Back to Journals » OncoTargets and Therapy » Volume 13
Knockdown of lncRNA PCAT6 Enhances Radiosensitivity in Triple-Negative Breast Cancer Cells by Regulating miR-185-5p/TPD52 Axis
Authors Shi R, Wu P, Liu M, Chen B, Cong L
Received 6 November 2019
Accepted for publication 6 February 2020
Published 8 April 2020 Volume 2020:13 Pages 3025—3037
DOI https://doi.org/10.2147/OTT.S237559
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Rui Shi, Peng Wu, Miaomiao Liu, Bing Chen, Longjiao Cong
Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning, People’s Republic of China
Correspondence: Rui Shi
Liaoning University of Traditional Chinese Medicine, No. 79, Chongshan Road, Huanggu District, Shenyang, Liaoning, People’s Republic of China
Tel +8624 31207114
Email [email protected]
Background: Long non-coding RNAs (lncRNAs) have been reported to play essential roles in regulating the radiosensitivity of cancers. Prostate cancer-associated transcript 6 (PCAT6) exerts oncogenic roles in several tumors. However, the roles of PCAT6 and its underlying mechanism in regulating the radiosensitivity of triple-negative breast cancer (TNBC) have not been investigated.
Methods: The expression levels of PCAT6, microRNA-185-5p (miR-185-5p) and tumor protein D52 (TPD52) were determined by quantitative real-time polymerase chain reaction (qRT-PCR). Cell viability, apoptosis and colony formation were assessed by Cell Counting Kit-8 (CCK-8) assay, flow cytometry and colony formation assay, respectively. The interaction between miR-185-5p and PCAT6 or TPD52 was predicted by bioinformatics analysis and verified by dual-luciferase reporter assay. Western blot was carried out to detect the protein level of TPD52.
Results: PCAT6 and TPD52 were highly expressed and miR-185-5p was lowly expressed in TNBC tissues and cells, which was associated with an aggressive tumor phenotype in patients, affecting lymph node metastasis and clinical stage. PCAT6 or TPD52 knockdown or miR-185-5p overexpression enhanced the radiosensitivity of TNBC cells via inhibiting proliferation and inducing apoptosis. PCAT6 directly interacted with miR-185-5p and negatively regulated miR-185-5p expression. Moreover, TPD52 was confirmed as a target of miR-185-5p. Besides, PCAT6 regulated the radiosensitivity of TNBC cells through acting as a molecular sponge of miR-185-5p to modulate TPD52 expression.
Conclusion: Knockdown of PCAT6 promoted the radiosensitivity of TNBC cells through regulating miR-185-5p/TPD52 axis, providing a vital theoretical basis to improve the radiotherapy efficiency of TNBC.
Keywords: triple-negative breast cancer, PCAT6, miR-185-5p, TPD52, radiosensitivity
Introduction
Breast cancer (BC) is one of the most lethal and prevalent cancers, and leading cause of cancer-related deaths among females. In 2012, it is estimated that there will be about 1.7 million cases and approximately 522,000 deaths.1 Triple-negative breast cancer (TNBC) acts as the most invasive and aggressive among the breast cancer subtypes.2 Despite outstanding advances have been made in the treatment of BC in the past few decades, the prognosis for TNBC is still poor.3,4 Radiotherapy has become an effective strategy for TNBC treatment; however, tumor recurrence commonly occurs after the development of radioresistance.5,6 Hence, better clarifying the underlying mechanism of radioresistance is especially important for developing more effective therapeutic strategies to treat TNBC.
Long non-coding RNAs (lncRNAs) are longer than 200 nucleotides and lack protein-coding ability, which are associated with radioresistance, metastasis, and other clinical outcomes in various cancers.7,8 Previous studies demonstrated that lncRNAs are tightly linked to the progression and development of many tumor types, including TNBC.9,10 For example, lncRNA MALAT1 promoted proliferation and invasion in TNBC cells by targeting miR-129-5p.11 Moreover, lncRNA ANRIL accelerated the progression of TNBC by sponging miR-199a.12 Besides, lncRNA CCAT1 has been suggested to be involved in the radiosensitivity of BC.13 Prostate cancer-associated transcript 6 (PCAT6) has been identified to play oncogenic roles in diverse cancers, such as gastric cancer,14 lung cancer,15 and cervical cancer.16 However, the functional roles of PCAT6 and its underlying mechanism in the radiosensitivity of TNBC have not been reported.
More and more reports have suggested that lncRNAs can serve as a microRNA (miRNA) sponge to competitively suppress miRNAs.17 MiRNAs are a class of non-coding RNAs with about 22 nucleotides and negatively modulate target genes expression through binding to the 3ʹ-untranslated regions (3ʹUTR) of mRNA containing complementary sequence.18 At present, emerging evidence revealed that miRNAs could affect cellular responses to radiation and modulate the radiosensitivity of many cancers.19 MiR-185-5p has been suggested to be dysregulated in many kinds of cancers, such as prostatic cancer,20 hepatocellular carcinoma,21 clear cell renal cell carcinoma.22 Moreover, previous study suggested that miR-185-5p was expressed at a low level in BC cells.23 Nevertheless, the functional effects of miR-185-5p on regulating the radiosensitivity of TNBC remain largely unknown.
It is well known that miRNAs exert biological function through directly binding to target mRNAs.24 Tumor protein D52 (TPD52), an oncogene, was isolated from the amplification region of human chromosome 8q21 and highly expressed in many cancers.25,26 In addition, the expression of TPD52 was also overexpressed in BC.27 However, the interactions among miR-185-5p, TPD52 and PCAT6 in the radiosensitivity of TNBC have not been investigated.
In our study, the effects of PCAT6, miR-185-5p and TPD52 on the radiosensitivity of TNBC cells were first measured. Additionally, we explored the PCAT6/miR-185-5p/TPD52 regulatory network in TNBC cells or the cells under irradiation, providing novel insights into improving the radiotherapy efficiency of TNBC.
Materials and Methods
Tissue Collection
In our study, 70 pairs of TNBC tissues and adjacent normal tissues were provided by the patients who underwent surgery at Liaoning University of Traditional Chinese Medicine and were diagnosed with TNBC (stage I, II, and III) based on histopathological evaluation. In these patients, lymph node metastasis had occurred in 46 cases. These patients had never received chemotherapy or radiotherapy before surgery, and these tissues were promptly frozen in liquid nitrogen and kept in −80°C until experiments were carried out. Every patient provided written informed consent in this study. And the research was approved by the Research Ethics Committee of Liaoning University of Traditional Chinese Medicine.
Cell Culture and Transfection
TNBC cells (MDA-MB-468 and MDA-MB-231) and breast epithelial cells (MCF-10A) were bought from American Tissue Culture Collection (ATCC; Manassas, VA, USA). These cells were grown in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) in an incubator with 5% CO2 at 37°C.
The small interfering RNA against PCAT6 or TPD52 (si-PCAT6 or si-TPD52) and their negative control (si-NC), miR-185-5p mimics (miR-185-5p) and its negative control (NC), miR-185-5p inhibitors (anti-miR-185-5p) and its negative control (anti-NC), pcDNA-PCAT6 overexpression vector (pc-PCAT6) and its negative control (pc-NC) were provided by RiBoBio (Shanghai, China). TNBC cells (2×105 cells/well) were placed in six-well plates and transfected with the oligonucleotides or vector (siRNA 50 nM, mimics/inhibitors 50 nM, vector 4 μg) using Lipofectamine 3000 (Invitrogen).
Ionizing Radiation
Transfected cells were exposed to various doses of 6-MV X-ray (0 Gy, 2 Gy, 4 Gy, 6 Gy and 8 Gy) using a linear accelerator (Varian 2300EX, Varian, Palo Alto, CA, USA) at a dose rate of 200 cGy/min.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA from frozen tissues or cultured cells was extracted with TRIzol reagent (Invitrogen), and then first-strand complementary DNA (cDNA) synthesis was performed using the PrimeScript RT reagent Kit (Takara, Tokyo, Japan). After that, qRT-PCR was carried out with SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) and TaqMan MicroRNA Assay Kit (Applied Biosystems) on the ABI 7500 Real-Time PCR System (Applied Biosystems), respectively. The relative expression of PCAT6, TPD52, and miR-185-5p was evaluated with 2−ΔΔCt method, and the expression of PCAT6 and TPD52 was normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and miR-185-5p level was normalized by U6. In this study, the sequences of primers were listed as below: PCAT6 (Forward, 5ʹ-CAGGAACCCCCTCCTTACTC-3ʹ; Reverse, 5ʹ- CTAGGGATGTGTCCGAAGGA-3ʹ), miR-185-5p (Forward, 5ʹ-TCCGCTGGAGAGAAAGGC-3ʹ; Reverse, 5ʹ-ATGGAGGCTGAGGAGCACTG-3ʹ), TPD52 (Forward, 5ʹ- AACAGAACATTGCCAAAGGGTG-3ʹ; Reverse, 5ʹ-TGACTGAGCCAACAGACGAAA-3ʹ), GAPDH (Forward, 5ʹ-CGCTCTCTGCTCCTCCTGTTC-3ʹ; Reverse, 5ʹ- ATCCGTTGACTCCGACCTTCAC-3ʹ), U6 (Forward, 5ʹ-CTCGCTTCGGCAGCACATATACT-3ʹ; Reverse, 5ʹ-ACGCTTCACGAATTTGCGTGTC-3ʹ).
Cell Viability Assay
Cell Counting Kit-8 (CCK-8; Sangon Biotech, Shanghai, China) was utilized to evaluate the cell viability. Briefly, TNBC cells (100 μL) were placed in 96-well plates and transfected with the indicated vectors, and then exposed to 4 Gy dose of X-ray. At 0 h, 24 h, 48 h, or 72 h after irradiation, CCK-8 (10 μL) reagent was added to the wells and placed in the incubator for 3 h. Finally, the absorbance of the wells was examined with a microplate reader (Bio-Rad, Hercules, CA, USA) at 450 nm.
Cell Apoptosis Assay
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Sangon Biotech) was utilized to detect cell apoptosis. TNBC cells were seeded in 6-cm dishes and then transfected with indicated vectors, and then exposed to 4 Gy dose of X-ray. After irradiation for 24 h, cells were collected and double-stained using the Annexin V- FITC (10 μL) and PI (5 μL) for 20 min in the darkness. Lastly, cell apoptosis was assessed using a flow cytometry (Guava Technologies, Hayward, CA, USA).
Colony Formation Assay
TNBC cells were placed in six-well plates overnight and then transfected with indicated vectors, and then exposed to various doses of X irradiation (0 Gy, 2 Gy, 4 Gy, 6 Gy and 8 Gy). After irradiation for 24 h, the medium was changed with fresh medium and updated every 3 days. After 2 weeks, TNBC cells were carefully washed with pre-cold phosphate-buffered saline (PBS) and fixed with the paraformaldehyde (4%, 1mL) for 30 min at 4°C. Subsequently, the cells were washed with PBS and stained with 0.1% crystal violet for 1 h, and again washed with PBS until per well was clear. If a colony exceeded 50 cells, the colony was counted.
Dual-Luciferase Reporter Assay
Putative binding sites of miR-185-5p and PCAT6 or TPD52 were predicted by DIANA-LncBase v2 or TargetScan. The PCAT6 or TPD52 3ʹUTR fragment containing putative or mutated miR-185-5p binding sites was synthesized and cloned into pmirGlO luciferase reporter vector (Promega, Madison, WI, USA), namely PCAT6-wt and TPD52-wt or PCAT6-mut and TPD52-mut. The miR-185-5p or NC and reporter plasmid were co-transfected into TNBC cells for 48 h. After that, dual-luciferase reporter assay system (Promega) was employed to assess the luciferase activity, followed by normalizing to Renilla luciferase activity.
Western Blot Assay
Transfected cells were lysed using RIPA lysis buffer (Thermo Fisher, Wilmington, DE, USA) containing protease inhibitors (Beyotime, Shanghai, China) to extract the total protein. After quantification by using bicinchoninic acid (BCA) protein assay kit (Tanon, Shanghai, China), protein samples (about 30 μg) were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After that, the gels were transferred onto the polyvinylidene fluoride (PVDF; Beyotime) membranes. Then, 5% non-fat milk (Sangon Biotech) in PBS containing 0.1% Tween 20 (PBST) was used to block the membranes, and then the membranes were immunoblotted for 12 h at 4°C by primary antibodies against TPD52 (1:5000, ab155296, Abcam, Cambridge, UK) or β-actin (1:2000, ab8227, Abcam). Following washing with PBST, membranes were incubated by secondary antibodies (1:4000, D110058, Sangon Biotech). Immunoreactive bands were examined by enhanced chemiluminescence (ECL) reagent (Tanon). Protein expression of TPD52 was evaluated using ImageJ software and normalized to the level of β-actin.
Statistical Analysis
All data in this study were expressed as the mean ± standard deviation from at least independent experiments. Student’s t-test was employed to determine statistical differences between the two groups. Spearman rank correlation was applied to explore the correlation between miR-185-5p and PCAT6 or TPD52 in TNBC tissues. All statistical analyses were performed by GraphPad version 5.0 program (GraphPad Software, San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
The Expression of PCAT6 Was Upregulated in TNBC Tissues
To explore the potential roles of PCAT6 in TNBC, the expression of PCAT6 in 70 pairs of TNBC tissues and matched adjacent normal tissues was measured by qRT-PCR. Results displayed that the expression of PCAT6 was markedly increased in TNBC tissues in contrast to matched adjacent normal tissues (Figure 1A). Additionally, patients with higher PCAT6 level were more likely to have lymph node metastasis (Figure 1B). Moreover, PCAT6 expression was higher in patients with II/III clinical stages than those with I clinical stage, especially in III clinical stages (Figure 1C). Furthermore, the relationship between the relative expression of PCAT6 and clinical characteristics in patients with TNBC was analyzed. As shown in Supplementary Table 1, high expression level of PCAT6 was significantly associated with lymph node metastasis and tumor stage, but not with age, menopause and tumor size. These results indicated that PCAT6 might be involved in the progression of TNBC.
Knockdown of PCAT6 Promoted Radiosensitivity of TNBC Cells
The expression of PCAT6 was further explored in TNBC cells, and qRT-PCR analysis revealed that PCAT6 level was evidently increased in TNBC cells (MDA-MB-468 and MDA-MB-231) compared with that in breast epithelial cells (MCF-10A) (Figure 2A). Bases on the upregulation of PCAT6 in TNBC tissues and cells, we supposed that PCAT6 might influence the radiosensitivity of TNBC cells. MDA-MB-468 and MDA-MB-231 cells transfected with si-PCAT6 were used to assess the knockdown efficiency for PCAT6. The qRT-PCR analysis suggested that the abundance of PCAT6 was decreased in MDA-MB-468 and MDA-MB-231 cells transfected with si-PCAT6 (Figure 2B), indicating that successful introduction of si-PCAT6 into MDA-MB-468 and MDA-MB-231 cells. Subsequently, MDA-MB-468 and MDA-MB-231 cells transfected with si-NC or si-PCAT6 were exposed to 4Gy irradiation treatment. CCK-8 assay demonstrated that PCAT6 deficiency or radiation therapy alone suppressed the viability of MDA-MB-468 and MDA-MB-231 cells, and PCAT6 knockdown together with radiation therapy obviously repressed cell viability in MDA-MB-468 and MDA-MB-231 cells (Figure 2C). MDA-MB-468/C4 and MDA-MB-231/C4 are radioresistant TNBC cells surviving from MDA-MB-468/WT and MDA-MB-231/WT cells after fractionated radiation (2 Gy × 30). In addition, knockdown of PCAT6 also inhibited cell viability of MDA-MB-468/C4 and MDA-MB-231/C4 cells (Supplementary Figure 1A and B). Moreover, interference of PCAT6 or radiation therapy alone promoted apoptosis in MDA-MB-468 and MDA-MB-231 cells, and combination of si-PCAT6 and radiation therapy conspicuously accelerated apoptosis of MDA-MB-468 and MDA-MB-231 cells (Figure 2D). Furthermore, we found that interference of PCAT6 also facilitated apoptosis of MDA-MB-468/C4 and MDA-MB-231/C4 cells, but radiation therapy had no significant effect on apoptosis of MDA-MB-468/C4 and MDA-MB-231/C4 cells (Supplementary Figure 1C and D). Besides, colony formation analysis disclosed that PCAT6 knockdown led to an obvious decrease of colony survival fraction in MDA-MB-468 and MDA-MB-231 cells treated with irradiation (Figure 2E). And we observed that PCAT6 silence or radiation therapy alone increased the percentage of cells in G0/G1 phase but decreased the percentage of cells in S phase, combination of si-PCAT6 and radiation therapy conspicuously enhanced the percentage of cells in G0/G1 phase (Supplementary Figure 2A and B and Supplementary material and methods), suggesting PCAT6 knockdown and radiation therapy blocked cell cycle progression at the G0/G1 phase. All these data revealed that downregulation of PCAT6 elevated sensitivity of TNBC cells to ionizing radiation via inhibiting cell survival and promoting cell apoptosis.
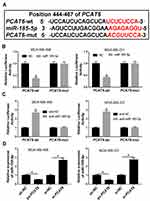
PCAT6 Directly Interacted with miR-185-5p and Negatively Regulated miR-185-5p Expression in TNBC Cells
Previous report has demonstrated that lncRNAs commonly exhibit their biological roles by acting as sponges of miRNAs.17 Through online bioinformatics database (DIANA-LncBase v2), PCAT6 contained potential binding sites of miR-185-5p (Figure 3A). Subsequently, the prediction was validated by dual-luciferase reporter assay. Results showed that overexpression of miR-185-5p significantly suppressed the luciferase activity of PCAT6-wt, whereas luciferase activity of PCAT6-mut was not evidently affected after transfection with miR-185-5p in MDA-MB-468 and MDA-MB-231 cells (Figure 3B). However, knockdown of miR-185-5p presented an opposite effect on luciferase activity of PCAT6-wt in MDA-MB-468 and MDA-MB-231 cells (Figure 3C). Next, the effect of PCAT6 on miR-185-5p expression was investigated in MDA-MB-468 and MDA-MB-231 cells. Results proved that overexpression of PCAT6 led to a significant decrease of miR-185-5p expression and its knockdown had an opposite effect in MDA-MB-468 and MDA-MB-231 cells (Figure 3D). Our findings suggested that PCAT6 acted as a molecular sponge of miR-185-5p and negatively modulated miR-185-5p expression in TNBC cells.
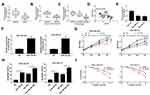
Overexpression of miR-185-5p Enhanced Radiosensitivity of TNBC Cells
To further investigate the roles of miR-185-5p in TNBC, the level of miR-185-5p in 70 pairs of TNBC tissues and matched adjacent normal tissues was determined by qRT-PCR. As presented in Figure 4A–C, the expression of miR-185-5p was reduced in TNBC tissues, patients with lymph node metastasis, and patients with advanced clinical stage. In addition, the association between the relative expression of miR-185-5p and clinical characteristics in patients with TNBC was assessed. As displayed in Supplementary Table 2, low expression level of miR-185-5p was significantly associated with lymph node metastasis and tumor stage, but not with age, menopause and tumor size. And we found that miR-185-5p expression was negatively correlated with PCAT6 level in TNBC tissues (R2=0.223, P<0.05) (Figure 4D). Besides, the abundance of miR-185-5p was also decreased in TNBC cells (MDA-MB-468 and MDA-MB-231) relative to breast epithelial cells (MCF-10A) (Figure 4E). QRT-PCR analysis showed that the expression of miR-185-5p was increased in MDA-MB-468 and MDA-MB-231 cells transfected with miR-185-5p mimics (Figure 4F), suggesting that successful introduction of miR-185-5p mimics into MDA-MB-468 and MDA-MB-231 cells. Subsequently, the effects of miR-185-5p on the radiosensitivity of TNBC cells were explored. CCK-8 assay showed that either miR-185-5p overexpression or irradiation exposure inhibited cell viability with respect to NC group, and combination of miR-185-5p restoration and radiation therapy strikingly suppressed cell viability compared with miR-185-5p group or 4Gy + NC group (Figure 4G). Moreover, MDA-MB-468 and MDA-MB-231 cells transfected with miR-185-5p or exposed to radiation therapy increased cell apoptosis, and apoptosis rate was remarkably enhanced in cells transfected with miR-185-5p and treated with radiation simultaneously (Figure 4H). Furthermore, miR-185-5p upregulation decreased colony formation rate in MDA-MB-468 and MDA-MB-231 cells exposed to irradiation (Figure 4I). Taken together, our results showed that miR-185-5p played an essential role in increasing the radiosensitivity of TNBC cells.
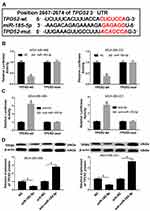
TPD52 Was a Direct Target of miR-185-5p in TNBC Cells
MiRNAs have been suggested to exert biological function via modulating their molecular targets.24 Hence, TargetScan software online was used to predict target genes of miR-185-5p. As shown in Figure 5A, TPD52 contained a putative target sequence for miR-185-5p in its 3ʹ-UTR. To confirm whether TPD52 was a downstream target of miR-185-5p, dual-luciferase reporter assay was performed. Results demonstrated that overexpression of miR-185-5p strongly suppressed the luciferase activity in TPD52-wt group, whereas the effect was little with respect to TPD52-mut group in MDA-MB-468 and MDA-MB-231 cells (Figure 5B). However, luciferase activity was prominently enhanced in MDA-MB-468 and MDA-MB-231 cells co-transfected with TPD52-wt and anti-miR-185-5p, while it was also not changed in TPD52-mut group (Figure 5C). Next, we observed the effect of miR-185-5p on TPD52 protein expression. Western blot assay proved that accumulation of miR-185-5p inhibited the protein expression of TPD52 and its knockdown presented an opposite effect (Figure 5D). These results together indicated that TPD52 was a direct target of miR-185-5p and was negatively regulated by miR-185-5p in TNBC cells.
Silencing TPD52 Elevated Radiosensitivity of TNBC Cells
Next, TPD52 expression was measured in TNBC tissues and cells by qRT-PCR. As expected, the results disclosed that the level of TPD52 was increased in TNBC tissues, patients with lymph node metastasis, and patients with advanced clinical stage (Figure 6A–C). Likewise, the association between the relative expression of TPD52 and clinical characteristics in patients with TNBC was also evaluated. As presented in Supplementary Table 3, high expression level of TPD52 was significantly associated with lymph node metastasis and tumor stage, but not with age, menopause and tumor size. Moreover, the TPD52 abundance was negatively associated with miR-185-5p expression in TNBC tissues (R2=0.123, P<0.05) (Figure 6D). Similarly, the mRNA and protein expression of TPD52 were also upregulated in TNBC cells (MDA-MB-468 and MDA-MB-231) compared with that in breast epithelial cells (MCF-10A) (Figure 6E). Western blot assay revealed that the protein level of TPD52 was reduced in MDA-MB-468 and MDA-MB-231 cells transfected with si-TPD52, suggesting that successful introduction of si-TPD52 into MDA-MB-468 and MDA-MB-231 cells (Figure 6F). CCK-8 analysis showed that TPD52 inhibition or treatment of 4Gy irradiation suppressed cell viability in MDA-MB-468 and MDA-MB-231 cells, and the cells transfected with si-TPD52 and treated with 4Gy irradiation further inhibited cell viability compared with those cells transfected with si-TPD52 or exposed to 4Gy irradiation (Figure 6G). Besides, TPD52 deficiency or 4Gy irradiation exposure induced apoptosis of MDA-MB-468 and MDA-MB-231 cells, and combination of TPD52 knockdown and irradiation significantly enhanced apoptosis rate in contrast to si-TPD52 or 4Gy + si-NC group (Figure 6H). In a word, these data indicated that TPD52 downregulation enhanced the radiosensitivity of TNBC cells by inhibiting cell viability and promoting apoptosis.
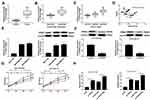
PCAT6 Regulated Radiosensitivity of TNBC Cells Through Acting as a Molecular Sponge of miR-185-5p to Modulate TPD52 Expression
Further, we explored whether PCAT6 regulated miR-185-5p/TPD52 axis in TNBC cells. The protein expression of TPD52 was investigated by Western blot in TNBC cells transfected with si-NC, si-PCAT6, si-PCAT6 + anti-NC, or si-PCAT6 + anti-miR-185-5p. Western blot assay proved that the protein expression of TPD52 was reduced in MDA-MB-468 and MDA-MB-231 cells transfected with si-PCAT6, while the effect was partially reversed by knockdown of miR-185-5p (Figure 7A). Similarly, the mRNA and protein expression of TPD52 were also decreased in MDA-MB-468/C4 and MDA-MB-231/C4 cells after transfection of si-PCAT6, which was abolished by downregulating miR-185-5p (Supplementary Figure 3A–D). CCK-8 analysis showed that interference of miR-185-5p weakened the inhibitory effect of PCAT6 knockdown on the viability of MDA-MB-468 and MDA-MB-231 cells treated with 4Gy irradiation as well as radioresistant TNBC cells (MDA-MB-468/C4 and MDA-MB-231/C4) (Figure 7B and Supplementary Figure 3E–F). Moreover, promotive effect of PCAT6 deficiency on apoptosis was abolished by silencing miR-185-5p in MDA-MB-468 and MDA-MB-231 cells exposed to 4Gy irradiation (Figure 7C). Furthermore, knockdown of miR-185-5p attenuated the suppressive effect of PCAT6 downregulation on colony formation in both DA-MB-468 and MDA-MB-231 cells treated with 4Gy irradiation (Figure 7D). All these data implied that PCAT6 knockdown promoted the radiosensitivity of TNBC cells via sponging miR-185-5p to affect TPD52 expression.
Discussion
Currently, radiotherapy is commonly considered a primary strategy for TNBC treatment. However, the development of radioresistance has become a main obstacle for treatment of cancer patients, resulting in tumor recurrence and repressing the effectiveness of radiation therapy.28 Thus, identifying new therapeutic targets is so critical to improve radiosensitivity in patients with TNBC.
In recent years, increasing evidence suggested that lncRNAs played regulatory roles in cell behaviors, cancer occurrence and pathogenesis, chemotherapy and radiotherapy resistance.29,30 Previous studies indicated that PCAT6 acted as a tumor promoter in various cancers. For instance, Cui et al stated that PCAT6 accelerated the progression of non-small cell lung cancer via regulating miR-330-5p.31 Xu et al presented that PCAT6 was overexpressed in gastric cancer tissues and promoted progression of gastric cancer via regulating miR-30.14 Besides, Wu et al found that the PCAT6 abundance was enhanced in colorectal cancer tissues and cell lines, and its knockdown alleviated colorectal cancer chemoresistance to 5-FU by regulating miR-204/HMGA2/PI3K.32 In our study, results proved that the abundance of PCAT6 was enhanced in TNBC tissues and cells, and we found that the level of PCAT6 was positively associated with lymph node metastasis and clinical stage. Moreover, the data revealed that downregulation of PCAT6 promoted the radiosensitivity of TNBC cells to irradiation through inhibiting cell growth and promoting apoptosis.
Notably, accumulating evidence suggested that lncRNA stability and function could be potently regulated by miRNAs.33 Here, DIANA-LncBase v2 was used to predict the potential target of PCAT6, and the data displayed that miR-185-5p and PCAT6 had potential binding sites. Subsequently, we confirmed miR-185-5p was a downstream target of PCAT6. MiR-185-5p dysregulation was observed in some cancers. For example, Tian et alstated that the miR-185-5p was lowly expressed in prostate cancer tissues and cells.34 Niu et al presented that miR-185-5p restoration impeded metastasis of hepatocellular carcinoma cells.35 Pei et al pointed out that miR-185-5p accumulation elevated the sensitivity of lung cancer cells to cisplatin through inhibiting cell growth and promoting apoptosis.36 Besides, Yin et al proved that miR-185-5p limited BC cell invasion and epithelial-mesenchymal transition (EMT).23 However, there is no report on the function of miR-185-5p in the radiosensitivity of TNBC cells. In our research, miR-185-5p abundance was reduced in TNBC tissues, and the TNBC patients with low expression of miR-185-5p had an invasive tumor phenotype. Moreover, we found that miR-185-5p upregulation resulted in an obvious decrease of cell growth and increase of cell apoptosis after irradiation, implying that miR-185-5p overexpression enhanced the radiosensitivity of TNBC. To explore how miR-185-5p influenced the radiosensitivity of TNBC, potential target was predicted by the online tool. TargetScan software online showed that TPD52 contained binding sites of miR-185-5p, which was then confirmed in TNBC cells through dual-luciferase reporter assay.
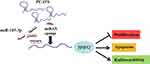
TPD52 is a tumor-promoting gene and is frequently overexpressed in multiple cancers, such as ovarian cancer,37 prostate cancer,38 colorectal cancer.39 Due to the oncogenic role of TPD52 in many cancers, TPD52 should be further studied. Moreover, accumulating evidence revealed that TPD52 was involved in cellular transformation, proliferation, apoptosis, and metastasis.40,41 Yang et al demonstrated that knockdown of TPD52L2 (a member of the TPD52) suppressed the proliferation and colony formation in BC cells.42 Zhang et al found that TPD52 expression was markedly increased in BC tissues and cells, and deficiency of miR-449 promoted proliferation and metastasis of BC cells through regulating TPD52.27 In our research, results uncovered that TPD52 expression was enhanced in TNBC tissues and cells. Besides, interference of TPD52 inhibited cell viability and induced apoptosis in TNBC cells under irradiation, disclosing that TPD52 interference enhanced the radiosensitivity of TNBC cells. It is widely reported that lncRNAs act as competing endogenous RNA (ceRNA) to affect genes expression by sponging miRNAs.43 Our present study displayed that TPD52 was positively regulated by PCAT6 and negatively regulated by miR-185-5p. Moreover, our results proved that silencing miR-185-5p abated the effects of PCAT6 downregulation on suppression of cell proliferation and promotion of apoptosis in TNBC cells under irradiation. These findings indicated PCAT6 knockdown promoted the radiosensitivity of TNBC cells via sponging miR-185-5p to affect TPD52 expression (Figure 8).
In conclusion, our research first explored the functional roles and underlying mechanism of PCAT6 in the radiosensitivity of TNBC cells. These data revealed that silencing PCAT6 promoted the radiosensitivity of TNBC cells via reducing cell proliferation and facilitating apoptosis by upregulating miR-185-5p expression and downregulating TPD52 expression. These findings might provide a new therapeutic target to enhance radiotherapy efficiency for TNBC patients.
Disclosure
The authors report no funding and no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23(Suppl 6):vi7–v12. doi:10.1093/annonc/mds187
3. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi:10.1158/1078-0432.CCR-06-3045
4. Tseng LM, Hsu NC, Chen SC, et al. Distant metastasis in triple-negative breast cancer. Neoplasma. 2013;60(3):290–294. doi:10.4149/neo_2013_038
5. He MY, Rancoule C, Rehailia-Blanchard A, et al. Radiotherapy in triple-negative breast cancer: current situation and upcoming strategies. Crit Rev Oncol Hematol. 2018;131:96–101. doi:10.1016/j.critrevonc.2018.09.004
6. Gasparini P, Lovat F, Fassan M, et al. Protective role of miR-155 in breast cancer through RAD51 targeting impairs homologous recombination after irradiation. Proc Natl Acad Sci U S A. 2014;111(12):4536–4541. doi:10.1073/pnas.1402604111
7. Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Mol Cancer. 2016;15(1):43. doi:10.1186/s12943-016-0530-6
8. Chi HC, Tsai CY, Tsai MM, et al. Roles of long noncoding RNAs in recurrence and metastasis of radiotherapy-resistant cancer stem cells. Int J Mol Sci. 2017;18(9):1903. doi:10.3390/ijms18091903
9. Soudyab M, Iranpour M, Ghafouri-Fard S. The role of long non-coding RNAs in breast cancer. Arch Iran Med. 2016;19(7):508–517. doi:0161907/AIM.0011
10. Qiu L, Tang Q, Li G, et al. Long non-coding RNAs as biomarkers and therapeutic targets: recent insights into hepatocellular carcinoma. Life Sci. 2017;191:273–282. doi:10.1016/j.lfs.2017.10.007
11. Zuo Y, Li Y, Zhou Z, et al. Long non-coding RNA MALAT1 promotes proliferation and invasion via targeting miR-129-5p in triple-negative breast cancer. Biomed Pharmacother. 2017;95:922–928. doi:10.1016/j.biopha.2017.09.005
12. Xu ST, Xu JH, Zheng ZR, et al. Long non-coding RNA ANRIL promotes carcinogenesis via sponging miR-199a in triple-negative breast cancer. Biomed Pharmacother. 2017;96:14–21. doi:10.1016/j.biopha.2017.09.107
13. Lai Y, Chen Y, Lin Y, et al. Down-regulation of LncRNA CCAT1 enhances radiosensitivity via regulating miR-148b in breast cancer. Cell Biol Int. 2018;42(2):227–236. doi:10.1002/cbin.v42.2
14. Xu Y, Sun JY, Jin YF, et al. PCAT6 participates in the development of gastric cancer through endogenously competition with microRNA-30. Eur Rev Med Pharmacol Sci. 2018;22(16):5206–5213. doi:10.26355/eurrev_201808_15718
15. Shi X, Liu Z, Liu Z, et al. Long noncoding RNA PCAT6 functions as an oncogene by binding to EZH2 and suppressing LATS2 in non-small-cell lung cancer. EBioMedicine. 2018;37:177–187. doi:10.1016/j.ebiom.2018.10.004
16. Lv XJ, Tang Q, Tu YQ, et al. Long noncoding RNA PCAT6 regulates cell growth and metastasis via Wnt/beta-catenin pathway and is a prognosis marker in cervical cancer. Eur Rev Med Pharmacol Sci. 2019;23(5):1947–1956. doi:10.26355/eurrev_201903_17233
17. Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34:9–14. doi:10.1016/j.semcdb.2014.05.015
18. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
19. Sun Q, Liu T, Yuan Y, et al. MiR-200c inhibits autophagy and enhances radiosensitivity in breast cancer cells by targeting UBQLN1. Int J Cancer. 2015;136(5):1003–1012. doi:10.1002/ijc.29065
20. Ostadrahimi S, Fayaz S, Parvizhamidi M, et al. Downregulation of miR-1266-5P, miR-185-5P and miR-30c-2 in prostatic cancer tissue and cell lines. Oncol Lett. 2018;15(5):8157–8164. doi:10.3892/ol.2018.8336
21. Wen Y, Han J, Chen J, et al. Plasma miRNAs as early biomarkers for detecting hepatocellular carcinoma. Int J Cancer. 2015;137(7):1679–1690. doi:10.1002/ijc.29544
22. Ma X, Shen D, Li H, et al. MicroRNA-185 inhibits cell proliferation and induces cell apoptosis by targeting VEGFA directly in von Hippel-Lindau-inactivated clear cell renal cell carcinoma. Urol Oncol. 2015;33(4):
23. Yin C, Zhang G, Sun R, et al. miR1855p inhibits F-actin polymerization and reverses epithelial mesenchymal transition of human breast cancer cells by modulating RAGE. Mol Med Rep. 2018;18(3):2621–2630. doi:10.3892/mmr.2018.9294
24. Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi:10.1016/j.addr.2015.05.001
25. Han G, Fan M, Zhang X. microRNA-218 inhibits prostate cancer cell growth and promotes apoptosis by repressing TPD52 expression. Biochem Biophys Res Commun. 2015;456(3):804–809. doi:10.1016/j.bbrc.2014.12.026
26. Byrne JA, Frost S, Chen Y, et al. Tumor protein D52 (TPD52) and cancer-oncogene understudy or understudied oncogene? Tumour Biol. 2014;35(8):7369–7382. doi:10.1007/s13277-014-2006-x
27. Zhang Z, Wang J, Gao R, et al. Downregulation of MicroRNA-449 promotes migration and invasion of breast cancer cells by targeting Tumor Protein D52 (TPD52). Oncol Res. 2017;25(5):753–761. doi:10.3727/096504016X14772342320617
28. Li JY, Li YY, Jin W, et al. ABT-737 reverses the acquired radioresistance of breast cancer cells by targeting Bcl-2 and Bcl-xL. J Exp Clin Cancer Res. 2012;31(1):
29. Yang P, Yang Y, An W, et al. The long noncoding RNA-ROR promotes the resistance of radiotherapy for human colorectal cancer cells by targeting the p53/miR-145 pathway. J Gastroenterol Hepatol. 2017;32(4):837–845. doi:10.1111/jgh.2017.32.issue-4
30. Fang Y, Fullwood MJ, Roles. Functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteomics Bioinf. 2016;14(1):42–54. doi:10.1016/j.gpb.2015.09.006
31. Cui LH, Xu HR, Yang W, et al. lncRNA PCAT6 promotes non-small cell lung cancer cell proliferation, migration and invasion through regulating miR-330-5p. Onco Targets Ther. 2018;11:7715–7724. doi:10.2147/OTT
32. Wu H, Zou Q, He H, et al. Long non-coding RNA PCAT6 targets miR-204 to modulate the chemoresistance of colorectal cancer cells to 5-fluorouracil-based treatment through HMGA2 signaling. Cancer Med. 2019;8(5):2484–2495. doi:10.1002/cam4.2019.8.issue-5
33. Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. (BBA)-Gene Regul Mech. 2016;1859(1):169–176. doi:10.1016/j.bbagrm.2015.06.015
34. Tian C, Deng Y, Jin Y, et al. Long non-coding RNA RNCR3 promotes prostate cancer progression through targeting miR-185-5p. Am J Transl Res. 2018;10(5):1562–1570.
35. Niu Y, Tang G. miR-185-5p targets ROCK2 and inhibits cell migration and invasion of hepatocellular carcinoma. Oncol Lett. 2019;17(6):5087–5093. doi:10.3892/ol.2019.10144
36. Pei K, Zhu JJ, Wang CE, et al. MicroRNA-185-5p modulates chemosensitivity of human non-small cell lung cancer to cisplatin via targeting ABCC1. Eur Rev Med Pharmacol Sci. 2016;20(22):4697–4704.
37. Byrne JA, Balleine RL, Schoenberg Fejzo M, et al. Tumor protein D52 (TPD52) is overexpressed and a gene amplification target in ovarian cancer. Int J Cancer. 2005;117(6):1049–1054. doi:10.1002/(ISSN)1097-0215
38. Ummanni R, Teller S, Junker H, et al. Altered expression of tumor protein D52 regulates apoptosis and migration of prostate cancer cells. FEBS J. 2008;275(22):5703–5713. doi:10.1111/j.1742-4658.2008.06697.x
39. Li J, Li Y, Liu H, et al. The four-transmembrane protein MAL2 and tumor protein D52 (TPD52) are highly expressed in colorectal cancer and correlated with poor prognosis. PLoS One. 2017;12(5):e0178515. doi:10.1371/journal.pone.0178515
40. Zhao Z, Liu H, Hou J, et al. Tumor protein D52 (TPD52) inhibits growth and metastasis in renal cell carcinoma cells through the PI3K/Akt signaling pathway. Oncol Res. 2017;25(5):773–779. doi:10.3727/096504016X14774889687280
41. Chen H, Xu H, Meng YG, et al. miR-139-5p regulates proliferation, apoptosis, and cell cycle of uterine leiomyoma cells by targeting TPD52. Onco Targets Ther. 2016;9:6151–6160. doi:10.2147/OTT
42. Yang M, Wang X, Jia J, et al. Retracted: tumor protein D52-like 2 contributes to proliferation of breast cancer cells. Cancer Biother Radiopharm. 2015;30(1):1–7. doi:10.1089/cbr.2014.1723
43. Kartha RV, Subramanian S. Competing endogenous RNAs (ceRNAs): new entrants to the intricacies of gene regulation. Front Genet. 2014;5:8. doi:10.3389/fgene.2014.00008
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.