Back to Journals » Advances in Medical Education and Practice » Volume 12
Knowledge, Attitude, and Practice of Medical, Pharmacy, and Nursing Students Towards Pharmacovigilance and Adverse Drug Reaction Reporting at University of Gondar College of Medicine and Health Sciences, Northwest Ethiopia: A Cross-Sectional Study
Authors Tekel MT , Bekalu AF , Sema FD
Received 8 July 2021
Accepted for publication 23 September 2021
Published 2 October 2021 Volume 2021:12 Pages 1129—1139
DOI https://doi.org/10.2147/AMEP.S327739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Md Anwarul Azim Majumder
Masho Tigabe Tekel, Abaynesh Fentahun Bekalu, Faisel Dula Sema
Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Faisel Dula Sema Email [email protected]; [email protected]
Background: The adequate knowledge, attitude, and practice (KAP) of pharmacovigilance and ADRs reporting is crucial for health care students.
Objective: This study aimed at assessing the KAP of final-year medical, pharmacy, and nursing (MPN) students towards pharmacovigilance and ADRs reporting at the University of Gondar, College of Medicine and Health Sciences, Northwest Ethiopia.
Methods: A cross-sectional study was conducted among 296 final-year MPN students at the University of Gondar College of Medicine and Health Sciences from November 1, 2020 to January 30, 2021. A close-ended, structured, self-administered questionnaire was used for data collection prospectively. SPSS® (IBM Corporation) version 24 was used to analyze the data with descriptive and inferential statistics. The comparison of the KAP of groups was made by using a Kruskal–Wallis test and Mann–Whitney U-test. Statistical significance was declared at a p-value < 0.05.
Results: Among 296 participants, the majority of them had a poor level of knowledge (69.9%), practice (95.9%), and moderate attitude (62.5%) towards pharmacovigilance and ADRs reporting. The median (interquartile range) score of the students’ knowledge (maximum score = 15), attitude (maximum score = 50), and practice (maximum score = 5) towards PV and ADR reporting was 6 (5– 8), 32 (28.25– 35), and 1 (0– 1), respectively. The KAP of the students has shown differences with age, sex, hearing of the term PV, and discipline. A lack of training on ADRs (49%) reporting and not knowing where and how to report ADRs (47.3%) were among the main reasons of MPN students for not reporting ADRs.
Conclusion: A majority of final-year MPN students had poor knowledge, practice, and a moderate attitude towards PV and ADRs reporting. The school of medicine, pharmacy, and nursing should adequately cover the issue of PV and ADRs reporting in the undergraduate curriculum.
Keywords: knowledge, attitude, practice, pharmacovigilance, adverse drug reaction, students
Introduction
Adverse drug reactions (ADRs) are defined by the World Health Organization (WHO) as “any type of response caused by a drug that is unintentional, noxious, and takes place at the drug doses which are used for diagnosing, prophylaxis, or treatment of a disease or due to the medications for the physiological functions”.1
ADRs create a heavy burden to the health care system by increasing the risk of patient injury, hospitalization, readmission, prolonged length of hospital stays, high healthcare costs, morbidity, and mortality.2–9 According to a systematic review on the prevalence of medication-related problems and ADRs, the overall median prevalence of ADRs in Ethiopia is 36.6% with a range of 10.0 to 85.7%.10 A prospective cross-sectional study conducted in Southwest Ethiopia also reported that ADRs were a common cause of hospitalization and are a reason for the high in-hospital mortality rate.11
Maintaining and monitoring drugs efficacy and safety is a critical point in clinical practice. Thus, pharmacovigilance is an essential clinical discipline to ensure the appropriate use of medicines and patient safety, worldwide. WHO defines pharmacovigilance (PV) as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem”.1 Therefore, spontaneous reporting of ADRs is the backbone of the PV program.
Healthcare professionals are mainly responsible for identifying and reporting important ADRs. If they have confidence in their ability to diagnose, manage, and prevent such reactions, they will more likely to identify and report important ADRs.12–16 Several studies, however, reported that there is under-reporting of ADR globally. The studies also showed the lack of adequate knowledge, attitude, and practice (KAP) about PV activities and ADR reporting is among the main reasons for underreporting of ADRs.15,17,18
To ensure the safe use of medicines, PV and ADRs reporting educations are important competencies to all healthcare students, and incorporating the PV course in their curriculum is mandatory.19–22 It is also very important to ensure that they are well trained and have adequate knowledge about PV and ADR reporting to reduce the under-reporting of ADRs, to minimize the incidence of ADR, and to provide quality of care to patients.16,19,22–26 However, many studies conducted outside of Ethiopia indicated that health care students, specifically medical, pharmacy, and nursing (MPN) students have insufficient knowledge of pharmacovigilance and ADRs reporting.16,20,21,27–31
In Ethiopia, a limited number of studies have shown that health care professionals have a low level of KAP on PV and ADR reporting.17,18,32–35 Whereas, there is a lack of evidence on health care students to the best of the authors’ literature review. Therefore, this study aimed to assess the KAP of final-year MPN Students towards PV and ADRs reporting at the University of Gondar, college of medicine and health sciences, Northwest Ethiopia. This study provides baseline information on the KAP of MPN students on PV and ADRs reporting in Ethiopia. It may help to target the focus of higher education and healthcare policymakers and authorities on the reinforcement of plans and training initiatives for health care students on PV and ADRs, ultimately for the benefit of the patients.
Materials and Methods
Study Design and Settings
This cross-sectional study was conducted at the University of Gondar, College of Medicine and Health Sciences from November 1, 2020 to January 30, 2021. The University of Gondar is one of the oldest Universities in the country. It is located in Gondar Town, 738 km away from the capital city of Ethiopia (Addis Ababa). The University of Gondar teaches around 40,000 students who come from different regions of the country. Currently, it has five major campuses namely the college of medicine and health sciences, Maraki, Atse Tedros, Tseda, and Atse Fasil. College of medicine and health sciences is one of the major campuses of the University of Gondar where different healthcare students including MPN students get their health sciences education.
Population
All final-year MPN students in the University of Gondar College of Medicine and Health sciences were the source population. Whereas, all final-year MPN students in the University of Gondar College of Medicine and Health Sciences in the 2020–2021 academic year were the study population.
Inclusion and Exclusion Criteria
Final-year MPN students were included in the study. Whereas, students who were not willing to participate in the study; did not return, incompletely filled, or unanswered the questioners, and were absent from class due to sickness or some other reason during the data collection period were excluded from the study.
Sample Size Determination and Sampling Procedure
A census of all final-year MPN students who fulfilled the inclusion criteria was done.
Study Variables
The dependent variables of the study were the KAP of final-year MPN students regarding PV and ADRs reporting. Whereas, the independent variables of the study were the socio-demographic characteristics of the participants such as gender, age, discipline, hearing of the terms ADRs and PV.
Data Collection Procedure and Quality Control
A 36 item close-ended, structured, self-administered questionnaire adopted from previously validated published studies on PV and ADR reporting among MPN students was used.20,28,29,31,36,37 The questionnaire was pretested by administering it to a sample of 30 MPN students who were not involved in the main study. The data collected for the pretest was not included in the final analysis.
The questioner contained five main sections. The first section was about socio-demographic characteristics of the students such as age, gender, discipline, and questions on whether the students have ever heard of the terms ADRs and PV. The second section included 15 multiple-choice questions designed to measure knowledge about PV and ADR reporting. A knowledge score was prepared as a guiding tool to assess knowledge, one point for the correct answer and zero for the wrong answer. The sum of all items gives a maximum score of 15. Students were categorized based on their overall knowledge scores using the modified Bloom’s cutoff points as “good knowledge” if a score ranges 80–100% (12–15 points), “moderate knowledge” if a score ranges 50–79% (7.5–11.85 point), and “poor knowledge” if a score ranges <50% (<7.5 points) of the maximum score. The third section comprises 10 questions to evaluate the attitude of the students toward PV activities and ADR reporting. Respondents were asked to indicate their level of agreement or disagreements on a five-point Likert scale containing “Strongly agree”, “agree”, “neutral”, “disagree”, and “Strongly disagree” on the scale, valued 5 to 1 respectively. The sum of all items gives a maximum score of 50. The overall level of attitude was categorized using original Bloom’s cut-off point, as a “positive attitude” if the score was 80–100% (40–50 points), “moderate attitude” if the score was 60–79% (30–39.5 points) and “negative attitude” if the score was less than 60% (< 30 points). The fourth section contains 5 practice-related questions with yes/no options. A score of 1 was assigned to the “yes” answer and 0 to the “no” answer. The total practice score was categorized using the original Bloom’s cut-off point, as “good practice” if the score was 80–100% (4–5 points) and “poor practice” if the score was < 80% (< 4 points). The fifth section contains one question which asks the students the factors that discouraged ADR reporting, and multiple selections were presented.
Data Entry and Statistical Analysis
Data were edited, cleaned, coded, entered, and analyzed using Statistical Package for Social Sciences version 24. Descriptive statistics like mean (± standard deviation (SD)) for normally distributed data and median (interquartile range (IQR)) for non-normally distributed data were used to summarize Continuous variables. However, frequency and proportion were used to summarize categorical data. The normality of the data were tested using the Kolmogorov–Smirnov test and a skewness test. Comparisons of the KAP of the participants for each KAP question were done based on their age, gender, discipline, and hearing of the term ADR and PV (Supplementary Tables). Comparison of the KAP participants was made by a Kruskal–Wallis test for groups having more than two categories and a Mann–Whitney U-test for groups with two categories. Since the distributions of the KAP scores were not normally distributed across the different socio-demographic characteristics of the students, the results of the Mann–Whitney U-test and Kruskal–Wallis test were interpreted as mean rank score, and a statistical significance was declared at a p-value < 0.05.
Ethical Consideration
Ethical clearance was obtained from the University of Gondar, College of Medicine and Health Sciences, School of Pharmacy, Research and Ethical Review Committee before data collection. A permission letter to conduct the study was obtained from the dean of the College of Medicine and Health Sciences, University of Gondar. This study was conducted in accordance with the Declaration of Helsinki. After the purpose of the study was explained to the students, both oral and written consent was obtained from each participant and had been approved by the ethical committee. Confidentiality was secured by using codes to identify the study subjects, and the collected data were stored in a locked cabinet to be accessed by the authorized persons for only the purpose of this research.
Result
Socio-Demographics Characteristics of Final-Year MPN Students
Out of 308 students, 296 (143 medical, 86 pharmacy, and 67 nurse students) completed the questioner, yielding a response rate of 96%. The mean (± SD) age of the respondents was 23.31 (± 1.48) years, ranging from 21–28 years. The majority (65.5%) of the participants were males. More than three fourth (79.4%) and a quarter (32.4%) of the students have heard about the term ADR and PV, respectively (Table1).
Knowledge of Final-Year MPN Students Towards PV and ADR Reporting
The median (IQR) score of the students’ knowledge about PV and ADR reporting was 6 (5–8). The majority (69.9%) (95% CI = 64.7, 75.0) of the participants had a poor level of knowledge (Table 2). About half (50.7%) (95% CI = 45.3, 56.3) of the participants correctly defined the term ADR. However, only around one-third (35.8%) (95% CI = 27.4, 37.0) of the students correctly define the term PV. Moreover, only about a quarter (27.7%) (95% CI = 22.3, 33.1) of the respondents understand the identification of safe drugs is the most important purpose of PV (Table 3).
 |
Table 2 The KAP of Final-Year Medical, Pharmacy, and Nurse Students Regarding PV and ADR Reporting at the University of Gondar College of Medicine and Health Sciences |
 |
Table 3 The Knowledge of Final-Year Medical, Pharmacy, and Nurse Students Regarding PV and ADR Reporting at the University of Gondar College of Medicine and Health Sciences |
The Attitude of Final-Year MPN Students Towards PV and ADR Reporting
The median (IQR) attitude score of the students’ towards PV and ADR reporting was 32 (28.25–35). More than half (62.5%) (95% CI = 56.9, 68.2) of the students had a moderate attitude (Table 2). More than three-fourth (78.1%) (95% CI = 72.8, 83.1) of the students agreed that the PV concept should be included as a core topic in their health education, and 71.6% (95% CI = 67.0, 77.4) of them agreed that information on how to report ADR should be taught to students. However, about two-thirds (66.6%) (95% CI = 61.1, 71.8) of the respondents disagreed that the topic of PV is well-covered in their study curriculum. About 60.5% (95% CI = 54.5, 66.5) of the participants disagreed with the statement that “with their current knowledge, they are well prepared to report any ADRs notice in their future practice” (Table 4).
 |
Table 4 Attitude of Final-Year Medical, Pharmacy and Nurse Students Towards PV and ADR Reporting at the University of Gondar College of Medicine and Health Sciences |
The Practice of Final-Year MPN Students Towards ADR Reporting
The median (IQR) total practice score of the students was 1 (0–1). Among the students, 284 (95.9%) (95% CI = 93.2, 98.3) had poor practice towards ADR reporting (Table 2). Nearly one-third (30.4%) (95% CI = 25.3, 35.8) of the participants have encountered a patient with ADR during their clinical attachment. However, only 13.5% (95% CI = 9.5, 17.6) of them reported ADRs to their supervisors (Figure 1).
Comparison of the Overall KAP of Final-Year MPN Students Based on Their Gender, Age, Discipline, and Hearing of the Term ADR and PV
A statistically significant difference was seen in the mean rank knowledge score of participants based on age, gender, discipline, and hearing of the term PV. Students age ≥ 23 years old had significantly higher knowledge scores as compared to students with age < 23 years olds (p = 0.004). Similarly, a significantly higher knowledge score was recorded on male students than female students (p-value < 0.001), pharmacy students than medical and nursing students (p-value < 0.001), and students who had heard the term PV than students who had not heard the term PV (p-value = 0.004). Nursing students had a higher mean rank attitude score than pharmacy, and medical students (P=0.017). However, pharmacy students (180.89) had higher practice scores than medical and nurse students (p < 0.001). Students who had heard the term PV had a higher practice score than students who had not heard the term PV (p-value = 0.004) (Table 1).
Reasons of Final-Year MPN Students for Not Reporting ADRs
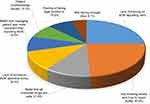
In this study, the lack of training on ADR (49%) (95% CI = 43.4, 55.3) followed by not knowing where and how to report ADRs (47.3%) (95% CI = 42.2, 53.0) was the main reason for not reporting ADRs by the students (Figure 2).
Discussion
This study aimed at assessing the KAP of final-year MPN students towards PV and ADR reporting at the University of Gondar, College of Medicine and Health Sciences. However, all previous studies done in Ethiopia focused on the KAP of healthcare professionals towards ADRs reporting. In addition, none of the previous studies in Ethiopia assessed the knowledge of the participants on PV.17,18,32–35,38 This is the first study that assessed the KAP of final-year healthcare students towards PV and ADR in a higher academic institution in Ethiopia.
The KAP of Final-Year MPN Students Towards PV and ADR Reporting
This study revealed that the majority (69.9%) of the participants had a poor level of knowledge on PV and ADR reporting. This is consistent with many previous studies conducted globally.20,27,30,31,39–41 In this study, less than half (34.5%) of the students heard about the term PV. It is similar to the study conducted among health care professionals in Northeast Ethiopia (20.2%).17 In addition, only one-third (35.8%) of students correctly defined the term PV. It is consistent with the study done in Malaysia.42 Moreover, only a quarter (27.7%) of the respondents gave the correct response to the purpose of PV. This is lower than studies conducted in Saudi Arabia (65.2%)22 and Malaysia (51.29%).26 The absence or inadequate coverage of PV courses in their health curriculum and lack of adequate training during clerkship could be a reason for the lower level of knowledge.39 So that incorporation of PV courses may help to improve the knowledge of students on PV and ADR reporting. A study done by Shrestha et al showed that the knowledge and attitude scores were increased following an educational intervention.43
Despite the lower knowledge, more than half (62.5%) of the students had a moderate attitude. This is consistent with many previous studies.20,31,42 Moreover, more than three-fourth (78.1%) of the students agreed that the PV concept should be included as a core topic in their health curriculum. This is supported by many previous studies as well.26,27,44 Around three-fourth (71.6%) of the participants also agreed that information on how to report ADR should be taught to the students. It is supported by the study reports from Malaysia.27,42
In this study, however, about two-thirds (66.6%) of the respondents disagreed that the topic of PV is well-covered in their study curriculum. About 45% of the participants also disagreed with the statement that “with their current knowledge, they are well prepared to report any ADRs noticed in their future practice”. These findings are consistent with the results of the Oman study (48.3%)44 This may greatly affect the students’ motivation in reporting ADRs encounter on different occasions which results in underreporting of ADRs. This is evidenced by this study that the majority (95.9%) of students had a poor ADR reporting practice. However, nearly one-third (30.4%) of the students encountered a patient with ADR during their clinical attachment, and only 13.5% of them reported ADRs to their supervisors.
Comparison of the Overall KAP of Final-Year MPN Students Towards PV and ADR Reporting
In this study, male students were found to have a higher knowledge of PV and ADR reporting than female students (P < 0.001). This finding is consistent with other previous studies.36,37 However, it is in contrast to the finding of the study done in Nigerian.20 Similarly, students with the age category of ≥ 23 years had a better knowledge score as compared to those who were in the age category of < 23 years (p=0.004). This is supported by the study conducted at the University of Gondar Hospital and Felege Hiwot Referral Hospital, Northwest Ethiopia.38
Pharmacy students showed a higher level of knowledge (p-value <0.001) on PV and ADR reporting than medical and nursing students. This result is similar to many previous studies.22,29,31,37 This may be because pharmacy students hear the issue of PV and ADR more frequently in their courses. Additionally, in this study participants who heard about PV had a higher knowledge score than those who did not hear the term PV. The study done in Malaysia reported that attending courses on PV and ADR reporting was associated with an increase in pharmacy students’ level of knowledge about ADR reporting.27
There is a significant difference in the ADR reporting practice based on the discipline of the students; pharmacy students had a better practice than medical and nursing students. The better knowledge of pharmacy students in this study may partially explain the difference. In addition, the focus of pharmaceutical care on the prevention, identification, and resolution of drug-related problems during their clerkship attachments may encourage them to report the ADR encounters to their preceptors.
Reasons of Final-Year MPN Students for Not Reporting ADRs
Another important finding in this study is that the lack of training on ADR (49%) and not knowing where and how to report ADRs (47.3%) were the major discouraging factors for reporting ADRs. So that provision of training for the students on the issues of PV and ADR reporting, where and how to report ADRs in particular may help to increase the KAP of the students.
ADR results in excessive healthcare costs through increased morbidity, mortality, and hospital admissions. A good KAP of all health care students on PV and ADRs reporting can contribute to minimizing the factors contributing to ADR underreporting. Since knowledge is a very important factor that influences attitude and practice, MPN students need to be well trained on how to recognize, prevent, and report ADRs as they are future health care professionals.
Strengths and Limitations of the Study
Despite it is the first study on the KAP of final-year MPN students towards PV and ADR reporting in Ethiopia, being a single-centered study may limit the generalizability of the study outcome to the national level. In addition, this study did not include all health science students other than MPN students. So that it may not be possible to extrapolate to other healthcare students. We did not include pre-final-year students, this may be the other limitation of the study.
Conclusion
The study concluded that the majority of final-year MPN students had poor knowledge, practice, and moderate attitude towards PV and ADRs reporting. The KAP of the students showed differences based on the students’ age, sex, hearing of the term PV, and discipline. Pharmacy students had significantly better knowledge and practice than medical and nursing students towards PV and ADRs reporting. A lack of training on ADRs reporting and not knowing where and how to report ADRs were among the main reasons for the students not reporting ADRs.
The schools of medicine, pharmacy, and nursing should include and adequately cover the issue of PV and ADRs reporting under the curriculum of undergraduate study. Providing special training for final-year MPN students may increase the KAP of the students towards PV and ADRs. Future scientists could conduct a multicenter study including all final-year health science students to produce strong data at the national level which may help to get the attention of policymakers.
Data Sharing Statement
All relevant data are in the manuscript. However, the minimal data underlying all the findings in the manuscript will be available upon request.
Acknowledgments
We would also like to express our deepest gratitude to the University of Gondar College of medicine and health final-year students in the 2020–2021 academic year for their cooperation in the data collection. The authors are grateful to data collectors too.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research is self-sponsored. No external fund was received by any of the authors.
Disclosure
The authors declare that they have no competing interests.
References
1. World Health Organization. The Importance of Pharmacovigilance. Geneva: World Health Organization; 2002.
2. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ. 2004;329(7456):15–19. doi:10.1136/bmj.329.7456.15
3. Pouyanne P, Haramburu F, Imbs JL, Bégaud B. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. French Pharmacovigilance Centres. BMJ. 2000;320(7241):1036. doi:10.1136/bmj.320.7241.1036
4. Geer MI, Koul PA, Tanki SA, Shah MY. Frequency, types, severity, preventability and costs of Adverse Drug Reactions at a tertiary care hospital. J Pharmacol Toxicol Methods. 2016;81:323–334. doi:10.1016/j.vascn.2016.04.011
5. Giardina C, Cutroneo PM, Mocciaro E, et al. Adverse drug reactions in hospitalized patients: results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study. Front Pharmacol. 2018;9(350). doi:10.3389/fphar.2018.00350.
6. Fasipe OJ, Akhideno PE, Owhin OS. The observed effect of adverse drug reactions on the length of hospital stay among medical inpatients in a Nigerian University Teaching Hospital. Toxicol Res Appl. 2019;3:2397847319850451. doi:10.1177/2397847319850451
7. Patel PB, Patel TK. Mortality among patients due to adverse drug reactions that occur following hospitalisation: a meta-analysis. Eur J Clin Pharmacol. 2019;75(9):1293–1307.
8. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Safety. 2015;38(5):437–453. doi:10.1007/s40264-015-0281-0
9. Mekonnen AB, Alhawassi TM, McLachlan AJ, Brien J-E. Adverse drug events and medication errors in African Hospitals: a systematic review. Drugs Real World Outcomes. 2018;5(1):1–24. doi:10.1007/s40801-017-0125-6
10. Kefale B, Degu A, Tegegne GT. Medication-related problems and adverse drug reactions in Ethiopia: a systematic review. Pharmacol Res Perspect. 2020;8(5):e00641. doi:10.1002/prp2.641
11. Angamo MT, Curtain CM, Chalmers L, Yilma D, Bereznicki L, Ramagopalan SV. Predictors of adverse drug reaction-related hospitalisation in Southwest Ethiopia: a prospective cross-sectional study. PLoS One. 2017;12(10):e0186631. doi:10.1371/journal.pone.0186631
12. Varallo FR, Guimarães Sde O, Abjaude SA, Mastroianni Pde C. [Causes for the underreporting of adverse drug events by health professionals: a systematic review]. Rev Esc Enferm USP. 2014;48(4):739–747. Portuguese. doi:10.1590/S0080-623420140000400023
13. Olsson S, Pal SN, Stergachis A, Couper M. Pharmacovigilance activities in 55 low- and middle-income countries: a questionnaire-based analysis. Drug Safety. 2010;33(8):689–703. doi:10.2165/11536390-000000000-00000
14. Moinuddin K, Ali S, Al-Aqqad AQ, et al. Knowledge and attitude of health-care professionals toward adverse drug reactions reporting at King Saud Medical City. J Pharm Bioallied Sci. 2018;10(1):29–34. doi:10.4103/jpbs.JPBS_234_17
15. AlShammari TM, Almoslem MJ. Knowledge, attitudes & practices of healthcare professionals in hospitals towards the reporting of adverse drug reactions in Saudi Arabia: a multi-centre cross sectional study. Saudi Pharm J. 2018;26(7):925–931. doi:10.1016/j.jsps.2018.04.012
16. Gaude OS, De Sa S. Assessment of knowledge, attitude, and practices of pharmacovigilance and adverse drug reaction reporting among final year medical students-A questionnaire-based study in a tertiary care hospital in Goa. Natl J Physiol Pharm Pharmacol. 2018;8(9):1657–1661. doi:10.5455/njppp.2018.8.0930506102018
17. Kassa Alemu B, Biru TT. Health care professionals’ knowledge, attitude, and practice towards adverse drug reaction reporting and associated factors at selected public hospitals in Northeast Ethiopia: a cross-sectional study. BioMed Res Int. 2019;2019:8690546.
18. Gidey K, Seifu M, Hailu BY, Asgedom SW, Niriayo YL. Healthcare professionals knowledge, attitude and practice of adverse drug reactions reporting in Ethiopia: a cross-sectional study. BMJ Open. 2020;10(2):e034553. doi:10.1136/bmjopen-2019-034553
19. Reddy VL, Pasha SJ, Rathinavelu M, Reddy YP. Assessment of knowledge, attitude and perception of pharmacovigilance and adverse drug reaction (ADR) reporting among the pharmacy students in south India. IOSR J Pharm Biol Sci. 2014;9(2):34–43.
20. Patrick OK, Olubunmi AM. Evaluation of the knowledge and perceptions about pharmacovigilance activities among pharmacy students in Nigeria: a cross-sectional study. Bangla Pharm J. 2017;20(1):1–13. doi:10.3329/bpj.v20i1.32082
21. Meher BR, Joshua N, Asha B, Mukherji D. A questionnaire based study to assess knowledge, attitude and practice of pharmacovigilance among undergraduate medical students in a Tertiary Care Teaching Hospital of South India. Perspect Clin Res. 2015;6(4):217–221. doi:10.4103/2229-3485.167102
22. Alwhaibi M, Alhindi G, Alshamrani M, Essa MB, Al Aloola N, Alhawassi TM. Pharmacovigilance in healthcare education: students’ knowledge, attitude and perception: a cross-sectional study in Saudi Arabia. BMC Med Educ. 2020;20(1):210. doi:10.1186/s12909-020-02116-2
23. Alwhaibi M, Al Aloola NA, Wieland LS. Healthcare students’ knowledge, attitude and perception of pharmacovigilance: a systematic review. PLoS One. 2020;15(5):e0233393. doi:10.1371/journal.pone.0233393
24. Marko S. A study of knowledge, attitude, and practice of pharmacovigilance among medical students at a tertiary care teaching hospital in Madhya Pradesh, India. Natl J Physiol Pharm Pharmacol. 2019;9(9):851–855.
25. Era N, Mukherjee S, Bordoloi SK. Assessment of knowledge, attitude, and practice of pharmacovigilance among undergraduate medical students in a tertiary care teaching hospital of Eastern India: a questionnaire-based study. Natl J Physiol Pharm Pharmacol. 2020;10(6):460–463.
26. Sivadasan S, Yuong NY, Chyi NW, et al. Knowledge and perception towards pharmacovigilance and adverse drug reaction reporting among medicine and pharmacy students. World J Pharm Pharm Sci. 2014;3(3):1652–1676.
27. Elkalmi RM, Hassali MA, Ibrahim MIM, Widodo RT, Efan QM, Hadi MA. Pharmacy students’ knowledge and perceptions about pharmacovigilance in Malaysian public universities. Am J Pharm Educ. 2011;75(5):96. doi:10.5688/ajpe75596
28. Al-lela OQ, Elkalmi R, Salih AS, Yousif DY, Ali HW, Shammo NO. Pharmacovigilance knowledge and perceptions among pharmacy, medical and nurse students in University of Duhok. J Pharm Pract Commun Med. 2018;4(2):60–65.
29. Alshakka M, Bahattab OA, Ali H, et al. Comparison of the knowledge and perception of pharmacovigilance among pharmacy, dental and medical students in Aden-Yemen. J Pharm Pract Commun Med. 2017;3:254–261. doi:10.5530/jppcm.2017.4.68
30. Odai M, Ghaida A. Knowledge and perception towards pharmacovigilance and adverse drug reporting among pharmacy and nursing students in Al Jouf University. Int J Pharm Bio Sci. 2019;9:36–44. doi:10.22376/ijpbs/lpr.2019.9.4.P36-44
31. Raza A, Jamal H. Assessment of knowledge, attitudes and practice among the medical and pharmacy students towards pharmacovigilance and adverse drug reactions in Abbottabad, Pakistan. J Pharmacovigil. 2015;3:1–5.
32. Gurmesa LT, Dedefo MG. Factors affecting adverse drug reaction reporting of healthcare professionals and their knowledge, attitude, and practice towards ADR reporting in Nekemte Town, West Ethiopia. Biomed Res Int. 2016;2016:5728462. doi:10.1155/2016/5728462
33. Seid MA, Kasahun AE, Mante BM, Gebremariam SN. Healthcare professionals’ knowledge, attitude and practice towards adverse drug reaction (ADR) reporting at the health center level in Ethiopia. Int J Clin Pharm. 2018;40(4):895–902. doi:10.1007/s11096-018-0682-0
34. Mulatu WN, Worku A. Assessment of knowledge, attitude and practice of health professionals towards adverse drug reaction reporting and factors associated with reporting. J Pharmacovigil. 2014;2:4.
35. Shanko H, Abdela J. Knowledge, attitudes, and practices of health care professionals toward adverse drug reaction reporting in Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia: a cross-sectional study. Hosp Pharm. 2018;53(3):177–187. doi:10.1177/0018578717737430
36. Rajiah K, Maharajan MK, Nair S. Pharmacy students’ knowledge and perceptions about adverse drug reactions reporting and pharmacovigilance. Saudi Pharm J. 2016;24(5):600–604. doi:10.1016/j.jsps.2015.03.021
37. Khan MU, Ahmad A, Ejaz A, et al. Comparison of the knowledge, attitudes, and perception of barriers regarding adverse drug reaction reporting between pharmacy and medical students in Pakistan. J Educ Eval Health Prof. 2015;12. doi:10.3352/jeehp.2015.12.28
38. Adimasu A. Nurses knowledge related to adverse drug reaction reporting and associated factors at Felegehiwot Referral Hospital and University of Gondar Teaching Hospital, Northwest Ethiopia. Am J Health Res. 2014;2(4):164–170. doi:10.11648/j.ajhr.20140204.20
39. Showande JS, Oyelola FT. The concept of adverse drug reaction reporting: awareness among pharmacy students in a Nigerian university. Internet J Med. 2013;8(1):24–30.
40. Vora MB, Paliwal NP, Doshi VG, Barvaliya MJ, Tripathi C. Knowledge of adverse drug reactions and pharmacovigilance activity among the undergraduate medical students of Gujarat. Int J Pharm Sci Res. 2012;3(5):1511.
41. Hema N, Bhuvana K. Pharmacovigilance: the extent of awareness among the final year students, interns and postgraduates in a government teaching hospital. J Clin Diagn Res. 2012;6(7):1248–1253.
42. Sivadasan S, Sellappan M. A study on the awareness and attitude towards pharmacovigilance and adverse drug reaction reporting among nursing students in a private university, Malaysia. Int J Curr Pharm Res. 2015;7(1):84–89.
43. Shrestha S, Sharma S, Bhasima R, Kunwor P, Adhikari B, Sapkota B. Impact of an educational intervention on pharmacovigilance knowledge and attitudes among health professionals in a Nepal cancer hospital. BMC Med Educ. 2020;20:1–10. doi:10.1186/s12909-020-02084-7
44. Al-Shekail NAS, Haridass S, Azmi M, Hassali NA. Knowledge and Perceptions of Pharmacy Students about Pharmacovigilance in Oman. 2017.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.