Back to Journals » Nature and Science of Sleep » Volume 15
Lack of Efficacy of Suvorexant in People with Insomnia and Poorly Controlled Type 2 Diabetes
Authors Winkelman JW , Wipper B , Zackon J, Hoeppner BB
Received 5 August 2023
Accepted for publication 12 December 2023
Published 23 December 2023 Volume 2023:15 Pages 1117—1128
DOI https://doi.org/10.2147/NSS.S434058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sarah L Appleton
John W Winkelman,1,2 Benjamin Wipper,2 Jordana Zackon,1 Bettina B Hoeppner1
1Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA; 2Harvard Medical School, Boston, MA, USA
Correspondence: John W Winkelman, Email [email protected]
Objective/Background: Sleep disturbance is a common and underappreciated feature of diabetes and sleep may contribute to glycemic control in people with type 2 diabetes (T2D). We conducted a 3-month trial to examine the efficacy of suvorexant in improving sleep and health outcomes in people with suboptimally controlled T2D and insomnia.
Participants/Methods: This parallel, double-blind, randomized placebo-controlled trial was conducted using the sequential parallel comparison design (SPCD). Sixty-nine people with poorly controlled T2D (HbA1c ≥ 6.5) were randomized to placebo and/or suvorexant (10– 20 mg). The primary outcome was subjective total sleep time (sTST), and secondary outcomes were Insomnia Severity Index (ISI) score and wake time after sleep onset (WASO). Exploratory outcomes included sleep efficiency, hemoglobin A1c (HbA1c), and C-reactive protein (CRP). Exploratory analyses were conducted on relationships between sleep and diabetes outcomes.
Results: There were no significant improvements in sTST (p = 0.27), ISI (p = 0.86), or WASO (p = 0.94) among participants taking suvorexant compared to placebo. There were also no significant changes in any of the exploratory endpoints. Improvements in sleep were associated with improvements in both objective (ie, HbA1c) and subjective (ie, Diabetes Distress Scale) measures of diabetes, as well as reductions in depressive symptoms, independent of treatment assignment.
Conclusion: The study did not find evidence that suvorexant is efficacious for insomnia in people with poorly controlled T2D. The associations of improved sleep with improvements in both diabetes-related metrics and depressive symptoms across groups highlight the importance of identifying and treating sleeping difficulties in this population.
CT Registration #: Nct03818581.
Keywords: insomnia, type 2 diabetes, sleep disorders, suvorexant
Introduction
Type 2 diabetes (T2D) results from a progressive insulin secretory defect on the background of insulin resistance. The prevalence of this disease has risen dramatically in recent decades and is currently a leading cause of morbidity and mortality throughout the world.1 Sleep disturbance and reduced sleep duration are underappreciated and important features of type 2 diabetes.2–5 People with type 2 diabetes have many potential disease-related etiologies for nocturnal awakenings from, and difficulty returning to, sleep (eg, nocturia, neuropathy, blood sugar assessments, anxiety, sleep apnea), producing sleep-maintenance insomnia and reduced total sleep duration. Numerous studies have attempted to assess the prevalence of insomnia and/or insomnia symptoms in populations of people with type 2 diabetes; a recent meta-analysis analyzing 78 studies found the prevalence of insomnia symptoms in people with type 2 diabetes to be 39% (95% CI: 34%, 44%).6
In those with established type 2 diabetes, there is emerging evidence from cross-sectional studies that sleep disturbance affects glycemic control. Recent meta-analyses have found associations between insomnia symptoms in people with type 2 diabetes and poor glycemic control.6,7 Additionally, the Coronary Artery Risk Development in Young Adults (CARDIA) study found that both actigraphic-determined sleep fragmentation and a combined measure of insomnia from actigraphy and self-reported sleep disturbance were substantially associated with abnormalities in fasting glucose, insulin, and insulin resistance in those with established type 2 diabetes.8
Hyperglycemia defines diabetes, and glycemic control is fundamental to the management of diabetes. Numerous longitudinal studies have demonstrated that maintenance of optimal glycemic control over time reduces the risk of microvascular complications (eg, retinopathy, nephropathy, neuropathy), cardiovascular disease, and overall death from any cause in people with type 1 and type 2 diabetes.9–12 Thus, a consensus among clinical guidelines has emerged that normalizing glycemic control reduces the risk and extent of diabetes mellitus complications.
Therefore, improvement of sleep in people with type 2 diabetes may enhance glycemic control and reduce the risk of negative health outcomes. Indeed, one randomized controlled trial assessing a sleep hygiene educational program in people with impaired fasting glucose or type 2 diabetes found this intervention to improve both subjectively reported sleep duration and quality and improve fasting glucose and HbA1c values.13 Further, a limited number of studies have examined the effects of insomnia medication treatment in people with type 2 diabetes.14 One small study (n = 32) found slight improvements in sleep quality but no change in HbA1c after three months of daily ramelteon administration in people with type 2 diabetes (mean baseline HbA1c = 6.7%).15 However, after participants were randomly assigned to either continue or discontinue ramelteon for the following three months, those who discontinued saw a slight increase in HbA1c levels compared to no change in the continuation group.
To our knowledge, the most popular on-label and off-label agents for insomnia (benzodiazepine-receptor agonists, antidepressants, and antipsychotic agents) have not been investigated for treatment of insomnia in the context of type 2 diabetes. Many of these agents have side effects (weight gain, exacerbation of obstructive sleep apnea, risk of cognitive dysfunction, and glucose and lipid abnormalities) that are of particular concern in this population.16 Suvorexant, a recently approved orexin antagonist, provides an important therapeutic option to treat insomnia in the context of type 2 diabetes.17 It has demonstrated long-term efficacy, particularly in shortening the duration of nocturnal awakenings and increasing total sleep time,18 while having a comparatively benign side effect profile compared to many of the agents described above. Furthermore, an orexin antagonist may have particular efficacy in people with type 2 diabetes and insomnia; in addition to its impact on sleep-wake function, orexin plays a key role in energy metabolism, and has known functions in both promotion of appetite and the regulation of glucose homeostasis.19,20 A study of suvorexant administration in diabetic db/db mice showed that the medication had beneficial effects on sleep and glucose metabolism over 2–4 weeks, with the authors concluding that this latter effect was mediated through CNS suppression of sympathetic influences on hepatic gluconeogenesis.21 It seems possible that similar beneficial effects would be seen with nocturnal administration of suvorexant in humans with type 2 diabetes considering that excessive hepatic gluconeogenesis is a major contributor to hyperglycemia in people with this condition.22 Multiple open-label, single-arm trials assessing suvorexant treatment in people with type 2 diabetes and insomnia found improvements in subjective sleep efficiency and glycemic control.23,24
The present randomized controlled trial examined whether suvorexant would improve sleep quality and quantity in people with suboptimally controlled type 2 diabetes. The primary hypothesis was that suvorexant is superior to placebo in increasing subjective total sleep time at 3 months of treatment in this population. Exploratory hypotheses included greater active treatment effects on wake after sleep onset as well as greater improvement in ISI. Furthermore, we hypothesized that benefits of improved sleep would be reflected in improved glycemic control, as measured by HbA1c.
Methods
Study Design
This was a parallel, double-blind, randomized placebo-controlled 3-month trial using the sequential parallel comparison design25 (SPCD), investigating the effects of suvorexant 10–20 mg on subjective total sleep time in people with suboptimally controlled type 2 diabetes. The SPCD is a clinical trial design that uses a two-phase approach to address the placebo response rate. In the first phase, more people are randomized to placebo than treatment (2:1) with the goal of identifying placebo non-responders. In the second phase, the focus is on placebo non-responders, who have already “failed” placebo, and thus are less likely to exhibit a placebo response during the second phase, thereby increasing the observable size of the treatment effect, if present. In line with this design, in this trial, people were randomized to placebo versus suvorexant (2:1) for the initial 6-week treatment period. For the purpose of identifying placebo non-responders, treatment response was defined as improving total sleep time by at least 15%, as assessed through the use of sleep diaries completed by participants during a two-week period prior to randomization, and the last two weeks of the treatment period. Suvorexant and placebo were both received from the study sponsor, Merck, and were packaged identically. Those randomized to placebo who failed to achieve this treatment response during the first phase were re-randomized 1:1 to placebo or suvorexant for the second 6-week period (see Figure 1). Participants identified as responders to placebo as well as those initially randomized to suvorexant were maintained on their initial treatment for the second 6-week period to maintain the blind. Randomization was computer generated (using randomization.com) and done in blocks of 6 for Phase 1 and blocks of 4 for Phase 2. All study procedures were approved by the Massachusetts General Brigham Institutional Review Board.
 |
Figure 1 Consort diagram. Notes: Adapted from Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3): e1000251. Copyright: © 2010 Schulz et al. Creative Commons Attribution License.26 |
Interested participants had an initial phone call with the research coordinator where they provided verbal informed consent and were screened to rule out any immediate exclusion factors. They then underwent a two-week screening period, during which time they completed daily sleep diaries. If deemed eligible based on their self-reported sleep, participants were invited to the Screening Visit, at which they provided written informed consent and were enrolled in the study. Following the Screening Visit, participants completed another two weeks of sleep diaries and had their blood drawn to test HbA1c and C-Reactive Protein levels before being randomized to the trial.
Both treatment periods lasted 6 weeks and followed the same structure. During the first week, participants took one 10 mg pill of suvorexant or placebo nightly, which was increased to 20 mg for the remaining 5 weeks unless side effects were present. Participants who took suvorexant for both study periods received 20 mg during the first week of the second treatment period (ie, there was no dose reduction back to 10 mg). Participants completed sleep diaries again for the last two weeks of treatment. At the end of the treatment period, participants had their blood drawn and met with the study coordinator and physician to review changes in their sleep and fill out surveys. Participants were also questioned on changes in diabetes medication and other changes in health.
Participants
Men and women between the ages of 25 and 75 with suboptimally controlled type 2 diabetes (ie, HbA1c between 6.5% and 10.0%), a DSM-5 diagnosis of Insomnia Disorder, at least a mild level of insomnia symptoms (ie, ISI >10), a self-reported total sleep time (TST) ≤6.5 hours and a combined sleep onset latency (SOL) and WASO > 45 minutes on 7 or more of the 14 nightly sleep logs during both the initial 2-week screening period and the two-week screening run-in period prior to randomization were eligible for participation. Those who met the following criteria were excluded: diagnosis of severe obstructive sleep apnea and not using CPAP machine, other untreated primary sleep disorder, shift workers, unwillingness to not use sedative-hypnotics (other than suvorexant) during the study period, unwillingness to maintain stable diabetes medication unless medically indicated, positive urine toxic screen for substance use other than nicotine, alcohol and marijuana at the Screening Visit, consumption of greater than six cups of coffee per day, current major depressive episode, active or unstable major psychiatric disorder, current alcohol or substance use disorder, renal or hepatic disease, pregnancy, malignancy within past 2 years, surgery within past 3 months, unstable neurological disorder or cardiovascular disease, concomitant medications with important drug interaction with suvorexant (eg, strong CYP3A inhibitors), history of being treated with suvorexant, and traveling across two time-zones during the week prior to enrollment.
Participants were recruited through advertisements on the Partners Clinical Trials webpage (Rally), flyers in local medical clinics, advertisements on Facebook and Google, referral by providing clinicians, and through a research coordinator stationed at a diabetes clinic at Brigham and Women’s Hospital. Prospective subjects were also identified through the MGH Diabetes Research Center Patient Registry; patients were sent a study information packet and an opt-out letter, and all patients who did not opt out were contacted by a research coordinator. Lastly, participants were recruited using Mass General Brigham’s Research Study Volunteer Program Registry, in which patients meeting basic criteria were sent information about the study and instructed to contact the research team.
Assessments
Sleep quality and quantity were assessed using the Insomnia Severity Index27 (ISI), the PSQI (specifically the item relating to sleep quality),28 and sleep diaries. Participants were encouraged to complete sleep diaries as soon as they woke up in the morning to ensure accuracy of responses. They reported the time they tried to go to sleep, any medications taken to help with sleep (including the study drug), their last awakening in the morning, sleep onset latency (SOL), the number of times they awakened from sleep, the amount of time they spent awake after sleep onset (WASO), and their subjective total sleep time (sTST). Calculated total sleep time (cTST) was determined by subtracting SOL and WASO from the amount of time spent in bed attempting to sleep. Sleep efficiency was calculated by dividing cTST by the time spent in bed. In cases where a participant spent less than 7 hours in bed, researchers referred to the question, “Did you have your final wake up before your alarm went off?” If the participant responded “Yes”, the difference in time between their alarm and their awakening was added to their WASO calculation. If the participant responded, “I don’t use an alarm”, the difference between 7 hours and their time in bed was added to their WASO calculation. If the participant responded “No”, the WASO calculation was not changed.
Insomnia severity was assessed by the Patient Global Impression for Insomnia29 (PGI-I) and the Clinical Global Impression30 for Insomnia (CGI-I). The PGI-I assessed participants’ subjective impression of their change in insomnia symptoms on a 7-point Likert scale; those reporting to be “much better” or “very much better” were deemed to be PGI-I responders. On the CGI-I, the study physician assessed participants’ change in insomnia symptoms and overall symptom severity on a 7-point Likert scale; those with ratings of change of “much improved” or “very much improved” were said to be CGI-I responders.
Data pertaining to participants’ experience with diabetes was collected using the Diabetes Quality of Life questionnaire31 (DQOL) and the Diabetes Distress Scale32 (DDS). Depression symptoms were assessed using the Beck Depression Inventory33 (BDI).
Serum values for HbA1c and C-Reactive Protein levels were assessed via blood draw before participants were given study medication, at the end of the first treatment period, and at the end of the second treatment period.
Statistical Analyses
The primary outcome variable was sTST. Secondary outcomes were ISI and WASO. The same analytic procedure was used across these outcomes. Namely, in line with the SPCD, the outcomes of phases 1 and 2 were first analyzed separately, and then combined in a weighted statistical test. For this trial, the ordinary least squares approach recommended by Chen et al was used.34 This approach uses linear regression models for both phases, as implemented here using the Proc REG procedure in the statistical software package. In each phase, the dependent variable was the outcome variable of interest as observed at treatment end (eg, sTST score at treatment end). The predictor variables were this outcome’s baseline assessment (eg, sTST score at baseline) and a binary indicator for randomized group assignment during this phase (ie, 1 = suvorexant, 0 = placebo). Phase 2 analyses only included Phase 1 placebo non-responders. Using standard SPCD practices, these results were then combined in a final weighted analysis, using the simple weighted ordinary least square test statistic ZOLS recommended by Chen et al.34 As per the study protocol, a 50:50 weighting was used. A statistically significant ZOLS value (ie, which combined Phase 1 and 2 estimates) was interpreted as a significant treatment effect, using alpha = 0.05. No corrections were made for multiple comparisons, as there was only one primary outcome, and the secondary outcomes were exploratory. Missing data occurred in 9% (6 out of 67 randomized participants) of the cases during Phase 1 as a result of study drop-out, and 0% (0 out of 34 Phase 2 randomized participants) during Phase 2. As missing data rates of less than 10% are deemed unlikely to produce biased results,35 no further action was taken to address missing data. In total, we expected to randomize 72 participants to treatment with 62 completed subjects (31 per treatment group). Using the SPCD calculator, to achieve 80% power to detect an effect size of 0.40, at a statistical significance level of 0.05, 70 randomized subjects were required.
Along with the SPCD analysis, exploratory statistical analysis was also conducted which included Chi-square tests (for categorical variables) and Spearman correlations (for continuous variables). This analysis was conducted using GraphPad Prism Version 9.4.1.
Clinical Trials registration number: NCT03818581
Results
Of the 69 participants randomized, 67 were given medication (Figure 1). Of these 67 participants, there was post-randomization data only available for 65 participants (66.2% female, 84.6% white, 92.3% non-Hispanic) as two participants dropped out prior to their first post-randomization visit. Of these randomized participants, 6 terminated study participation early, yielding a completion rate of 91.0%. Of note, one of the 65 participants with available data did not complete sleep diaries, meaning n = 64 for the primary endpoint, sTST. At entry to the study, participants were, on average, sleeping for under 6 hours per night, with an average combined SOL and WASO of just under two hours. The mean ISI score was 16.1, indicating moderate insomnia severity. The mean HbA1c for participants upon entry to the study was 7.6%. At the time of study entry, 32.3% (n = 21) of participants had a current diagnosis of sleep apnea. Of those, 66.7% (n = 14) were regularly using a CPAP or BiPap machine. The participants not using a CPAP or BiPap machine were confirmed to have mild or moderate (ie, not severe) sleep apnea. Other baseline characteristics can be found in Table 1.
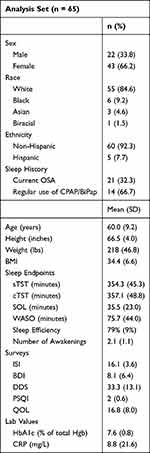 |
Table 1 Participant Baseline and Demographic Information |
Primary Outcome: sTST
The SPCD analysis results indicated that suvorexant was not significantly more effective than placebo in increasing sTST (p = 0.27; combining data from both phases 1 and 2) (Figure 2, Table 2). In both phases, participants assigned suvorexant had greater improvements in sTST than those assigned placebo (49.2-minute improvement vs 26.5-minute improvement in phase 1, 11.5-minute improvement vs 6.8-minute improvement in phase 2). As change in sTST was independently associated with baseline sTST (those with lower sTST improved more) and the suvorexant group had lower sTST at baseline, these group effects, however, were not significant when taking baseline values into account. During the final 2 weeks of phase 1, the average dose of suvorexant was 19.5 mg (21 of 22 participants increased to 2 pills), while during the final 2 weeks of phase 2, the average dose of suvorexant was 20.0 mg (all participants increased to 2 pills).
 |
Table 2 SPCD Analysis of Primary, Secondary, and Exploratory Outcomes |
Secondary Outcomes: ISI and WASO
There was no significant difference in change in neither ISI nor WASO between participants on suvorexant and placebo. These results are summarized in Table 2. Only 4.6% of the participants (n = 3) had severe insomnia at baseline, according to the ISI.
Exploratory Outcomes
There were no significant differences between groups in subjective quality of sleep, subjective sleep efficiency, PSQI, Beck Depression Inventory, or the DQOL or DDS. There were similarly no significant changes in serum levels of HbA1c or CRP between treatment groups (Table 2).
Exploratory Analyses
There were associations between improvements in sleep and improvements in various diabetes-related metrics independent of treatment assignment. During the first six-week treatment phase, among participants with baseline HbA1c ≥7.0, those with a >15% increase in sTST (our treatment response criterion) were more likely to see clinically significant HbA1c decreases of 0.5% or more (33.3% vs 9.4%; p = 0.05). Additionally, when looking at all participants during this first treatment phase, more CGI-I responders (ie, assessed by study physician to have “much improved” or “very much improved” insomnia) compared to non-responders showed a clinically significant reduction in HbA1c (23.1% vs 6.3%; p = 0.06). Reductions in the number of awakenings per night during the first treatment phase were also associated with reductions in HbA1c (r = 0.33; p = 0.01). When looking at all participants over the entire 12-week study period, CGI-I responders were more likely to have a reduction in HbA1c than non-responders (58.6% vs 31.8%; p = 0.06).
In addition to HbA1c, there were associations between improved sleep and subjective diabetes measures. Improvement in insomnia as indicated by a reduced ISI score was associated with DDS improvement in the first phase (r = 0.31; p = 0.01), second phase (r = 0.30; p = 0.02), and over the entire 12-week period (r = 0.25; p = 0.05). During the second phase, ISI improvements were associated with DQOL improvements (r = 0.24; p = 0.07), as were improvements on the PSQI (r = 0.35; p = 0.01).
Improvements in sleep over the entire 12-week study period were also associated with reductions in depressive symptoms as indicated by scores on the BDI (after omitting the BDI question inquiring about sleeping difficulties): SOL (r = 0.27; p = 0.06), ISI (r = 0.45; p < 0.01), and sTST (r = −0.27; p = 0.05) were all associated with improvements on the BDI in terms of percentage change. Additionally, participants who were CGI-I responders at 12-weeks were significantly more likely to see >50% reductions in BDI score (60.7% vs 26.1%; p = 0.01). PGI-I responders (ie, those who reported their insomnia to be “much better” or “very much better”) over the 12-week study period were also more likely to see >50% BDI score reductions (58.3% vs 30.8%; p = 0.05).
Safety and Tolerability
The study medication was well tolerated in this study sample. Adverse events are summarized in Table 3. Over four-fifths of the participants in each study group reported ≤1 adverse event. The most common side effect in the suvorexant treatment group during each study period was grogginess (36.4% in T1 and 13.9% in T2). There was only one serious adverse event, which was a toe amputation secondary to diabetes, unrelated to the study medication.
 |
Table 3 Summary of Adverse Events Over Each of the 6-Week Treatment Periods |
Discussion
In this randomized, double-blind, placebo-controlled study of people with chronic insomnia and suboptimally controlled type 2 diabetes, we did not find evidence that suvorexant is efficacious in improving sleep over a 12-week period in this population. Neither our primary sleep endpoint, sTST, nor our secondary sleep endpoints, WASO and ISI, demonstrated significant improvement in insomnia symptoms among participants taking 10–20mg suvorexant, compared to those taking placebo. We also found no impact of suvorexant on any of our exploratory endpoints, including cTST, number of awakenings, PSQI, SOL, sleep efficiency, BDI, DQOL, DDS, HbA1c, or CRP.
These findings showing no improvement in sleep in subjects taking suvorexant are inconsistent with those of Herring et al,36 which demonstrated the efficacy of suvorexant in improving symptoms of insomnia in two placebo-controlled 3-month trials, particularly by increasing subjective TST and decreasing WASO. We find it plausible that the increased medical complexity of this sample may help explain why our findings are discordant with these previous results. Specifically, as many participants in this study had medical conditions and symptoms disrupting sleep (eg, frequent urination related to hyperglycemia, mild-moderate obstructive sleep apnea, neuropathy, other chronic pain, etc.), suvorexant may have a more limited effect in improving sleep given these comorbidities. Additionally, the current study sample had notably higher body mass indexes than those in the previous trials (mean of 34 in this study versus means of 25 and 26 in those conducted by Herring et al).36 It is therefore possible that suvorexant serum concentrations among participants in this study were lower than those at similar doses used in the previous research, leading to a reduced benefit of the medication. Furthermore, participants in the Herring et al trials had more severe initial insomnia than those in this study: although participants in the two trials had nearly identical baseline ISI scores as those in this study, those in our study had baseline sTST durations that were over 30 minutes longer, as well as shorter baseline SOL durations (mean of 35.5 in our study versus >60 minutes in the previous trials). Lastly, the study may have been underpowered to identify a statistically significant difference between groups;34 our effect size for sTST was 0.44; significant differences in this primary endpoint might be observed in a larger trial.
Similarly, our results are distinct from those of the two previously mentioned trials that found improvements in sleep, as well as measures of glycemic control and/or obesity-related parameters, with administration of suvorexant in people with type 2 diabetes and insomnia.36 Both of these trials observed these positive findings with smaller sample sizes compared to the present study (n = 18 and n = 13). Furthermore, both trials were open-label and uncontrolled, and their results must therefore be interpreted with caution. Our notably larger, randomized, and placebo-controlled trial is an important addition to the growing literature on this topic.
We hypothesized that participants taking suvorexant might see improvements in glycemic control related to both improved sleep and modulation of the orexin system. Such an effect was not seen, although improvements in HbA1c were larger in the suvorexant group than those in the placebo group for both phases of the trial. Similarly, the small Yoshikawa et al study, which saw improvements in sleep efficiency in people with type 2 diabetes with suvorexant administration, did not find changes in HbA1c, which the authors attribute to their limited sample size.24 Of note, the short Toi et al study did find improvements in continuous glucose monitor (CGM) measured mean glucose levels after suvorexant administration, though this may be at least partially related to their highly controlled environment compared to our study (eg, all participants were hospitalized throughout the study and were given a regimented diet).23 The short time frame of our study may have limited ability to detect decreases in HbA1c levels. Specifically, HbA1c (as opposed to more immediate metrics of glycemic control such as CGM measured glucose levels) is widely interpreted as an average of blood glucose levels over the preceding two or three months,37 and the treatment periods in the current study were only six weeks. Future large studies should assess the impact of suvorexant on HbA1c over a longer time frame than was assessed in the current study.
Notably, exploratory analyses revealed significant relationships between improved sleep and decreased HbA1c across treatment groups. These findings linking improved sleep with reductions in blood sugar add to a growing body of literature showing the importance of adequate sleep for optimal glycemic control.5–8,13,38 For instance, in our previous 8-week blinded placebo-controlled study of eszopiclone in the treatment of primary insomnia in people who mostly did not have type 2 diabetes, strong correlations were observed between improvement in diary-reported total sleep time and improvement in HbA1c in both the eszopiclone and placebo groups.38 Our current findings linking improved sleep to reductions in HbA1c are especially noteworthy considering the relatively short time frame of this study and the longer period during which HbA1c integrates glycemic values, mentioned above. We find it plausible that such reductions may have been even larger with a longer treatment period. Furthermore, these associations between improved sleep and reductions in blood sugar levels were seen with a relatively limited sample (HbA1c values from several participants were not obtained due to the onset of the Covid-19 pandemic midway through the study, causing an inability to collect some laboratory samples).
Certain improvements in HbA1c with improved sleep were specifically seen in participants with a baseline HbA1c of 7.0% or greater, and not in those with HbA1c values between 6.5% and 6.9% at baseline. Although it is certainly possible that individuals with relatively lower blood sugar levels do see positive impacts of improved sleep on glycemic control, these results suggest the presence of a floor effect in which individuals with lower initial levels see less dramatic decreases in blood sugar with improved sleep.
Improved sleep was also associated with improvements on both the Beck Depression Inventory and diabetes-related distress as indicated by the Diabetes Distress Scale. The former finding is consistent with numerous studies linking improved sleep to decreased depressive symptoms.39 This observed reduction in depressive symptoms further underscores the importance of treating sleep difficulties in this population, in which clinical depression prevalence is about twice the rate of the general population.40
This study has certain limitations. The majority of the sample was non-Hispanic (92.3%) and white (84.6%), limiting the generalizability of these results to Latinx and non-white people. Additionally, this study only assessed subjective sleep metrics (rather than objective metrics obtained through either actigraphy or polysomnography) and these results are therefore susceptible to self-report biases. Further, although people with severe sleep apnea not using CPAP machine were excluded from participation, information on CPAP adherence and effectiveness among the included participants was not collected. Lastly, as alluded to above, the sudden onset of the Covid-19 pandemic midway through the study led to premature termination of several participants (refer to methods for discussion on missing data).
Conclusion
In conclusion, we did not find evidence that suvorexant improved sleep in this sample of people with type 2 diabetes, nor was suvorexant associated with improvements in glycemic control, CRP levels, or diabetes-related quality of life metrics. Independent of treatment with suvorexant and/or placebo, improvements in sleep were related to improvements in both glycemic control and subjective diabetes measures as well as reductions in depressive symptoms, suggesting the importance of identifying and treating sleep difficulties in this population.
Data Sharing Statement
No further data will be shared. This study is in compliance with the Declaration of Helsinki.
Acknowledgment
The abstract of this paper was presented at Sleep 2023 as a poster presentation with interim findings. The poster’s abstract was published in Sleep Volume 46, 2023: https://academic.oup.com/sleep/article/46/Supplement_1/A176/7182032?searchresult=1
Funding
Supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The opinions expressed inthis paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Disclosure
John Winkelman receives consultation fees from Emalex, Noctrix, and Disc Medicine; personal fees from Idorsia and Disc Medicine; grants from American Regent; and research support from NIDA, the RLS Foundation, and the Baszucki Brain Research Fund. All other authors report no disclosures.
References
1. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi:10.2991/jegh.k.191028.001
2. Khandelwal D, Dutta D, Chittawar S, Kalra S. Sleep disorders in type 2 diabetes. Indian J Endocrinol Metab. 2017;21(5):758–761. doi:10.4103/ijem.IJEM_156_17
3. Garg H. Role of optimum diagnosis and treatment of insomnia in patients with hypertension and diabetes: a review. J Family Med Prim Care. 2018;7(5):876–883. doi:10.4103/jfmpc.jfmpc_337_17
4. Schipper SBJ, Van Veen MM, Elders PJM, et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: a review of the literature. Diabetologia. 2021;64(11):2367–2377. doi:10.1007/s00125-021-05541-0
5. Knutson KL. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Archives of Internal Medicine. 2006;166(16):1768–1774. doi:10.1001/archinte.166.16.1768
6. Koopman ADM, Beulens JW, Dijkstra T, et al. Prevalence of insomnia (symptoms) in T2D and association with metabolic parameters and glycemic control: meta-analysis. J Clin Endocrinol Metab. 2019;105(3):614–643. doi:10.1210/clinem/dgz065
7. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91–101. doi:10.1016/j.smrv.2016.02.001
8. Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34(5):1171–1176. doi:10.2337/dc10-1962
9. Diabetes control and complications trial research group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. NEJM. 2022. doi:10.1056/NEJM199309303291401
10. UK prospective diabetes study (UKPDS) group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865. doi:10.1016/S0140-6736(98)07037-8
11. UK prospective diabetes study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. doi:10.1016/S0140-6736(98)07019-6
12. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi:10.1056/NEJMoa0806470
13. García-Serrano C, Pujol Salud J, Aran-Solé L, et al. Enhancing night and day circadian contrast through sleep education in prediabetes and type 2 diabetes mellitus: a randomized controlled trial. Biology. 2022;11(6):893. doi:10.3390/biology11060893
14. Garfinkel D, Zorin M, Wainstein J, Matas Z, Laudon M, Zisapel N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab Syndr Obes. 2011;4:307–313. doi:10.2147/DMSO.S23904
15. Tsunoda T, Yamada M, Akiyama T, et al. The effects of ramelteon on glucose metabolism and sleep quality in type 2 diabetic patients with insomnia: a pilot prospective randomized controlled trial. J Clin Med Res. 2016;8(12):878–887. doi:10.14740/jocmr2754w
16. Winkelman JW. Insomnia disorder. N Engl J Med. 2015;373(15):1437–1444. doi:10.1056/NEJMcp1412740
17. Tan X, van Egmond L, Chapman CD, Cedernaes J, Benedict C. Aiding sleep in type 2 diabetes: therapeutic considerations. Lancet Diabetes Endocrinol. 2018;6(1):60–68. doi:10.1016/S2213-8587(17)30233-4
18. Herring WJ, Connor KM, Snyder E, et al. Suvorexant in patients with insomnia: pooled analyses of three-month data from phase-3 randomized Controlled clinical trials. J Clin Sleep Med. 2016;12(09):1215–1225. doi:10.5664/jcsm.6116
19. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi:10.1016/s0092-8674(00)80949-6
20. Tsuneki H, Wada T, Sasaoka T. Role of orexin in the central regulation of glucose and energy homeostasis. Endocr J. 2012;59(5):365–374. doi:10.1507/endocrj.ej12-0030
21. Tsuneki H, Kon K, Ito H, et al. Timed inhibition of orexin system by suvorexant improved sleep and glucose metabolism in type 2 diabetic db/db mice. Endocrinology. 2016;157(11):4146–4157. doi:10.1210/en.2016-1404
22. Rines AK, Sharabi K, Tavares CDJ, Puigserver P. Targeting hepatic glucose output in the treatment of type 2 diabetes. Nat Rev Drug Discov. 2016;15(11):786–804. doi:10.1038/nrd.2016.151
23. Toi N, Inaba M, Kurajoh M, et al. Improvement of glycemic control by treatment for insomnia with suvorexant in type 2 diabetes mellitus. J Clin Transl Endocrinol. 2018;15:37–44. doi:10.1016/j.jcte.2018.12.006
24. Yoshikawa F, Shigiyama F, Ando Y, et al. Chronotherapeutic efficacy of suvorexant on sleep quality and metabolic parameters in patients with type 2 diabetes and insomnia. Diabetes Res Clin Pract. 2020;169:108412. doi:10.1016/j.diabres.2020.108412
25. Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. PPS. 2003;72(3):115–127. doi:10.1159/000069738
26. Schulz KF, Altman DG, Moher D. CONSORT statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251.
27. Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi:10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
29. Snyder ES, Tao P, Svetnik V, Lines C, Herring WJ. Use of the single-item patient global impression-severity scale as a self-reported assessment of insomnia severity. J Sleep Res. 2021;30(1):e13141. doi:10.1111/jsr.13141
30. Berk M, Ng F, Dodd S, et al. The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J Eval Clin Pract. 2008;14(6):979–983. doi:10.1111/j.1365-2753.2007.00921.x
31. Shen W, Kotsanos JG, Huster WJ, Mathias SD, Andrejasich CM, Patrick DL. Development and validation of the diabetes quality of life clinical trial questionnaire. Med Care. 1999;37(4):AS45–AS66. doi:10.1097/00005650-199904001-00008
32. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi:10.2337/diacare.28.3.626
33. Jackson-Koku G. Beck depression inventory. Occup Med. 2016;66(2):174–175. doi:10.1093/occmed/kqv087
34. Chen YF, Yang Y, Hung HMJ, Wang SJ. Evaluation of performance of some enrichment designs dealing with high placebo response in psychiatric clinical trials. Contemp Clin Trials. 2011;32(4):592–604. doi:10.1016/j.cct.2011.04.006
35. Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health. 2001;25(5):464–469. doi:10.1111/j.1467-842X.2001.tb00294.x
36. Herring WJ, Connor KM, Ivgy-May N, et al. Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016;79(2):136–148. doi:10.1016/j.biopsych.2014.10.003
37. Florkowski C. HbA1c as a diagnostic test for diabetes mellitus – reviewing the evidence. Clin Biochem Rev. 2013;34(2):75–83.
38. Buxton OM, Pavlova MK, O’Connor SP, Wang W, Winkelman JW. Lack of change in glucose metabolism in eszopiclone-treated primary insomnia patients. Nat Sci Sleep. 2017;9:187–198. doi:10.2147/NSS.S130505
39. Scott AJ, Webb TL, Martyn-St James M, Rowse G, Weich S. Improving sleep quality leads to better mental health: a meta-analysis of randomised controlled trials. Sleep Med Rev. 2021;60:101556. doi:10.1016/j.smrv.2021.101556
40. Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142(Suppl):S8–21. doi:10.1016/S0165-0327(12)70004-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

