Back to Journals » Clinical Ophthalmology » Volume 18
Latanoprostene Bunod 0.024% in Patients with Open-Angle Glaucoma Switched from Prior Pharmacotherapy: A Retrospective Chart Review
Authors Okeke CO, Cothran NL , Brinkley DA, Rahmatnejad K, Rodiño FJ, Deom JE
Received 19 October 2023
Accepted for publication 15 January 2024
Published 7 February 2024 Volume 2024:18 Pages 409—422
DOI https://doi.org/10.2147/OPTH.S442940
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Constance O Okeke,1 Nora Lee Cothran,2 Desirae A Brinkley,3 Kamran Rahmatnejad,4 Frank J Rodiño,5 James E Deom6
1Virginia Eye Consultants/CVP, Norfolk, VA, USA; 2The Eye Institute of West Florida, Largo, FL, USA; 3Eye Specialty Group, Memphis, TN, USA; 4Eastern Virginia Medical School, Norfolk, VA, USA; 5Churchill Outcomes Research, Red Bank, NJ, USA; 6Hazleton Eye Specialists, Hazle Township, PA, USA
Correspondence: Constance O Okeke, Virginia Eye Consultants/CVP, 241 Corporate Blvd, Norfolk, VA, 23320, USA, Tel +1 757-622-2200, Fax +1 757-622-4866, Email [email protected]
Introduction: Latanoprostene bunod 0.024% (LBN, Vyzulta®) is a nitric oxide-donating prostaglandin analog (PGA). We investigated the real-world efficacy and safety of LBN in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT) who switched their existing intraocular pressure (IOP)-lowering treatment(s) to LBN.
Methods: This non-interventional, multicenter (United States), retrospective chart review included patients aged ≥ 18 years with OHT and/or mild-to-moderate OAG diagnoses taking 1– 2 IOP-lowering treatments at the time of switch to LBN (index visit). Chart-extracted data included demographics, diagnoses, IOP and ocular assessments, other IOP-lowering treatments, adverse events (AEs), and reasons for discontinuation. The main study outcome was IOP change from the index visit to each of the next 2 chart-recorded follow-up visits. Analysis groups included the overall dataset and 2 subgroups of patients switched from PGA therapy to LBN: “PGA-all” subgroup [all patients previously on a PGA with/without another IOP-lowering product] and “PGA-monotherapy” subgroup [patients previously on a PGA alone]). Additional ocular outcomes (eg, visual acuity) were examined, if available.
Results: The overall dataset included 49 patients (46 had OAD alone, 2 had OHT alone, and 1 had both). The PGA-all subgroup and PGA-monotherapy subgroups had 41 and 32 patients, respectively. Switching to LBN led to a ~25% IOP reduction from the index visit to Visit 1 that was sustained at Visit 2. IOP findings in the PGA-all and PGA-monotherapy subgroups were consistent with the overall dataset. No meaningful changes in other ocular outcomes were found. Of 14 ocular AEs, 3 were recorded as such (mild in severity, considered unrelated to treatment), and 11 were identified through review of interval ocular histories (no severity/relatedness information); none led to discontinuation.
Conclusion: In this short-term retrospective chart review of mild-to-moderate OAG/OHT, switching prior IOP-lowering therapy to LBN produced an additional ~25% IOP reduction and appeared to be well tolerated.
Keywords: latanoprostene bunod, LBN, prostaglandin analog, PGA, ocular hypertension, OHT, open-angle glaucoma, OAG, intraocular pressure, IOP
Introduction
Glaucoma is a leading cause of blindness and affects approximately 3 million people in the United States, with open-angle glaucoma (OAG) being the most common form.1–4 Elevated intraocular pressure (IOP) or ocular hypertension (OHT) is an important and modifiable risk factor for OAG.5
Latanoprostene bunod 0.024% (LBN, Vyzulta®), the first topical nitric oxide (NO)-donating prostaglandin analog (PGA), is indicated for the reduction of IOP in patients with OAG or OHT.6 After topical ocular instillation, LBN is rapidly hydrolyzed by esterases to latanoprost acid, a prostaglandin F receptor agonist, and butanediol mononitrate, which subsequently releases the active component, nitric oxide. The combination of active moieties results in a dual mechanism for IOP lowering, with latanoprost acid increasing aqueous humor outflow through the uveoscleral pathway and nitric oxide increasing aqueous humor outflow through the trabecular meshwork (TM) and Schlemm’s canal.7
The clinical efficacy and safety of LBN in patients with OAG or OHT have been established in prospective clinical studies, with study durations of up to 1 year.8–11 Pooled data from two separate Phase 3 studies comparing once daily LBN 0.024% to twice daily timolol maleate 0.5% (LUNAR and APOLLO) demonstrated a reduction from baseline in IOP among patients treated with LBN ranging from 7.5 to 9.1 mm Hg.10 In both studies, LBN was noninferior to timolol. Further, reductions in mean IOP were significantly greater with LBN versus timolol at all 9 time points evaluated in APOLLO (all P≤0.002), and at 8 of 9 time points in LUNAR (8 visits; P≤0.025).10 In the VOYAGER study, once daily LBN 0.024% produced significantly greater IOP reductions from baseline as compared with once daily latanoprost 0.005% (–9.0 mm Hg vs –7.8 mm Hg; P=0.005) at 28 days.12
The randomized, controlled, prospective clinical studies conducted to obtain regulatory approval of LBN, included patients who were currently using or had recently used IOP-lowering medication(s); these patients were required, per-protocol, to undergo a “wash-out” period prior to initiating LBN treatment, during which they did not take their previously prescribed IOP-lowering medication(s).9,11,12 However, in clinical practice, it is likely that patients who are changing from existing IOP-lowering treatment to LBN will typically be switched directly to LBN, without a washout period, to avoid an interruption in treatment.
As LBN is increasingly being prescribed for patients with OAG or OHT, it is of interest to examine real-world usage and IOP-lowering efficacy of LBN in patients who switch from their current IOP-lowering medication(s) to LBN. Since receiving marketing approval, retrospective chart reviews of LBN have demonstrated additional IOP-lowering when LBN either replaced prior therapy in predominantly late-stage glaucoma patients or was used as an adjunctive therapy in patients predominantly on multiple medications.13–15 The current study was specifically conducted to investigate the real-world efficacy (IOP-lowering) and safety of LBN in a representative sample of patients with mild-to-moderate OAG/OHT who were on 1–2 topical IOP-lowering therapies and switched 1 or both of these existing treatments to LBN.
Methods
Study Design and Patients
This was a non-interventional, multicenter, retrospective chart review conducted in the US at 1 ophthalmology and 3 optometry sites. Longitudinal data reflecting routine care and follow-up were gathered.
The study protocol was reviewed by the Advarra Institutional Review Board (Columbia, MD, USA), which ruled that approval was not required for this study, granting a waiver of informed consent and exemption from ongoing IRB oversight on June 7, 2021. The study adhered to the tenets of the Declaration of Helsinki as well as the Health Insurance Portability and Accountability Act privacy requirements. Only necessary data sets were collected, all patient data were de-identified, and no unique patient identifiers were recorded or retained.
Patient charts were eligible for review if the patients were aged 18 years or older at the time of LBN treatment initiation with a confirmed diagnosis of OHT or OAG (primary or secondary) such as pre-glaucoma (International Statistical Classification of Diseases [ICD-]10 code H40.00), OAG with borderline findings low risk (H40.01), OAG with borderline findings, high risk (H40.02), unspecified OAG (H40.10), primary OAG (H40.11), pigmentary glaucoma (H40.13), capsular glaucoma with pseudo-exfoliation of lens (H40.14), and OHT (H40.05). Patients were required to have at least 1 visit prior to and 2 visits following commencement of LBN therapy (on or after September 1st 2018) recorded in the chart. Since the final IRB exemption was obtained on June 7, 2021, the 2nd follow-up visit had to occur prior to this date. Additionally, patients had to be on at least 1 to a maximum of 2 concurrent prior IOP-lowering therapies (a combination product was considered 1 therapy) for ≥1 month prior to starting LBN.
Patient charts were excluded for any of the following reasons: recording of advanced/severe glaucoma prior to initiation of LBN therapy, an ICD10 code for advanced glaucoma (H40.15, residual stage of OAG or H40.XXX3, which denotes severe disease), an ICD10 code for low-tension glaucoma [H40.12] or recording of normal tension glaucoma in the chart, prior glaucoma filtration surgery, trabeculectomy or tube shunt surgery, or initial LBN use prior to September 1st 2018.
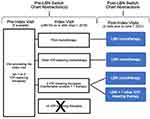
Every sequential qualifying chart was selected to minimize selection bias. Data were abstracted from charts by study authors or their delegates at the following time points: 1 to 2 pre-LBN visits (ie, visits recorded in the charts prior to initiation of LBN: the pre-index visit [if available] and the index visit [required]) and 2 post-index follow-up visits (ie, the first [Visit 1] and second [Visit 2] visits recorded in the charts after LBN initiation) through the end of the study (IRB determination) or surgical intervention/selective laser trabeculoplasty (SLT) (Figure 1). The index () visit was defined as the visit at which LBN was first prescribed and when patients were still on their prior IOP-lowering medication(s) (ie, the last visit just prior to switching to LBN treatment). Study site personnel were trained on the REDCap Cloud Electronic Data Capture (EDC) System (Encinitas, California) which utilized standardized and secure electronic case report forms (eCRFs). Key data that were extracted from the charts included: demographics (age, gender, race), ophthalmologic diagnoses, ocular assessments (eg, IOP), other IOP-lowering treatments (including documented additions/discontinuations), and adverse events (AEs).
Study Outcomes
The primary outcome of interest was the change in IOP from the index visit at each of the 2 sequential follow-up visits post-LBN initiation. Responder rate analyses were conducted based on the proportion of patients with IOP reductions of ≥2, ≥3, ≥4, and ≥5 mm Hg from the index visit to each follow-up visit.
Additional ocular assessment data that were extracted from the charts, if available, at the index visit and 2 post-index follow-up visits included visual acuity (VA), cup-to-disc ratio (CDR), visual fields, and central corneal thickness (CCT). AEs were reported separately for the pre- and post-LBN treatment periods. Information available in the interval ocular history portion of the eCRFs was reviewed to identify additional possible AEs that may not have been formally reported. Any discontinuations, including those due to AEs, were recorded.
For each patient, the eye with the higher pre-treatment IOP was considered the study eye; however, if the pre-treatment IOP was the same in both eyes, then the right eye was considered the study eye.
Statistical Analysis
Paired t-tests (which accounted for multiplicity) were used to compare IOP (one-tailed), VA (two-tailed), and CDR (two-tailed) between the index visit and follow-up visits after starting treatment with LBN. An alpha of 0.05 was considered significant.
Changes in IOP and responder rates were evaluated in the overall dataset as well as in 2 subgroups of patients who were receiving a PGA prior to LBN. The overall dataset included any patient who switched to LBN from 1 or both of the IOP-lowering products in their pre-LBN OAG/OHT regimen. The “PGA-all” subgroup included any patient using a PGA (ie, with or without another IOP-lowering product) prior to the switch from their PGA to LBN. The “PGA-monotherapy” subgroup included only patients using a PGA alone prior to the switch from that PGA to LBN. The primary efficacy analyses were based on study eyes. As an additional supportive analysis, IOP data in LBN-treated fellow eyes of the overall dataset were examined, if available, using the same statistical methodology as for study eyes.
Descriptive statistics were used to summarize the data with tabulation of frequencies and percentages for discrete variables and sample size, mean, standard deviation (SD), minimum, and maximum for continuous variables. Statistical analyses were conducted with Statistix 9 (Analytical Software, Tallahassee, FL, US) and Microsoft Excel (Microsoft Corporation, Redmond, WA, US).
Results
Study Patients
A total of 49 switch patients (49 study eyes; 44 treated fellow eyes) were identified from medical charts and formed the overall dataset for the study population. Of these 49 patients, 47 had a diagnosis of OAG (including 2 patients with pigmentary glaucoma and 1 patient with capsular glaucoma) and 3 had a diagnosis of OHT (1 patient had recorded diagnoses of OAG as well as OHT) (Table 1). Two patients were excluded from all Visit 1 assessments and 9 were excluded from all Visit 2 assessments due to protocol violations (Figure 2).
 |
Table 1 Demographics and Baseline Characteristics |
The most common diagnostic codes were for mild bilateral POAG (n=15; 30.6%), moderate bilateral POAG (n=10; 20.4%), and low-risk bilateral OAG with borderline findings (n=7; 14.3%). The mean (SD) age was 65 (13) years; 59.2% of patients were female and 79.6% were White. Comorbid conditions were present in 59.2% of patients, with systemic hypertension being most common (44.9%), followed by diabetes (16.3%).
Prior to initiating LBN, 41/49 patients (83.7%) were using another PGA (32 as monotherapy; 9 in combination with a non-PGA IOP-lowering product). The distribution of PGA-monotherapy was bimatoprost (n=11), latanoprost (n=10), travoprost (n=9), and tafluprost (n=2). Eight of the 9 PGA plus non-PGA regimens included latanoprost as the PGA (ie, with brimonidine, n=1; with brinzolamide, n=1; with timolol, n=1; with a brinzolamide/brimonidine product, n=2; with a brimonidine/timolol product, n=2; and as a latanoprost/netarsudil product with timolol, n=1); the remaining regimen was bimatoprost plus timolol. Among 8 patients using only non-PGA IOP-lowering treatments, 6 were using a single product (timolol/brimonidine combination product [n=3], brimonidine [n=1], brinzolamide [n=1], and timolol [n=1]) and 2 were on dual regimen of brimonidine plus dorzolamide. In 2 patients, LBN replaced 2 IOP-lowering treatments.
The most common reasons for discontinuation of prior IOP-lowering medications at index (ie, presumed reason for switching to LBN) were related to inadequate IOP-lowering (eg, “IOP target not being met”, “poor IOP control”, [treatment] “failed”, “not controlling IOP”, or “higher/worsening of IOP/glaucoma”; n=40); the remaining documented reasons for discontinuation were optic nerve changes/thin inferior rim (n=2), regimen simplification to maximize compliance (n=1); blurry vision (n=1), and an unspecified AE (n=1). In the 4 remaining patients, reasons for discontinuation were not recorded/unclear.
The median (IQR) days from the index visit to the first and second post-index follow-up visits were 28 (21–41) and 126 (80–160) days, respectively. The median (IQR) time between the first and second post-index visits was 84 (37–125) days.
Clinical Efficacy Assessments
Change in IOP
IOP measurements were available for 49 study eyes at both the pre-index and index visits, 47 study eyes at post-index Visit 1, and 40 at post-index Visit 2. Applanation tonometry was consistently used to measure IOP across all visits for all patients. The mean (SD) IOP in study eyes was 18.9 (3.9) mm Hg at the pre-index visit and increased to 21.9 (4.2) mm Hg at the index visit (P<0.001). After switching to LBN, study eye IOPs were reduced to 16.5 (3.7) mm Hg and 15.9 (4.3) mm Hg at post-index Visit 1 and Visit 2, respectively (Figure 3A and Table 2). Switching to LBN led to a mean (SD) reduction of 5.4 (3.3) mm Hg at post-index Visit 1 and 5.2 (4.8) mm Hg at post-index Visit 2 (P<0.001 for both vs index visit); thus, a ~25% reduction from index visit in IOP was observed at Visit 1 and maintained at Visit 2.
 |
Table 2 Change in IOP from Index Visit in Study Eyes of Patients Switched to LBN |
 |
Figure 3 Mean IOP in study eyes before and during LBN treatmenta. (A) All patients; (B) PGA-all subgroup (all patients who were using a PGA [with or without another IOP-lowering product] prior to switching to LBN); (C) PGA-monotherapy subgroup (patients who were using PGA monotherapy prior to switching to LBN). Whiskers represent standard deviations. Index Visit = visit at which IOP-lowering therapy was switched to LBN. aIn the overall dataset and PGA-all subgroup, 2 patients were excluded from post-index Visit 1 and 9 were excluded from Visit 2 (Figure 2); in the PGA-monotherapy subgroup, 1 and 8 patients were excluded from post-index Visits 1 and 2, respectively. Abbreviations: LBN, latanoprostene bunod 0.024%; IOP, intraocular pressure; PGA, prostaglandin analog. |
Data were available for 44 treated fellow eyes at the pre-index and index visits, 41 at post-index Visit 1, and 34 at post-index Visit 2. The magnitude of IOP lowering in fellow eyes was comparable to that in study eyes. The mean (SD) IOP in fellow eyes was 17.7 (3.3) mm Hg at the pre-index visit and increased to 20.2 (3.6) mm Hg at the index visit (P<0.001). After switching to LBN, fellow eye IOPs were reduced to 16.3 (4.0) mm Hg and 14.6 (3.1) mm Hg at post-index Visit 1 and Visit 2, respectively. Switching to LBN led to mean (SD) IOP reductions in fellow eyes of 4.0 (3.2) and 4.6 (3.7) mm Hg at post-index Visits 1 and 2, respectively (P<0.001 for both vs index visit); reductions in IOP from index visit were 20% and 24% at Visits 1 and 2, respectively.
The magnitude of IOP lowering in study eyes of the PGA subgroups was consistent with that in the overall dataset. In the PGA-all subgroup (pre-index and index, n=41; Visit 1, n=39; Visit 2, n=32), mean (SD) reductions from index visit to Visits 1 and 2 were 5.0 (3.1) and 4.9 (3.8) mm Hg, respectively (P<0.001 for both vs index visit). Thus, reductions in IOP were 23.1% and 23.9% from index visit to Visits 1 and 2, respectively (Figure 3B and Table 2). In the PGA-monotherapy group, (pre-index and index, n=32; Visit 1, n=31; Visit 2, n=24), mean (SD) reductions in study eye IOP measurements were 5.2 (3.4) and 5.4 (4.0) mm Hg at the first and second follow-up visits (P<0.001 for both vs index visit), with 23.5% and 25.5% IOP reductions from the index visit to Visit 1 and Visit 2, respectively (Figure 3C and Table 2).
Responder Analysis
The majority (89.4%) of patients had an IOP lowering of ≥2 mm Hg from the index visit to Visit 1; IOP lowering of ≥3, ≥4, and ≥5 mm Hg at Visit 1 was observed in 83.0%, 70.2%, and 59.6% of patients, respectively (Table 3A). This distribution of responders was similar at Visit 2. The responder analysis findings in the PGA subgroups were comparable to those in the overall dataset. In the PGA-all subgroup, IOP lowering from the index visit to Visit 1 of ≥2, ≥3, ≥4, and ≥5 mm Hg was observed in 87.2%, 79.5%, 69.2%, and 56.4% of patients (Table 3B), with a similar distribution of responders at Visit 2. In the PGA-monotherapy subgroup, IOP lowering from the index visit to Visit 1 of ≥2, ≥3, ≥4, and ≥5 mm Hg was observed in 83.9%, 80.6%, 71.0%, and 54.8% of patients (Table 3C), with a similar distribution of responders at Visit 2.
 |
Table 3 Responder Analysis of Patients Switched to LBN (Study Eyes) (A) All Patients; (B) PGA-All Subgroupa; (C) PGA-Monotherapy Subgroup |
Other Ocular Assessments
Documentation of VA was available at pre-index, index, Visit 1, and Visit 2 in 49, 49, 47, and 39 study eyes, respectively. Beyond the exclusions noted previously, VA data was not reported for some patients. VA was generally measured by the same method, typically Snellen, across all visits. The mean VA in study eyes was 20/52.1, 20/46.6, and 20/52.3 at the index visit, Visit 1, and Visit 2, respectively. There were no significant changes in VA from the index visit to either Visit 1 (mean [SD] change – 6.4 [57.6]; P=0.45) or Visit 2 (4.1 [20.4]; P=0.22).
Among study eyes with CDR data available (pre-index, n=37; index, n=31; Visit 1, n=21; Visit 2, n=27), the mean CDR was 0.49–0.54 across all time points, with no apparent changes from the index visit to either of the follow-up visits.
Limited numbers of patients with assessments of visual fields and CCT (ie, n ≤ 6 at follow-up visits) precluded clinically meaningful analysis.
Safety/Tolerability and Discontinuations
There were no systemic AEs reported during LBN treatment. Overall, nine (17.6%) patients had 14 ocular AEs (Table 4). Three AEs were formally identified as such on the eCRFs during the post-index period (dry eye in 1 patient; floaters and flashes in 1 patient); study investigators considered these formally reported AEs to be mild and unrelated to treatment. In the patient reporting dry eye, this AE, along with blurred vision and eye pruritus had also been documented while on the prior IOP-lowering therapy (ie, pre-LBN); in the patient with floaters and flashes, floaters had also been documented pre-LBN. Eleven possible AEs were found via review of interval ocular histories but not explicitly identified as AEs; no information regarding severity or relatedness to treatment was available for these events. Overall, AEs found in ≥2 patients included floaters (n=3; 6.1%) and eye dryness (n=2; 4.1%). None of the ocular AEs led to treatment discontinuation.
 |
Table 4 Ocular Adverse Events Reported in ≥1 Patient Chartsa |
Among 8 patients who discontinued LBN, the reasons provided were inadequate IOP-lowering in 6 patients (Visit 1, n=4; Visit 2, n=2) and cost/insurance-related issues in 2 patients (Visit 2). Among the 6 patients who discontinued LBN due to inadequate IOP-lowering, most (5/6) had OAG, their mean (SD) IOP was 23.7 (4.2) mm Hg, and most (5/6) were switched from PGA monotherapy at index; additional demographic and clinical characteristics for these patients are included in the supplement (Supplement, Table S1). As previously noted, patients who discontinued LBN at Visit 1 were considered protocol violations and, therefore, these patients were excluded from Visit 2 assessments.
Discussion
In this real-world multicenter retrospective chart review of 49 patients with mild-to-moderate OAG or OHT on up to 2 topical IOP-lowering products, the switch from 1 or both prior IOP-lowering treatment(s) to LBN produced a substantial additional IOP reduction and was well tolerated. Treatment with LBN reduced IOP by approximately 25% from the index visit to each follow-up visit. More than 85% of patients experienced IOP lowering ≥2 mm Hg after switching to LBN, and over half had IOP lowering ≥5 mm Hg.
Notably, when examining the 41 patients who were previously on another PGA, whether alone (73% of the dataset) or in combination with other IOP-lowering pharmacotherapy (18% of the dataset), switching from their prior PGA to LBN resulted in a significant IOP reduction from the time of the therapy switch. Our results suggest that the dual mechanism of action of LBN, through its 2 active metabolites, may be more effective for IOP lowering than a PGA alone.7 LBN lowers IOP by (1) a PGA-specific mechanism in which the latanoprost acid metabolite increases aqueous humor outflow through the uveoscleral pathway and (2) an NO-donating mechanism in which NO-release due to the butanediol mononitrate metabolite increases outflow through the TM and Schlemm’s canal.7 The finding that treatment with LBN produced greater IOP lowering than other PGAs is consistent with prior reports.12,16 The Phase 2 VOYAGER study showed that, on average, LBN produced an additional IOP reduction of 1.23 mm Hg than latanoprost;12 similar results were reported in another prospective comparative study.16
Although direct comparisons cannot be made due to differences in study populations, it is of interest to consider the findings from prior chart review-based investigations of LBN. The IOP reduction associated with a switch to LBN in the current study (~25%) is greater than that observed in a previous retrospective chart review which demonstrated ~15% additional IOP-lowering when LBN replaced prior therapy; however, the prior study included predominantly late-stage glaucoma patients.13 In the current chart review, we sought to examine IOP-lowering effects in patients diagnosed with mild/moderate OAG; to help assure we were targeting patients with earlier-stage disease, we specifically selected patients on only 1 to 2 IOP-lowering products. We speculate that use earlier in the OAG disease process may increase the potential for the NO-donating moiety released by LBN to relax TM tissue, further enhancing the increase in aqueous humor outflow.17–19 In fact, while the responder analysis in the prior study of late-stage patients showed substantial proportions of patients achieved IOP lowering of ≥2, ≥3, and ≥4 mm Hg (60%, 46%, and 34%, respectively),13 the responder analysis in the current study of patients with mild-to-moderate OAG/OHT showed that even higher proportions of patients achieved these levels of IOP lowering (≥80%, ≥75%, and ≥65%, respectively, at both post-index visits) in the overall dataset as well as in the subgroups who were switched from a prior PGA. Also of interest, in a retrospective analysis examining adjunctive use of LBN in patients with refractory glaucoma who were using 3 or more topical agents, addition of LBN produced an IOP reduction of 9% at 3 months,14 whereas in a retrospective chart review of LBN in treatment-naïve patients (mean baseline IOP, 21.7 mm Hg in study eyes), LBN produced mean IOP decreases of ~31% from baseline to each follow-up visit.19 Collectively, these real-world analyses demonstrate that LBN effectively lowers IOP across a wide range of OAG disease severities.
Limitations
Retrospective observational data have inherent limitations including lack of control groups and masking; although the use of an objective measurements such as IOP and sequential selection of charts reduces the risk of research bias.13,19 While there was no placebo or active control group in the current study, each patient did serve as their own historical control. Other limitations related to real-world data collection included not being able to control for time of day for IOP measurements, varying time intervals between study visits, and inconsistent/incomplete documentation of ocular assessments beyond IOP across study sites. The study had a relatively small sample size, a consequence of purposefully targeted eligibility requirements pertaining to prior treatment patterns and completeness of follow-up visit documentation in order to gather meaningful data. Despite the small sample size, the analyses of IOP change from LBN baseline to follow-up were statistically significant at P<0.001. Low representation of patients with OHT alone is another potential limitation. Also, CCT can impact IOP measurements,20 but the IOP measurements were not corrected for CCT. While renewed motivation for good medication adherence due to a newly prescribed treatment may have contributed to the significant IOP-lowering observed after switching to LBN, we note that the IOP-lowering effect with LBN persisted through the second follow-up visit weeks to months later, and the mean IOP continued to decrease, in fact. Similarly, patients can be motivated to improve medication adherence just prior to a scheduled doctor’s visit. In patients predisposed to this behavior, we would expect that would have similarly impacted IOP readings taken at pre-LBN office visits, as well, and not just the LBN-related visits. Another limitation is that this was a relatively short-term report, with a median time between the switch to LBN and the second post-index follow-up visit being 18 weeks. Finally, while the 3 formally reported AEs were characterized as mild and unrelated to treatment, the possible AEs found upon review of interval ocular histories did not have sufficient information to enable determinations of severity or relatedness.
A key strength of the current study is that it adds to our understanding of the real-word usage, efficacy, and safety of LBN in patients with mild-to-moderate OAG/OHT. Our findings support the needed effort for a future prospective series to investigate switch to LBN in this patient population.
Conclusion
In this retrospective chart review of patients with mild-to-moderate OAG/OHT, switching prior IOP-lowering therapy to LBN produced an additional ~25% IOP reduction. Comparable and significant reductions in IOP were achieved in the subgroups of patients who were switched from other PGA treatments to LBN. Switching to LBN in the real-world clinical practice setting appeared to be safe and well tolerated.
Summary Points
- Latanoprostene bunod 0.024% (LBN, Vyzulta®) is a topical nitric oxide-donating prostaglandin analog (PGA) indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).
- We sought to investigate the real-world efficacy and safety of LBN in patients with mild-to-moderate OAG or OHT who switched their IOP-lowering treatment(s) directly to LBN without a washout period, a population not specifically evaluated in clinical trials.
- LBN produced an additional ~25% IOP reduction, whether considering the overall dataset, the subgroup of patients previously on a PGA plus another IOP-lowering product or the subgroup of patients previously on a PGA alone; LBN appeared well tolerated with no new safety signals.
- These data may help inform clinicians on the potential effectiveness of LBN when switching patients with mild-to-moderate OAG/OHT from a prior PGA to LBN.
Data Sharing Statement
All data analyzed during this study are included in this article.
Ethics Approval and Informed Consent
The study protocol was reviewed by the Advarra Institutional Review Board (Columbia, MD, USA), which ruled that approval was not required for this study, granting a waiver of informed consent and exemption from ongoing oversight on June 7, 2021. The study adhered to the tenets of the Declaration of Helsinki as well as the Health Insurance Portability and Accountability Act privacy requirements.
Acknowledgments
The authors wish to acknowledge Megan Cavet, PhD and Heleen DeCory, PhD, employees at Bausch + Lomb at the time of study, for their work in protocol development, study design, and analysis . Statistical analyses were performed by Herbert F. Lewis, PhD (Stony Brook University, Stony Brook, NY, USA), funded by Bausch + Lomb. Medical writing assistance was provided by Kulvinder K. Singh, PharmD (KK Singh, LLC, Branchburg, NJ, USA), funded by Bausch + Lomb.
Data were presented at the 2023 American Glaucoma Society meeting (March 2-5, 2023).
Funding
This study was funded by Bausch + Lomb. The Article Processing Fee was funded by Bausch + Lomb.
Disclosure
Constance O. Okeke and Nora Lee Cothran report serving as consultants, researchers, and speakers for Bausch + Lomb. Nora Lee Cothran reports personal fees from Aerie, Alcon, Allergan/ AbbVie, Glaukos, Sight Sciences, outside the submitted work. Frank J. Rodiño is an employee of Churchill Outcomes Research that supported the study. James E. Deom reports serving as a consultant for Bausch + Lomb. Desirae A. Brinkley and Kamran Rahmatnejad have no conflicts to report.
References
1. Centers for Disease Control and Prevention. Vision health initiative: don’t let glaucoma steal your sight; 2020. Available from: https://www.cdc.gov/visionhealth/resources/features/glaucoma-awareness.html#:~:text=About%203%20million%20Americans%20have,know%20they%20have%20the%20disease.
2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
3. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi:10.1016/S2214-109X(17)30393-5
4. Gupta P, Zhao D, Guallar E, Ko F, Boland MV, Friedman DS. Prevalence of glaucoma in the United States: the 2005–2008 national health and nutrition examination survey. Invest Ophthalmol Vis Sci. 2016;57(6):2905–2913. doi:10.1167/iovs.15-18469
5. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi:10.1001/archopht.120.6.701
6. Bausch + Lomb, a division of Valeant Pharmaceuticals North America LLC. Vyzulta (Latanoprostene Bunod Ophthalmic Solution). Bridgewater, NJ: Bausch + Lomb, a division of Valeant Pharmaceuticals North America LLC; 2018.
7. Cavet ME, DeCory HH. The role of nitric oxide in the intraocular pressure lowering efficacy of latanoprostene bunod: review of nonclinical studies. J Ocul Pharmacol Ther. 2018;34(1–2):52–60. doi:10.1089/jop.2016.0188
8. Kawase K, Vittitow JL, Weinreb RN, Araie M, Study Group JUPITER. Long-term safety and efficacy of Latanoprostene Bunod 0.024% in Japanese subjects with open-angle glaucoma or ocular hypertension: the Jupiter study. Adv Ther. 2016;33(9):1612–1627. doi:10.1007/s12325-016-0385-7
9. Weinreb RN, Scassellati Sforzolini B, Vittitow J, Liebmann J. Latanoprostene Bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension: the APOLLO study. Ophthalmology. 2016;123(5):965–973. doi:10.1016/j.ophtha.2016.01.019
10. Weinreb RN, Liebmann JM, Martin KR, Kaufman PL, Vittitow JL. Latanoprostene Bunod 0.024% in subjects with open-angle glaucoma or ocular hypertension: pooled phase 3 study findings. J Glaucoma. 2018;27(1):7–15. doi:10.1097/IJG.0000000000000831
11. Medeiros FA, Martin KR, Peace J, Scassellati Sforzolini B, Vittitow JL, Weinreb RN. Comparison of Latanoprostene Bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension: the LUNAR study. Am J Ophthalmol. 2016;168:250–259. doi:10.1016/j.ajo.2016.05.012
12. Weinreb RN, Ong T, Scassellati Sforzolini B, Vittitow JL, Singh K, Kaufman PL. VOYAGER study group. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open-angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015;99(6):738–745. doi:10.1136/bjophthalmol-2014-305908
13. Radell JE, Sharma HK, Auyeung KL, et al. Two-year experience with Latanoprostene Bunod in clinical practice. J Glaucoma. 2021;30(9):776–780. doi:10.1097/IJG.0000000000001904
14. Zhou B, Bekerman VP, Khouri AS. Use of Latanoprostene Bunod as adjunctive glaucoma therapy in refractory glaucoma. J Curr Glaucoma Pract. 2022;16(3):166–169. doi:10.5005/jp-journals-10078-1386
15. Mehta AA, Kanu LN, Sood-Mendiratta S, et al. Experience with netarsudil 0.02% and latanoprostene bunod 0.024% as adjunctive therapy for glaucoma. Eur J Ophthalmol. 2022;32(1):322–326. doi:10.1177/1120672121998913
16. Wang Y, Liao Y, Nie X. Comparative evaluation of latanoprostene bunod, timolol maleate, and latanoprost ophthalmic solutions to assess their safety and efficacy in lowering intraocular pressure for the management of open-angle Glaucoma. Clinics. 2020;75:e1874.
17. Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52(5):2147–2152. doi:10.1167/iovs.10-6342
18. Liu B, McNally S, Kilpatrick JI, Jarvis SP, O’Brien CJ. Aging and ocular tissue stiffness in glaucoma. Surv Ophthalmol. 2018;63(1):56–74. doi:10.1016/j.survophthal.2017.06.007
19. Okeke CO, Burstein ES, Trubnik V, et al. Retrospective chart review on real-world use of Latanoprostene Bunod 0.024% in treatment-naïve patients with open-angle glaucoma. Ophthalmol Ther. 2020;9(4):1041–1053. doi:10.1007/s40123-020-00307-0
20. Francis BA, Varma R, Chopra V, Lai MY, Shtir C, Azen SP; Los Angeles Latino Eye Study Group. Intraocular pressure, central corneal thickness, and prevalence of open-angle glaucoma: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2008;146(5):741–746. doi:10.1016/j.ajo.2008.05.048
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.