Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Laboratory Surge Response to the COVID-19 Pandemic: An Incident Command System Approach
Authors Chuang HN, Shih CH, Tsai HW, Jiang RS, Hsiao TH, Liu PY , Jan YJ, Wang JM
Received 13 January 2022
Accepted for publication 21 April 2022
Published 11 May 2022 Volume 2022:15 Pages 1083—1088
DOI https://doi.org/10.2147/JMDH.S358096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Han-Ni Chuang,1,* Chien-Hung Shih,1,* Hung-Wen Tsai,2 Rong-San Jiang,1 Tzu-Hung Hsiao,2– 4 Po-Yu Liu,5 Yee-Jee Jan,6 Jiunn-Min Wang6
1Department of Medical Research, Taichung Veterans General Hospital, Taichung, 407204, Taiwan; 2Medical Administration Department, Taichung Veterans General Hospital, Taichung, 47204, Taiwan; 3Department of Public Health, Fu Jen Catholic University, New Taipei City, 24205, Taiwan; 4Institute of Genomics and Bioinformatics, National Chung Hsing University, Taichung, 40227, Taiwan; 5Division of Infection, Taichung Veterans General Hospital, Taichung, 47204, Taiwan; 6Department of Pathology & Laboratory Medicine, Taichung Veterans General Hospital, Taichung, 407, Taiwan
*These authors contributed equally to this work
Correspondence: Yee-Jee Jan; Jiunn-Min Wang, Department of Pathology & Laboratory Medicine, Taichung Veterans General Hospital, 1650 Taiwan Boulevard Sect. 4, Taichung, 40705, Taiwan, Tel +886-4-23592525, Fax +886-4-2359-5046, Email [email protected]; [email protected]
Abstract: The COVID-19 pandemic has reaffirmed the critical significance of effective diagnostics in outbreak response. In Taiwan, the COVID-19 wave in May 2021 led to a rapidly growing demand for SARS-CoV-2 diagnostic tests. To meet the challenge, an extensive system-wide emergency preparedness plan, hospital emergency incident command system (HEICS), was developed to deal with emergencies involving healthcare systems. During the wave of the COVID-19 outbreak, a 19.4-fold increase in SARS-CoV-2 PCR (polymerase chain reaction) diagnostic tests occurred in the hospital. The incident commander of TCVGH reviewed COVID-19 related events daily and purchased a high-throughput PCR machine for SARS-CoV-2 PCR diagnostic tests. In addition, the Department of Operations was responsible for staff scheduling and educational training. The turn-around times of SARS-CoV-2 diagnostic tests were shortened from 21.2 hours to 5.8 hours in the second week of the COVID-19 wave. Implementation of HEICS integrated resources could be helpful for expanding surge capacity during future outbreaks.
Keywords: COVID-19, hospital emergency incident command system, pandemics
Introduction
Coronavirus Disease 2019 (COVID-19) is a systemic disease commonly presented with cough, fever, fatigue, headache, myalgia, diarrhea, anosmia and dyspnea together with hypoxemia.1,2 SARS-CoV-2 has spread worldwide and was declared as a global pandemic in March 2020.3 The current standard for diagnosis of COVID-19 is detection of SARS-CoV-2 RNA taken from nasopharyngeal swabs or saliva by real-time Polymerase Chain Reaction (QPCR).1 During progression of the outbreak, there were a variety of commercial PCR-based diagnostic reagent kits and automatic platforms developed to improve the efficiency of SARS-CoV-2 testing. However, technological advancements may not be enough to meet the need during laboratory surges in testing during a pandemic. The significant increase in COVID-19 testing could further prolong turnaround time, the expected number of days required to collect and transport samples, testing, as well as the reporting of results for a specimen. These delays have an adverse impact on routine specimen processing in a laboratory, including the specimens of patients with a critical illness. To expand the scale and scope of COVID-19 testing and maintain appropriate turnaround times to meet patient needs, a systemic approach to coordinate a response to laboratory surges is essential.
The Hospital Emergency Incident Command System (HEICS) was developed in the 1980s as a foundational response to emergencies handled by the California Emergency Medical Services Authority.4 It is currently implemented by most hospitals and provides a foundation for emergency planning and configuration of administrative staff through the development of a logical management structure, duty descriptions, clear reporting channels, and a standard and simple nomenclature system.5 In the COVID-19 pandemic of 2021, Taichung Veterans General Hospital (TCVGH) established the TCVGH-HEICS system to address the surge in laboratory testing.
Here, we describe the clinical laboratory which utilization trends resulted from an influx of COVID-19 PCR cases, leading to the requirement for an HIS (Hospital Information System), laboratory staff and testing supplies. Therefore, the TCVGH-HEICS was established to address the challenges being faced during COVID-19, which also resolving the large influx of COVID-19 cases.
Results
HEICS Architecture in Taichung Veterans General Hospital
When COVID-19 pandemic, the Central Epidemic Command Center (CECC) of Taiwan raised epidemic warnings to Level 2 nationwide on May 11, 2021. After one week, CECC then raised epidemic warnings to Level 3 nationwide. For the large amount of PCR testing which occurred in TCVGH on May 18, staff in the Clinical Virology Laboratory were scheduled accordingly in order to maintain the testing needs during an around-the-clock effort.
TCVGH’s COVID-19 epidemic HEICS system, an extensive system-wide emergency preparedness plan, was developed to deal with emergencies significantly affecting the health system. The HEICS-TCVGH consists of Incident Commander, Deputy commander/Public Information Officer, Liaison officer, Security office, Secretariat, and Department of Operations (Figure 1). The staff duties in the HEICS-TCVGH are shown in Table 1. Incident commanders set up both the objectives and strategies for COVID-19 associated emergency, and later issue the appropriate order. The Deputy commander/Public Information officer was then responsible for supervising the internal staff of TCVGH. The Department of Operations implemented the objectives and policies, including maintenance of testing capacity, immediate testing and approval reports, replies to doctor examination results, and reduction in the number of frontline patients. The Secretariat formulated the infection control policies for the personnel, patients, employees, and visitors to TCVGH. In addition, they also developed a policy for restricting hospital access for non-essential individuals (visitors, vendors and media) and reporting infection control policies to the Taiwan Emergency Operations Center.
 |
Table 1 New Position Assignment During the COVID Pandemic in the HEICS |
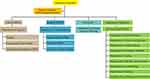 |
Figure 1 Development of hospital emergency incident command system (HEICS) in TCVGH. |
Liaison officers reported daily on hospital surveillance results to the city health bureau and Taiwan Center for Disease Control (TCDC). They were also responsible for the management and operation of the hospital, including personnel training, scheduling, inventory control, purchasing of inspection equipment and construction of an information system for the equipment, as well as other tasks. A security officer was responsible for purchasing high-throughput PCR machines such as the cobas® 4800 system (Roche), while also assigning personnel from other departments within the hospital to dispatch support. Incident commanders reviewed daily the data from emergency rooms, hospital facilities, and clinical laboratories and managed the real-time emergency events.
Laboratory Surge for SARS-CoV-2 PCR in TCVGH Clinical Virology Laboratory
As the COVD-19 pandemic continued in Taiwan during 2021, the volume of PCR testing for SARS-CoV-2 dramatically increased at clinical virology laboratory of TCVGH. The volume of SARS-CoV-2 PCR tests was 137 in the first week (prior to the COVID-19 pandemic). However, the volume of SARS-CoV-2 PCR had increased dramatically to 2661 tests in the third week, representing a 19.4-fold increase (Figure 2).
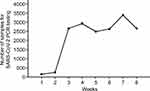 |
Figure 2 The laboratory surge in SARS-CoV-2 PCR tests in TCVGH at the third week. |
The Turnaround Times for the SARS-CoV-2 PCR Test Report Were Narrowed Down
Before the COVID-19 outbreak, the approximate turnaround times (TAT) SARS-CoV-2 PCR tests was 21.2 hours. When samples for SARS-CoV-2 PCR tests increased in the Clinical Virology Laboratory, the TCVGH-HEICS had adjusted the approximate TAT to 5.8 hours by the second week (Figure 3). During the third to eighth week, the approximate TAT for SARS-CoV-2 PCR testing was approximately 5.5 to 6 hours. The HEICS system had clearly worked to ease the influx of front-end patients by cutting down the turnaround times needed for SARS-CoV-2 PCR testing.
 |
Figure 3 Turnaround times for SARS-CoV-2 PCR test was decreased from 21.2 hours to 5.8 hours at the third week. |
Discussion
In 2021, the COVID-19 pandemic drove a dramatic demand for healthcare resources to occur in Taiwan. The Clinical Virology Laboratory of TCVGH need to provide much more testing for COVID-19 in addition to its routine inspection processing responsibilities. The SARS-CoV-2 testing represented an approximately an increased 19.4-fold increased daily testing volumes in the clinical laboratory. For the first two weeks, we prepared all infection control policies, including high-throughput PCR machines and staff scheduling of the assistants et al. We improved the turnaround time for SARS-CoV-2 testing from 21.2 to 5.8 hours. The TCVGH-HEICS had adjust its process of SARS-CoV-2 testing thus enabling it to relieve congestion in the frontline medical personnel: including HIS (Healthcare Information System), inventory control, staffing and technology requirements.
In the 2009 H1N1 pandemic, Crawford et al.6 Reported on their laboratory surge response in the New York City metropolitan area, including an increase in its workforce, the constructing of new physical practices, deploying high capacity molecular respiratory virus testing, building new laboratory information system and establishing solid communication with clinical personnel. A similar condition also occurred in recent years regarding the in Ebola virus disease7 and bioterrorism.8 A surge in demand for clinical laboratory capacity has also occurred during the SARS-CoV-2 pandemic. Durant et al9 conducted a retrospective assessment of lab utilization in an urban tertiary care medical center during the SARS-CoV-2 pandemic. They found that the volume of laboratory testing had decreased during the COVID-19 pandemic, while surge demand for SARS-CoV-2 testing, such as COVID-19-related markers, had increased. In Indonesia, Hendarwan et al10 reported that the primary constraints of increasing laboratory capacity were a lack of reagents and equipment, as well as limited human resources. In the United Kingdom, due to the National Health Service (NHS), the Department of Health and Social Care directed NHS hospitals to increase laboratory capacity. Bosworth et al11 reported that their rapid-establish workflow in Birmingham provided surge capacity. Overall, enhancing laboratory capacity during high-demand periods relies on multiple-aspect adjustments.
The HEICS was created in the late 1991 and serves as an essential foundation for hospitals in the United States when responding to various types of disasters.4 The HEICS is a hospital emergency management system that provides maximum efficiency, with the minor use of facilities and human resources. The HEICS is based upon five functional units involved in hospital emergency response, as well as improved patient safety with multiple positions organized under main sections: Administrative, Logistics, Operations, Planning, and Finance.12 Each of these roles has a specific mission during a crisis, and a list of position descriptions will guide the appointed staff during the crisis. The HEICS is necessary when handling hospital emergencies and has proven to be useful not only during the COVID-19 pandemic, but also when taking on other emergencies.
Conclusion
Outbreaks of highly contagious pathogens inevitably push up the demand for testing. To address this challenge, health care system could implement the HEICS as a potential solution to this concern.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi:10.1056/NEJMcp2009249
2. Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi:10.1056/NEJMcp2009575
3. Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and Control of COVID-19. Viruses. 2020;12(4):372. doi:10.3390/v12040372
4. Authority EMS. HEICS: the hospital emergency incident command system. Emergency Medical Services Authority Web site; 2004.
5. Jagminas L, Bubly G. The hospital emergency incident command system–are you ready? Med Health R I. 2003;86(7):193–195.
6. Crawford JM, Stallone R, Zhang F, et al. Laboratory surge response to pandemic (H1N1) 2009 outbreak, New York City metropolitan area, USA. Emerg Infect Dis. 2010;16(1):8–13. doi:10.3201/eid1601.091167
7. Katawera V, Kohar H, Mahmoud N, et al. Enhancing laboratory capacity during Ebola virus disease (EVD) heightened surveillance in Liberia: lessons learned and recommendations. Pan Afr Med J. 2019;33(Suppl 2):8. doi:10.11604/pamj.supp.2019.33.2.17366
8. Shapiro DS. Surge capacity for response to bioterrorism in hospital clinical microbiology laboratories. J Clin Microbiol. 2003;41(12):5372–5376. doi:10.1128/JCM.41.12.5372-5376.2003
9. Durant TJS, Peaper DR, Ferguson D, Schulz WL. Impact of COVID-19 pandemic on laboratory utilization. J Appl Lab Med. 2020;5(6):1194–1205. doi:10.1093/jalm/jfaa121
10. Hendarwan H, Syachroni S, Aryastami NK, et al. Assessing the COVID-19 diagnostic laboratory capacity in Indonesia in the early phase of the pandemic. WHO South East Asia J Public Health. 2020;9(2):134–140. doi:10.4103/2224-3151.294307
11. Bosworth A, Whalley C, Poxon C, et al. Rapid implementation and validation of a cold-chain free SARS-CoV-2 diagnostic testing workflow to support surge capacity. J Clin Virol. 2020;128:104469. doi:10.1016/j.jcv.2020.104469
12. Arnold JL, Dembry LM, Tsai MC, et al. Recommended modifications and applications of the hospital emergency incident command system for hospital emergency management. Prehosp Disaster Med. 2005;20(5):290–300. doi:10.1017/S1049023X00002740
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
