrophorum: a rare case of lemierre syndrome. J Microbiol Immunol Infect. 2017;50(4):552–554. doi:10.1016/j.jmii.2016.11.007
19. Li Y, Wang C, Jean S, Chen F, Lee W, Chang J. Lemierre syndrome complicating deep neck infection and descending necrotizing mediastinits caused by odontogenic infections. J Microbiol Immunol Infect. 2020;53(2):357–359. doi:10.1016/j.jmii.2019.10.002
20. Jones C, Siva TM, Seymour FK, O’Reilly BJ. Lemierre’s syndrome presenting with peritonsillar abscess and VIth cranial nerve palsy. J Laryngol Otol. 2006;120(6):502–504. doi:10.1017/S002221510600034X
21. Reymond B, Huette P, Roger P, et al. Fatal Fusobacterium necrophorum infection with gynecological Lemierre’s syndrome. Méd Maladies Infect. 2019;49(1):72–74. doi:10.1016/j.medmal.2018.09.006
22. Hedengran KK, Hertz J. Lemierre’s syndrome after evacuation of the uterus: a case report. Clin Case Rep. 2014;2(2):60–61. doi:10.1002/ccr3.62
23. Hirai J, Kuruma T, Sakanashi D, et al. Lemierre syndrome due to dialister pneumosintes: a case report. Infect Drug Resist. 2022;15:2763–2771. doi:10.2147/IDR.S359074
24. Repper DC, Arrieta AC, Cook JE, Renella P. A case of lemierre syndrome in the era of COVID-19: all that glitters is not gold. Pediatr Infect Dis J. 2020;39(12):e445–e447. doi:10.1097/INF.0000000000002939
25. Yaita K, Sugi S, Hayashi M, et al. The co-existence of Lemierre’s syndrome and Bezold’s abscesses due to Streptococcus constellatus: a case report. Medicine. 2018;16(1):97. doi:10.1186/s12916-018-1080-0
26. Mellor TE, Mitchell N, Logan J. Lemierre’s syndrome variant of the gut. BMJ Case Rep. 2017;2017:2017–221567.
27. Surapaneni BK. Fusobacterium necrophorum Septicemia Leading to Lemierre’s Syndrome in an Immunocompetent Individual: a Case Report. Cureus. 2020;2:12.
28. Jafri FN, Shulman J, Gomez-Marquez JC, Lazarus M, Ginsburg DM. Sore throat, fever, septic emboli, and acute respiratory distress syndrome: a case of lemierre syndrome. Case Rep Emerg Med. 2018;2018:7373914. doi:10.1155/2018/7373914
29. Radovanovic N, Dumic I, Veselinovic M, et al. Fusobacterium necrophorum subsp. necrophorum Liver Abscess with Pylephlebitis: an Abdominal Variant of Lemierre’s Syndrome. Case Rep Infect Dis. 2020;2020:9237267. doi:10.1155/2020/9237267
30. Valerio L, Zane F, Sacco C, et al. Patients with Lemierre syndrome have a high risk of new thromboembolic complications, clinical sequelae and death: an analysis of 712 cases. J Intern Med. 2021;289(3):325–339. doi:10.1111/joim.13114
31. Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. doi:10.1097/CCM.0000000000000330
32. Schlaberg R. Clinical Metagenomics—from Proof-of-Concept to Routine Use. Clin Chem. 2022;68(8):997–999. doi:10.1093/clinchem/hvac091
33. Hagelskjaer KL, Prag J. Human necrobacillosis, with emphasis on Lemierre’s syndrome. Clin Infect Dis. 2000;31(2):524–532. doi:10.1086/313970
34. David N, Johan E, Gustav T, Karin H. Jugular Vein Thrombosis and Anticoagulation Therapy in Lemierre’s Syndrome—A Post Hoc Observational and Population-Based Study of 82 Patients. Open Forum Infect Diseases. 2021;8(1):ofaa585. doi:10.1093/ofid/ofaa585
35. Mitchell R. Gore Lemierre Syndrome: a Meta-analysis. Int Arch Otorhinolaryngol. 2020;24(03):e379–e385. doi:10.1055/s-0039-3402433
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.